Currently, there is no agreement regarding if it would be necessary to perform an axillary lymph node dissection (ALND) in patients who have macrometastases in the sentinel lymph node (SLN). We studied the utility of the secondary node analysis (SN), defined as the following node after the SLN in an anatomical and lymphatic pathway, as a sign of malignant axillary involvement.
MethodsAn observational, retrospective and multicenter study was designed to assess the utility of the SN as a sign of axillary involvement. Among 2273 patients with breast cancer, a valid sample of 283 was obtained representing those who had the SN studied. Main endpoints of our study were: the SLN, the SN and the ALND histological pattern. Sensitivity, specificity and precision of the test were also calculated.
ResultsSN test, in cases with positive SLN, has a sensitivity of 61.1%, a specificity of 78.7%, a positive predictive value of 45.8% and a negative predictive value of 87.3% with a precision of 74.7%.
ConclusionThe study of the SN together with the technique of the SLN allows a more precise staging of the axillary involvement, in patients with breast cancer, than just the SLN technique.
En la actualidad no existe consenso en cuanto a la necesidad de realizar linfadenectomía axilar (LA) en los casos en que se detectan macrometástasis en el ganglio centinela (GC). En este estudio se presenta la utilidad del ganglio secundario (GS), una nueva técnica diagnóstica, como factor predictor de afectación axilar.
MétodosSe diseñó un estudio observacional, retrospectivo y multicéntrico con el objetivo de validar la técnica del GS, entendido como tal el siguiente ganglio a nivel anatómico y de difusión linfogammagráfica tras el GC, como predictor de la afectación axilar. Sobre un total de 2.273 pacientes afectas de cáncer de mama se obtuvo una muestra válida de 283 pacientes a las que se había analizado el estado del GS de forma adicional. Las variables principales del estudio fueron el estado histológico del GC, del GS y del vaciamiento axilar y se valoró la sensibilidad, especificidad y exactitud de la prueba.
ResultadosLa prueba del GS, con GC positivo, presenta una sensibilidad del 61,1%, una especificidad del 78,7%, un valor predictivo positivo del 45,8% y un valor predictivo negativo del 87,3%, con una exactitud del 74,7%.
ConclusiónEl estudio del GS junto con la técnica del GC permite realizar una estadificación más precisa del estado axilar, en pacientes con cáncer de mama, en comparación con el estudio único del GC.
The surgical management of invasive breast cancer has evolved dramatically toward increasingly conservative treatments, while still maintaining oncological standards. In the axillary area, the sentinel lymph node (SLN) technique constitutes the “gold standard” method for correct breast cancer staging and subsequent determination of the most appropriate treatment.1–4 This test has a high negative predictive value and, in addition, represents one of the most relevant predictive factors after the diagnosis of the disease.5–7
At present, it is accepted not to perform axillary lymph node dissection (ALND) in the presence of micrometastasis or isolated tumor cells.8,9 However, there is controversy regarding the need for ALND in cases where macrometastasis is detected, since in these cases the SLN is the only ganglion affected in approximately 70% of patients, which means that a large number of total axillary dissections (AD) are avoidable.10–12
There is also the reasonable doubt that, by carrying out ALND, an elevated number of patients with macrometastasis in the SLN without involvement in the rest of the axilla are being overtreated, with the associated morbidity that this implies. What is more, the Giuliano et al.13,14 study concludes that patients with 1–2 affected SLN do not require ALND if they are treated with chemotherapy or radiotherapy, as they have the same disease-free survival as patient who undergo AD. For this reason, the scientific community is attempting to determine which predictive method is currently able to define in which patients ALND should be avoided.
