This clinical practice guideline (CPG) emerges as an initiative of the scientific committee of the Spanish Society of Thoracic Surgery.
We formulated PICO (patient, intervention, comparison, and outcome) questions on various aspects of spontaneous pneumothorax.
For the evaluation of the quality of evidence and preparation of recommendations we followed the guidelines of the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) working group.
Esta guía de práctica clínica (GPC) surge como iniciativa del comité científico de la Sociedad Española de Cirugía Torácica.
Para elaborar dicha GPC se han formulado las preguntas PICO (paciente, intervención, comparación y outcome o variable resultado) sobre distintos aspectos del neumotórax espontáneo.
Para la evaluación de la calidad de la evidencia y elaboración de las recomendaciones se han seguido las directrices del grupo de trabajo Grading of Recommendations, Assessent, Development and Evaluation (GRADE).
External manuscript review:
- -
SECT scientific committee: Raúl Embún, Néstor Martínez, Sergi Call, Ricard Ramos, Beatriz de Olaiz
- -
Nicolás Moreno, Hospital Universitario Vírgen del Rocío (Seville)
- -
Laureano Molins, Hospital Universitario Clínic (Barcelona)
Pneumothorax is one of the most frequent pleural diseases treated in hospitals around the world, and it is responsible for 20% of hospitalizations in Thoracic Surgery Departments.1 Its incidence is quite varied, as age-adjusted figures for primary cases range from 16.8 cases per 100000 inhabitants/year (males: 24, and females: 9.8)2 collected in England to 4.2 cases per 100000 inhabitants/year in the USA,3 or 3.8 per 100000 inhabitants with secondary pneumothorax (males: 6.3, and females: 2).
The creation of these clinical practice guidelines (CPG) is justified, firstly, by the high incidence of spontaneous pneumothorax (SP) in the general (and actively employed) population, along with the consumption of resources of its initial management. Furthermore, the same patient with SP can be diagnosed and treated in different ways depending on the hospital or the doctor who is treating him/her. All this can produce different clinical results, unequal consumption of resources and some confusion in patients who consult different professionals to obtain a second opinion. The target population of these CPG includes patients with primary (PSP) and secondary (SSP) spontaneous pneumothorax.
The characteristics of this medical problem entail the need for adequate coordination between different specialists responsible for the care of patients with pneumothorax. It is precisely this target audience that these CPG are aimed at because, depending on the healthcare system of each autonomous community in Spain, patients will be treated by different medical professionals, including general emergency and primary care physicians, thoracic surgeons, pulmonologists or general surgeons. In addition, patients may be treated and their progress followed by any of the aforementioned professionals, so the guidelines aim to standardize the management criteria among the different medical professionals and create an environment of fluid communication and understanding. The main objective of these CPG is to summarize the best evidence currently available.
MethodologyThese CPG have emerged as an initiative of the scientific committee of the Spanish Society of Thoracic Surgery (Sociedad Española de Cirugía Torácica, SECT). PICO questions (patient, intervention, comparison and outcome) have been formulated about the different aspects of SP. A search was carried out using the TRIP database, Cochrane Database of Systematic Reviews (The Cochrane Library) and OVID platforms using MEDLINE and EMBASE resources. A flow chart was created for each PICO question. The search process was conducted until May 2015 and was not limited by any language.
To evaluate the quality of evidence and the development of the recommendations, the guidelines of the GRADE4 workgroup were followed (Appendix A).
An update of the guidelines is planned every 3–5 years maximum, or in a shorter period if new scientific evidence appears that could modify some of the recommendations offered by the guidelines.
ResultsEtiopathogenesisMany studies have tried to relate the appearance of pneumothorax with different factors. On the one hand are anthropological aspects, such as age, height or weight.5 On the other hand are the presence of toxic factors, such as tobacco and its destructive effects on the pulmonary parenchyma, which increase the risk of developing pneumothorax by 20-fold6 (GRADE recommendation 1B). Likewise, the presence of pulmonary diseases, such as chronic obstructive pulmonary disease, cystic fibrosis or interstitial lung diseases, etc., have been related to the appearance of secondary pneumothorax.7 It is worth mentioning that studies have tried to correlate the appearance of SP with changes in atmospheric pressure,8–11 without reaching conclusive results.