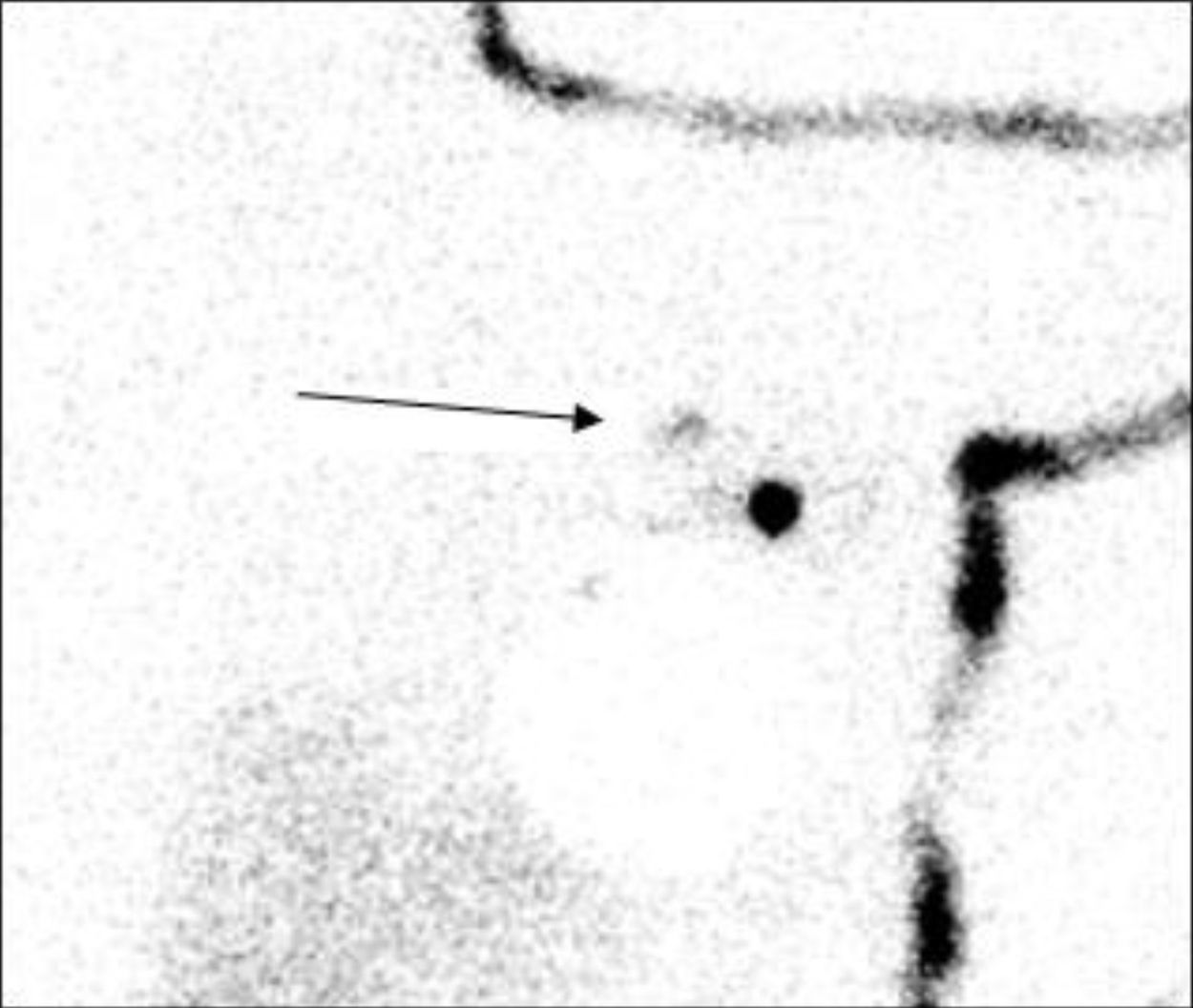
The objective of this study was to analyze the utility of the secondary node (SN) analysis as a predictive factor for axillary involvement to avoid unnecessary AD. The SN is the next lymph node both anatomically and in lymphatic drainage, located centripetally toward the thoracic duct, that metastasizes after the SLN (Fig. 1). In addition, to be defined as SN, it should present a radiotracer uptake of 10%–25% of that identified in the SLN as seen with a portable probe during surgery.
MethodsIn order to determine the validity of the SN to predict the involvement found in AD, an observational, retrospective and multicenter study was designed (Hospital Universitari Germans Trias i Pujol of Badalona, Hospital of Mataró, Municipal Hospital of Badalona, Hospital Sant Joan de Déu of Martorell, Hospital of Calella, Clínica Sagrada Familia of Barcelona and Hospital del Esperit Sant of Santa Coloma de Gramenet). Data was collected from the period between October 1997 and October 2010.
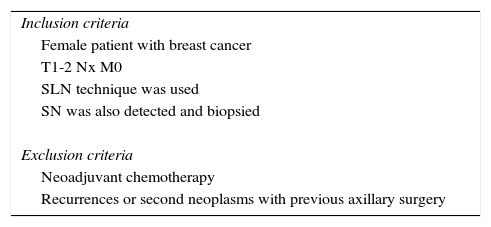
Out of a total of 2273 patients diagnosed with breast cancer, which was our reference population and control group, we obtained a valid n of 285, and the pretest probability of positive total axillary dissection was calculated in our population. The inclusion and exclusion criteria are listed in Table 1.
The main variables of the study were the histological status of the SLN, SN (independent variables) and AD (dependent variable). Secondary variables included age, tumor palpability, tumor location and radiological presentation. Histological variables were tumor size, histological type, presence of lymphatic/vascular invasion, status of estrogen and progesterone hormone receptors, and the presence of HER-2.
For the study of the SLN and SN, a nanocolloidal albumin marked with Tc99m was used as a tracer. Between 3 and 20h before surgery, a dose of 111 MBq of the peritumoral/intratumoral tracer was injected at a total volume of 0.4mL. Preoperative lymphoscintigraphy was done according to the current protocol from 14 to 16h before surgery (range 2–24h, exceptionally, in the event of an incident).
After excision of the tumor, the SLN and SN were biopsied. When the SLN was positive, conventional LA was performed.
The indication of extraction of the SN was carried out exploratively with the same portable gamma detection probe (Navigator, USSC, USA) as the SLN. The SN was defined as the next lymph node anatomically and in lymphatic drainage, in centripetal direction toward the thoracic duct, which metastasizes after the SLN. In addition, it should present radiotracer uptake 10%–25% of the uptake identified in the SLN with a portable probe during surgery.
The biopsy specimens of the SLN, SN and AD were processed following the standard procedure of the Anatomic Pathology Department at or hospital.
Statistical AnalysisThe sample size necessary for the study to reach a significance of 0.05 (95% confidence interval) was 226, with a precision of 6%.
The statistical analysis was calculated with SPSS® software (version 19.0) and the EPIDAT® program (version 3.1). For the validation of the SN as a predictive test for axillary lymph node tumor dissemination, we assessed:
- a)
the capacity of SN for detecting pathological AD
- b)
the capacity of SN for detecting non/pathological AD
- c)
the exactness of the SN test
In order to evaluate SN as a diagnostic test, 4 patient groups were created: patients with positive SLN and SN, patients with positive SLN and negative SN, patients with negative SLN and positive SN, and patients with negative SLN and SN.
Afterwards, we compared the results obtained in the histologic SN analysis with those obtained from the AD.
ResultsFrom the medical files of 2273 patients, we established an estimated prevalence of positive AD and, therefore, the probability of pretest total axillary dissection in our population of 8.8%.
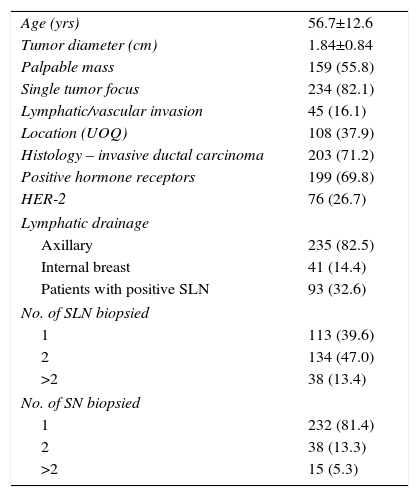
As previously mentioned, a valid sample of 285 cases was obtained. The results of the clinical–radiological variables of the sample are described in Table 2. In none of the variables studied were significant differences found between the values of the sample and those of the reference population.