Finally, we can emphasize that the physiopathological mechanisms of SP remain unknown, although it is assumed that the primary form is the result of the formation and subsequent rupture of subpleural blebs or blisters.12 There are hypotheses related with structural changes in the parenchyma (emphysematous changes): because the pressure gradient in the vertices is higher, this causes greater distension of the apical subpleural alveoli, with the consequent formation of blebs and their subsequent rupture12 (low level of evidence).
In any case, the pathophysiology is multifactorial and remains unknown, giving rise to the entry of air into the pleural cavity depending on the mechanism of each baseline disease.3,13
EvolutionWhat does seem to be clear is the tendency for recurrence of this disease according to the treatment used and follow-up period,12 although its evolution cannot be predicted. It is estimated that the number of recurrences of PSP is around 30%, with a range between 16% and 52%, and secondary pneumothorax ranges from 40% to 56%.5,12,14,15 Generally, recurrence usually occurs in the first 6 months after the first episode. After a second pneumothorax and without adequate treatment, the possibility of a third episode is nearly 80%.16
Factors that we found related with recurrence included pulmonary fibrosis detected in a radiological study, age over 60 years or high body mass index5 (low level of evidence). It has been shown that continued smoking is related with a greater number of pneumothorax episodes7 (GRADE recommendation 1B).
MortalityMortality associated with pneumothorax is low. For example, the mortality rate calculated in the United Kingdom is 0.62 per million inhabitants/year for women and 1.26 for men.3
When classified by type, PSP has an estimated rate of 0.09% in males and 0.06% in females,3 while in secondary spontaneous pneumothorax the mortality rate is higher due to the baseline pulmonary disease, as well as the lower respiratory reserve.
DiagnosisUsually, an appropriate anamnesis and a proper physical examination are enough to establish the diagnosis of SP. Complementary studies (such as simple chest radiography) are indicated when it is necessary to confirm the diagnosis or in certain circumstances that we will discuss further ahead.
The most characteristic symptom is ipsilateral pleuritic pain, often accompanied by a certain degree of dyspnea and irritative cough13; if the pneumothorax is small, these symptoms may disappear in the first 24h, even if the condition is not resolved.17 However, symptoms do not seem to be a reliable indicator of the pneumothorax size (low level of evidence),7 since the symptoms reported by the patient are more related with the functional respiratory reserve than the degree of lung collapse. Different criteria18 have been proposed to assess the clinical stability of patients, and obvious dyspnea is an important factor when determining the most appropriate clinical management (low level of evidence).7
The ACCP defines clinical stability as a respiratory rate lower than 24 breaths/min, heart rate between 60 and 120beats/min, systemic blood pressure within the normal range, arterial oxygen saturation breathing room air greater than 90%, and patient ability to pronounce complete sentences between breaths.18 The BTS adds the absence of dyspnea to these stability criteria.7 Patients who do not meet one or more of the previously mentioned parameters are considered clinically unstable.
The coexistence of previous episodes of pneumothorax (homo- or contralateral), baseline pulmonary disease or certain toxic habits (e.g., smoking) influences the progression of symptoms and the likelihood of recurrence, so they also have therapeutic implications. Finally, there are some special cases (recurrent pneumothorax in young women of reproductive age) in whom the presence of infertility, previous uterine surgery and more rarely hemoptysis or hemothorax related with menstruation may indicate a catamenial origin.19,20
Although the physical examination can be anodyne (when the pneumothorax is small), there is usually sinus tachycardia together with decreased motility of the affected hemithorax, reduction or absence of the vesicular murmur, increased resonance upon percussion and decreased voice transmission,17 sometimes with subcutaneous emphysema.
The systematic use of electrocardiography, baseline arterial blood gas or respiratory function tests is not indicated in patients with SP1 (GRADE recommendation 1C). The use of diagnostic imaging techniques is mainly justified by the need for diagnostic confirmation of the episode and, to a lesser extent, by other causes that we will see below. In addition, imaging techniques allow for pneumothorax to be classified according to its size.