Results of the Clinical–Radiological Variables of the Sample, n (%).
| Age (yrs) | 56.7±12.6 |
| Tumor diameter (cm) | 1.84±0.84 |
| Palpable mass | 159 (55.8) |
| Single tumor focus | 234 (82.1) |
| Lymphatic/vascular invasion | 45 (16.1) |
| Location (UOQ) | 108 (37.9) |
| Histology – invasive ductal carcinoma | 203 (71.2) |
| Positive hormone receptors | 199 (69.8) |
| HER-2 | 76 (26.7) |
| Lymphatic drainage | |
| Axillary | 235 (82.5) |
| Internal breast | 41 (14.4) |
| Patients with positive SLN | 93 (32.6) |
| No. of SLN biopsied | |
| 1 | 113 (39.6) |
| 2 | 134 (47.0) |
| >2 | 38 (13.4) |
| No. of SN biopsied | |
| 1 | 232 (81.4) |
| 2 | 38 (13.3) |
| >2 | 15 (5.3) |
UOQ: upper outer quadrant; SLN: sentinel lymph node; SN: secondary lymph node; HER-2: human epidermal growth factor receptor 2.
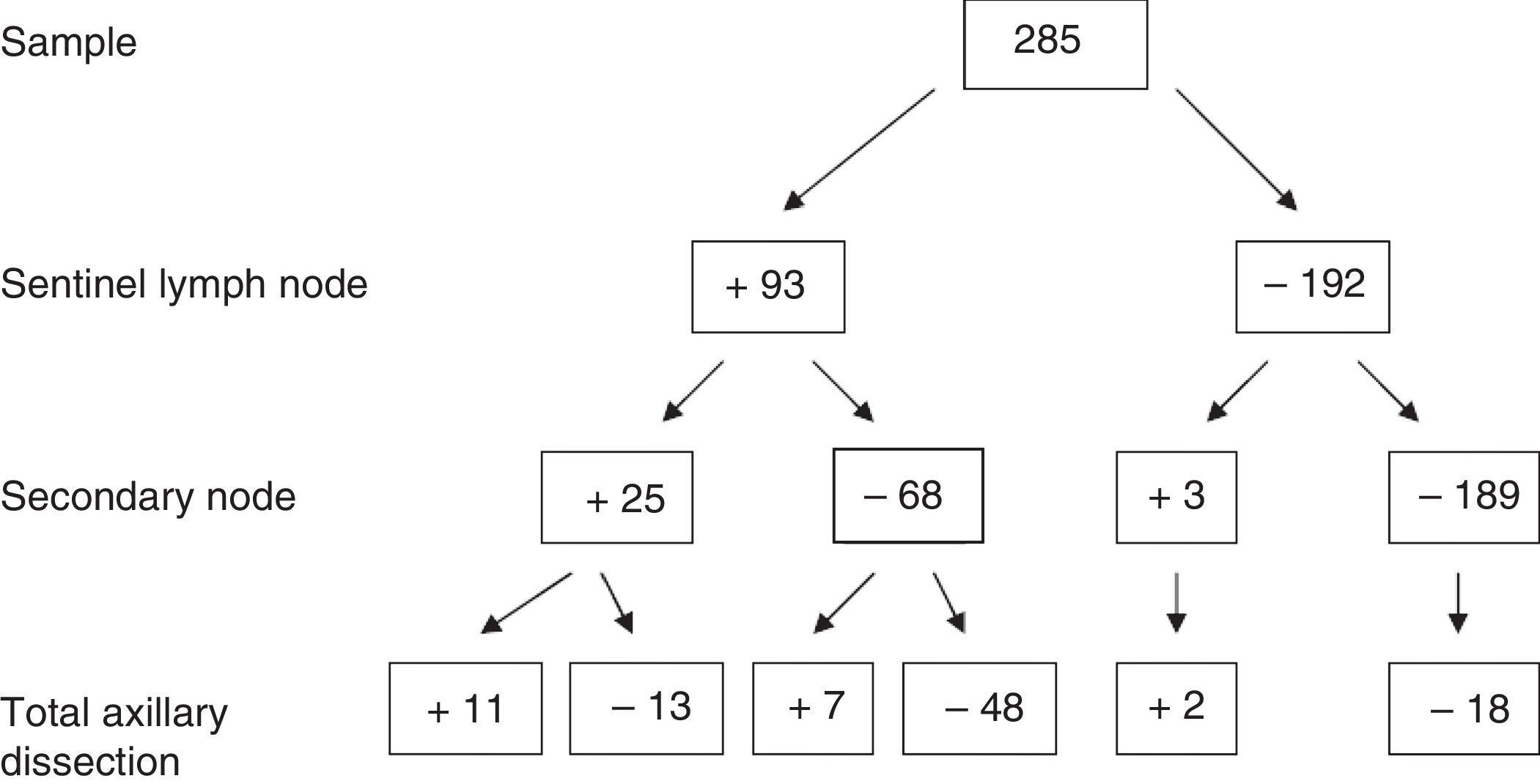
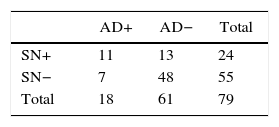
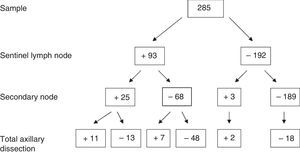
Regarding the absolute results of the sample for SLN, SN and axillary dissection, out of the 285 patients, 93 cases with positive SLN were obtained, of which 25 presented SN involvement and 68 negative SN. Of those with SN involvement, 10 also presented axillary involvement (40%). However, in the SN-negative group, only 7 had axillary infiltration (10.3%). Complete results are provided in Fig. 2.
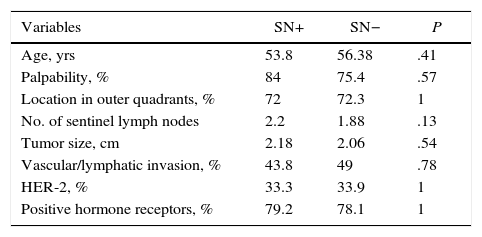