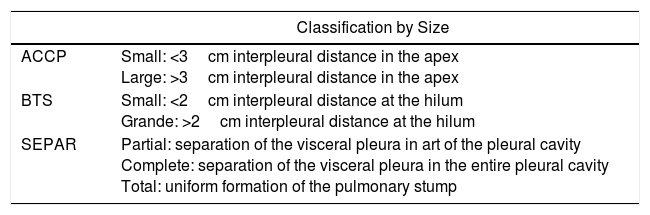
The diversity of methods published to quantify the size of the pneumothorax21–23 reflects the lack of consensus on the system to be used, since the inconstant and uneven conformation of the lung parenchyma during the episode generally leads to an underestimation of the pneumothorax volume. For the quantification of pneumothorax size, the most commonly used indices for the measurement of pulmonary collapse are those by Rhea et al.,21 Light22 and Collins et al.23 We consider that the classification proposed by SEPAR is that which best adapts to clinical use (Table 1).
Different Classifications According to Size of the Pneumothorax.
| Classification by Size | |
|---|---|
| ACCP | Small: <3cm interpleural distance in the apex Large: >3cm interpleural distance in the apex |
| BTS | Small: <2cm interpleural distance at the hilum Grande: >2cm interpleural distance at the hilum |
| SEPAR | Partial: separation of the visceral pleura in art of the pleural cavity Complete: separation of the visceral pleura in the entire pleural cavity Total: uniform formation of the pulmonary stump |
Simple chest radiography is usually the test of choice due to its availability, innocuity and low cost. Normally, it is enough to perform a standard projection (posteroanterior, standing and in forced inspiration), since the different published guidelines do not systematically recommend the forced expiration maneuver7,18,24,25 (GRADE recommendation 1B). A clear hyperdense line (corresponding to the line of the visceral pleura) is usually identified, with absence of bronchopulmonary trauma distal to it, although caution should be exercised given the possibility of “false pneumothorax” images due to skin folds, alterations of the thoracic wall or parenchymal lesions such as cysts or blisters. We should be equally cautious when using digitalized images (PACS), as small pneumothoraces may not be very apparent and may even go unnoticed.7 In the diagnosis of tension pneumothorax, complete pulmonary collapse is observed with contralateral mediastinal deviation and inversion of the homolateral hemidiaphragm.26
From 10% to 20% of cases have associated eosinophilic pleural effusion, secondary to the irritation of the parietal pleura by air; infrequently, it may present with hemothorax, usually secondary to rupture of vascularized pleural adhesions during lung collapse.
Ultrasound has been proposed as an alternative to radiotherapy for pneumothorax27 because of the possibility of its bedside use, greater sensitivity and equal specificity as conventional radiology. However, its accuracy seems highly dependent on the operator who performs it28 and on the existence of underlying lung disease. In any case, and once the pneumothorax has been detected by ultrasound, it seems prudent to perform a simple radiograph to quantify its size and detect possible underlying thoracic comorbidity.29 Thus, the use of ultrasound seems reasonable as a diagnostic tool to complement conventional radiography or in the follow-up of pneumothorax by experienced clinicians or radiologists (GRADE recommendation 2C).
Thoracic computed tomography (CT) is not recommended systematically for all patients with an initial episode of SP (GRADE recommendation 1B),18 and its indication is limited to certain specific cases. Thus, it could be useful in some of the following situations7:
- –
Detection of small-sized pneumothorax
- –
Estimation of the actual size of a pneumothorax, as it is the ideal technique for size determinations (GRADE recommendation 2A)7
- –
Existence of emphysema or bullous disease with possible indication for surgery
- –
Confirm or rule out incorrect position of a chest drain tube
- –
Diagnosis of other underlying pulmonary diseases
- –
Determination of the etiology of the pneumothorax in patients with recurrent episodes; for instance, in cases of catamenial pneumothorax, pneumoperitoneum, endometrial implants in the diaphragm or total/partial diaphragmatic rupture may occasionally appear.19,20
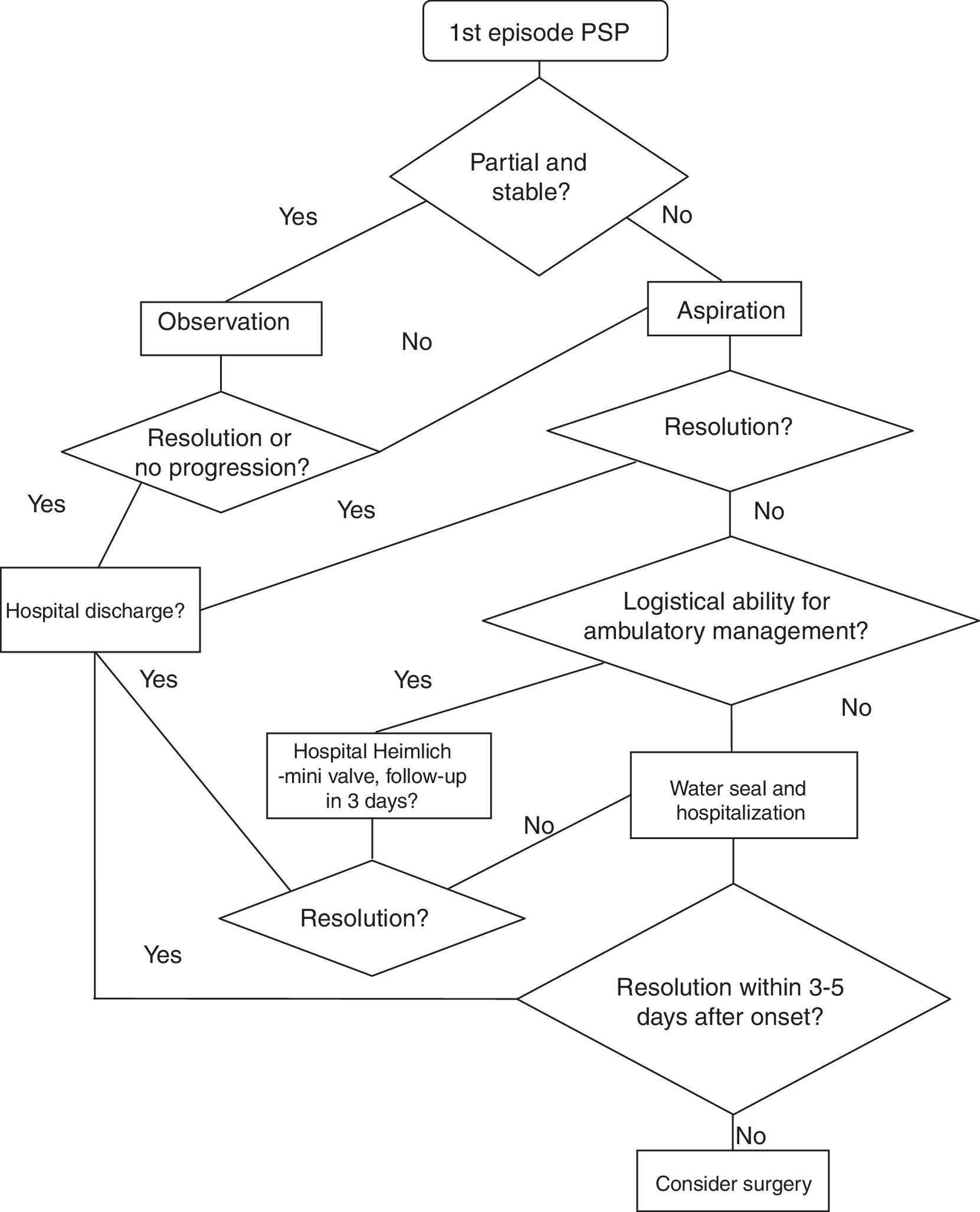
In patients with an initial episode of PSP and in those with no other indication requiring intervention, the following strategy is followed:
- a.
Partial pneumothorax and stable patient: observation. Thus, in these patients, it can be noticed again during the first 4–6 degrees in the emergency room. If there is no need for a clinical or radiological examination, that patient can be discharged to follow-up (<1 week). In case of worsening, it is a complete/total or unstable pneumothorax.
- b.
Non-partial or unstable pneumothorax: we must take actions to drain the air. Fig. 1 presents a PSP treatment algorithm.