In the homogeneity assessment between the groups with positive and negative SN, there were no significant differences between positive or negative SN in terms of age, secondary clinical variables, tumor variables, number of SLN analyzed or in the number of SN analyzed (Table 3). Based on these results, it can be stated that there is homogeneity between the groups with positive and negative SN, within the positive SLN sample.
Results of the Secondary Clinical–Radiological Variables for the Positive SLN Group.
| Variables | SN+ | SN− | P |
|---|---|---|---|
| Age, yrs | 53.8 | 56.38 | .41 |
| Palpability, % | 84 | 75.4 | .57 |
| Location in outer quadrants, % | 72 | 72.3 | 1 |
| No. of sentinel lymph nodes | 2.2 | 1.88 | .13 |
| Tumor size, cm | 2.18 | 2.06 | .54 |
| Vascular/lymphatic invasion, % | 43.8 | 49 | .78 |
| HER-2, % | 33.3 | 33.9 | 1 |
| Positive hormone receptors, % | 79.2 | 78.1 | 1 |
SN: secondary lymph node; HER-2: human epidermal growth factor receptor 2.
As a diagnostic test, the SN showed:
Group 1: positive SLN and SN (n=25); AD was done in those who presented macrometastasis, meaning 24. Involvement of the AD was 44% (n=11/24).
Group 2: positive SLN and negative SN (n=68); AD was done in the 55 who presented macrometastasis. Positive AD was observed in 12.7% (n=7/55).
Group 3: negative SLN and positive SN (n=3); AD was done in 2. The AD was positive in 66% of cases (n=2), detecting in this manner possible false negatives in the SLN.