There is sufficient evidence about needle-aspiration as an outpatient strategy for these patients without worsening needs or re-hospitalization with regard to pneumothorax resolutions (GRADE recommendation 1A).30,31 Because the studies on which this recommendation is based exclude patients with hemopneumothorax, tension pneumothorax and bilateral pneumothorax, we believe it is reasonable not to apply this recommendation in these situations. Likewise, 2 systematic reviews have compared needle-aspiration versus hospitalization with drainage and concluded that needle-aspiration should be the first step in cases with indication for the evacuation of air.32,33 Despite numerous references in the literature, in many countries this is not the strategy of choice when faced with a first episode of PSP.1,34
In order to observe the resolution, a follow-up radiograph is taken 2–4h after needle-aspiration. If the condition is not resolved, the patient is hospitalized and treated with a seal system. If the leak persists after 3–5 days, surgery is considered.
Another ambulatory strategy is based on management with a Heimlich valve/mini chest tube. A clinical trial has compared both outpatient strategies, showing similar success and satisfaction rates35 (GRADE recommendation 2A).
In addition, it is necessary to assess the logistical resources of the hospital and patients, since this strategy requires outpatient monitoring.
If the patient is discharged with portable drainage and the leaks persists after three days, the patient is re-hospitalized and evaluated for surgery (3–5 days after the initial episode).
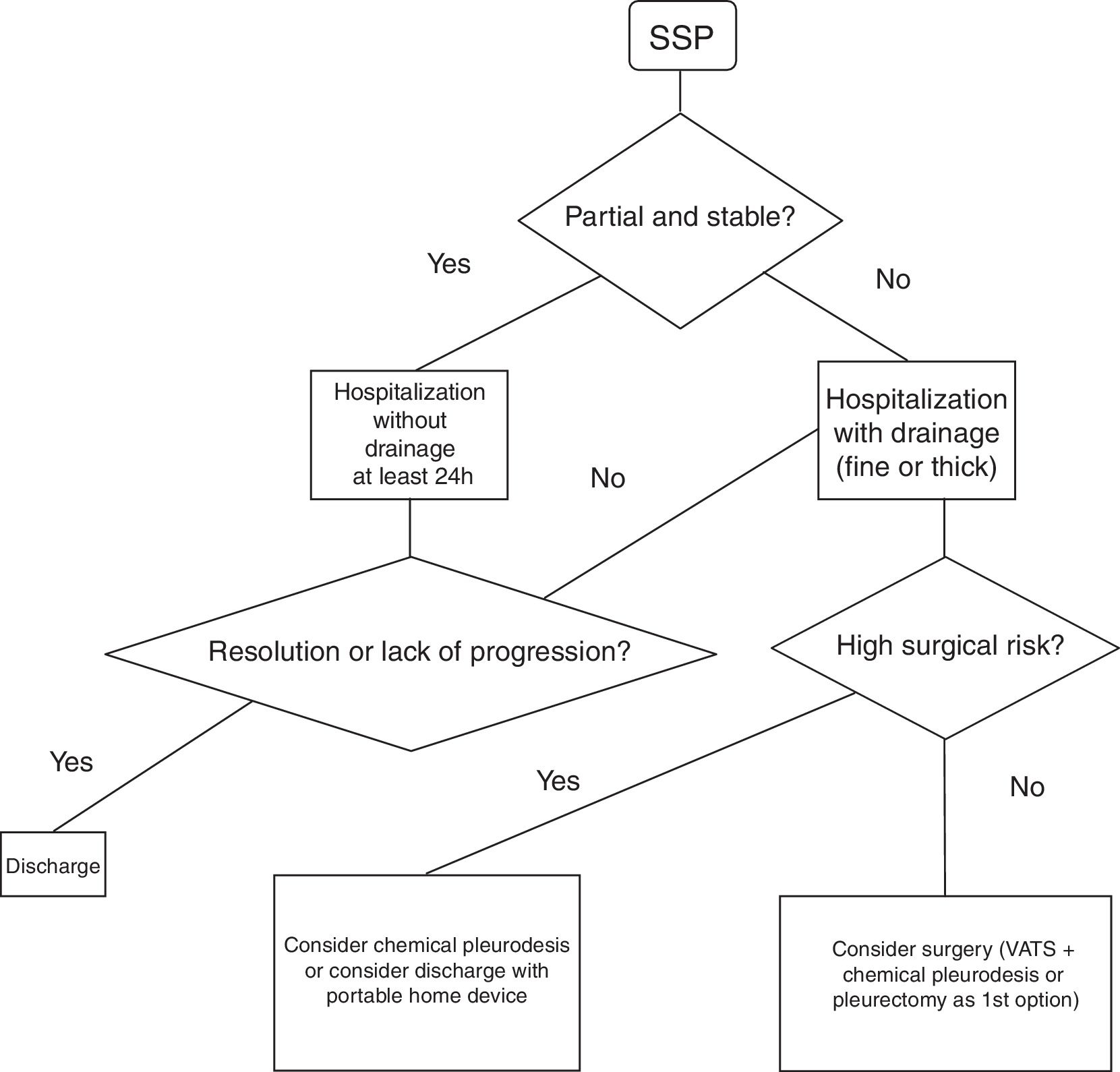
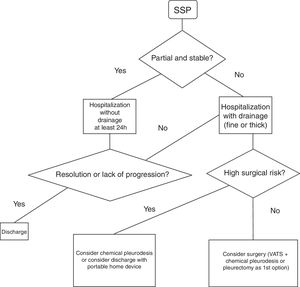
Spontaneous Secondary PneumothoraxHospital admission is recommended for at least 24h to control the pneumothorax and the baseline disease7 (GRADE recommendation 1C). It is unclear which type of drainage should be used in the treatment of these episodes. The 2010 BTS guidelines recommend (GRADE recommendation B) the use of fine-gauge drains based on the study published by Tsai et al.,36 where they conclude that fine-gauge drains should be used initially in SSP since they observed a success rate (success defined as no need for another drain or surgery) similar to the large-caliber drain group (success defined as no need for surgery).
Another retrospective study37 compared the use of the fine-gauge catheters with thick-gauge catheters in patients with primary and secondary pneumothorax. In the secondary group, failure was observed (need for a thick-gauge drain after the non-resolution of the case with a thin-gauge drain) of 52% (GRADE recommendation 2C).
Fig. 2 demonstrates a treatment algorithm for SSP.
Surgical Treatment of PneumothoraxCurrently, the accepted surgical indications for the treatment of pneumothorax are1,17:
- –
No resolution of the pneumothorax with the described strategy for prolonged air leak (3–5 days) or impossibility for lung re-expansion
- –
Second episode (ipsilateral or contralateral)
- –
Synchronous bilateral episode
- –
Hemothorax associated with pneumothorax38,39
- –
Professions at risk (pilots, divers)
While these are the formal indications in the case of PSP, in patients with SSP the indications described should be considered cautiously, since these patients have low respiratory functional reserve to tolerate single-lung ventilation, more associated comorbidities, a markedly bullous pulmonary parenchyma without a specific target area and with the possibility of extensive pleuropulmonary adhesions that can make surgery more difficult. Thus, in patients with SSP in whom the benefit-risk balance of the intervention is not so evident or those who present a formal contraindication for surgery, decisions should be made individually and non-surgical treatments assessed to avoid recurrences, such as chemical pleurodesis through the drain tube and, in the case of persistent air leaks, ambulatory management should be considered with a portable drainage system at home (Pneumostat, Minipleurevac, Heimlich valve, etc.).40
Currently, the surgical technique is based on the identification and resection of target areas or bullous areas using endostaplers and an associated pleurodesis technique, be it mechanical abrasion of the parietal pleura, chemical, or apical pleurectomy.41–44 Materials may be used such as bovine pericardium or PTFE to reinforce the mechanical sutures in very bullous parenchyma minimize the leak area in the suture area.45
VATS surgery has been shown to be a safe procedure with few complications and offers advantages in terms of better postoperative recovery, better pain control, shorter hospital stay and better esthetic results compared to approaches with thoracotomy.46–51 A meta-analysis52 of the literature reports a risk for recurrence of pneumothorax in thoracoscopic surgery 4 times greater than in open surgery (approximately 1% in open surgery vs 5% in VATS), when in both a similar type of pleurodesis is used; however, the assumable rate of recurrence through the thoracoscopic approach associated with the postoperative advantages that this approach offers compared to thoracotomy justify its use as a primary option7,52–54 with greater scientific evidence in the case of primary pneumothorax (GRADE recommendation 1A) than in secondary presentations (GRADE recommendation 1B). The results of the French database with 7396 patients go in the same direction with shorter hospitalization but a higher rate of recurrence (3.8% vs 1.8% of thoracotomies).55
On the other hand, bullous resection surgery should be associated with pleurodesis to reduce the probability of recurrence, since recurrence in patients treated with VATS without associated pleurodesis can reach 24%,56 although other studies have reported much lower figures in patients in whom no pleurodesis technique was associated (6.3%).57
Pleurodesis techniques are divided into mechanical or chemical.