Group 4: negative SLN and SN (n=189). All the cases with AD (n=18), which were the first cases at the beginning of the series when the SLN technique was in the validation phase, presented negative AD.
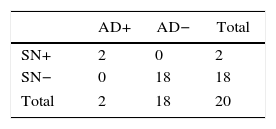
The stratified results according to SLN stage and SN are shown in Tables 4 and 5. Based on these results, it was determined that the SN test with positive SLN presented a sensitivity of 61.1%, specificity of 78.7%, PPV of 45.8% and NPV of 87.3%, with a precision of 74.7%. When the SLN was negative, the SN had a sensitivity, specificity PPV, NPV and exactness of 100%.
At present, the need to perform ALND in certain circumstances with positive SLN is being called into question.8,15,16 The current conflict is due to the rise of several studies in which it was observed that, in more than 60% of cases with positive SLN, the remaining AD lymph nodes were negative, which brings about unnecessary and unavoidable morbidity.17–19 Several methods have been proposed to discern which therapeutic approach would be best in these cases.
Giuliano et al., based on the ACOSOG-Z0011 study, claim that there were no significant differences in the disease-free survival times whether or not ALND was done in patients with SLN+ who had been administered axillary radiation therapy.13 However, the study mentioned has received multiple methodological criticisms in terms of its clinical applicability.9
Another proposed option is the use of nomograms that stratify the risk of axillary involvement according to the characteristics of the patients and neoplasms. One of the studies with better results, Tenon 5, has a sensitivity of 94% and a specificity of 59% with an NPV of 97.5% and a PPV of 38%.20 These values are among the best from all the options analyzed so far.10,21–26
Alternatively, it has been postulated that the intraoperative molecular study of the SLN could be an adequate indicator.27,28 As described in the article by Peg et al.,29 starting from a tumor load exceeding 15000, a sensitivity of 77% is obtained, the NPV was 85%, PPV 41% and specificity 55%. These values do not seem sufficiently valid to implement said technique as a “gold standard”.
Although our study has limitations related with the fact that it is retrospective and there may be selection bias involved, the results of our study on SN are promising since they are virtually equal to the results of Tenon 5 and surpass other options.
The application of this technique would allow us to detect a large part of the cases in which the SLN is the only affected lymph node, which could prevent approximately 73% of unnecessary AD (68/93), with a false negative rate somewhat higher than the SLN (12% vs 5%–10%).
In addition, a noteworthy fact about the SN analysis is that, as described in the results, it is possible to detect possible false negative SLN. This implies that it would be able to identify and more adequately treat patients who, otherwise, would be undertreated.
Therefore, if good sensitivity, specificity, PPV and NPV are taken into account, the SN technique has some of the best results among existing options, and its analysis could become a valid, more precise alternative for studying the axillary node status. It is a simple and safe technique that would enable the optimization of the treatment of axillary metastasis in breast cancer, while maintaining quality standards.
Authorship/collaborationManel Cremades: analysis and interpretation of the results, article composition.
Mireia Torres: study design, data collection, analysis and interpretation of the results.
Montse Solà: analysis and interpretation of the results, article composition.
Jordi Navinés: study design, analysis and interpretation of the results.
Icíar Pascual: study design, analysis and interpretation of the results.
Antonio Mariscal: study design, analysis and interpretation of the results.
Albert Caballero: article composition, critical review and approval of the final version.
Eva Castellà: study design, analysis and interpretation of the results
Miguel Ángel Luna: study design, analysis and interpretation of the results.
Joan Francesc Julian: study design, data collection, analysis and interpretation of the results, article composition, critical review and approval of the final version.
Conflict of InterestsThe authors have no conflict of interests to declare.
The authors would like to thank Dr. Jaime Fernández Llamazares and Dr. David Parés for their contribution to the development of the project and their collaboration with its later publication.
Please cite this article as: Cremades M, Torres M, Solà M, Navinés J, Pascual I, Mariscal A, et al. Ganglio secundario como indicador de linfadenectomía axilar en pacientes afectas de cáncer de mama. Cir Esp. 2017;95:536–541.