- 1.
Mechanical:
- a.
Abrasion: production of an inflammatory reaction on the parietal pleura that facilitates pleural symphysis once lung re-expansion is re-established57
- b.
Pleurectomy: total or partial excision of the parietal pleura. VATS pleurectomy has been shown to be comparable with pleurectomy by means of thoracotomy in the treatment of SP. A systematic review reports shorter hospital stays and less need for analgesia in the VATS option.53
- 2.
Chemical:
- a.
Talc: pulverized talc is instilled into the pleural cavity after bullectomy, causing an inflammatory foreign-body reaction in the pleural cavity.
- b.
Other agents: doxycycline, minocycline, bleomycin, povidone iodine and Picibanil (OK-432),58 produce an inflammatory reaction in the pleural cavity.
There is much controversy about which pleurodesis should be used, and in the literature there are articles with conclusions in opposing directions.
Two recent clinical trials compared mechanical abrasion after bullectomy versus no added pleurodesis technique and reinforcement of the stapleline with absorbable cellulose mesh and fibrin glue. Both conclude that abrasion does not improve the results in terms of recurrence rate. In the study comparing abrasion with glue,59 recurrences were observed in 9.3% of the glue coating group versus 11.4% in the abrasion group. In the comparison study with no added technique, recurrences were observed in 5.5% of the abrasion group and 6.3% in the group without abrasion.60
Another clinical trial compared mechanical abrasion with pleurectomy and concluded that both had a similar recurrence rate (6.2% in the abrasion group and 4.6% in the pleurectomy group) and that abrasion presented fewer complications (bleeding) than pleurectomy.61 In contrast, other studies conclude that the number of complications between pleurectomy and abrasion are similar and that pleurectomy has a lower recurrence rate.62,63
Mechanical pleurodesis has also been compared with chemical pleurodesis. A retrospective study with 432 consecutive patients with pleurectomy and the use of talc concluded that the benefit of the use of talc was the lower rate of recurrence (1.8% compared to 9.1%).64 In a recent clinical trial that randomized patients at high risk of recurrence, pleurectomy was compared to mechanical abrasion with added minocycline instillation, and low as well as similar relapse rates were observed (3.8% in both groups).65
Given all this, and with the necessary caution due to the differing results, we can conclude that it is not clear whether mechanical abrasion contributes any benefit and that pleurectomy seems to obtain better results in terms of recurrence, even though a study has shown a higher rate of bleeding. As for chemical pleurodesis, talc seems to reduce the number of recurrences, although there are many groups that do not use it for its side effects, and minocycline after abrasion presents results similar to pleurectomy.
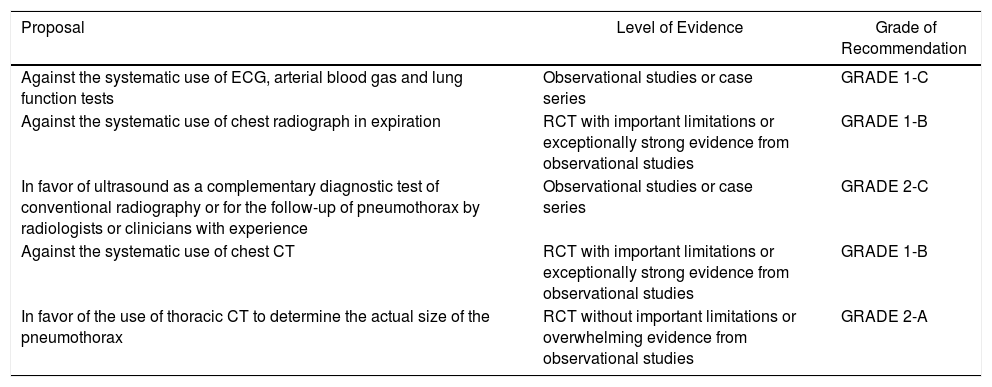
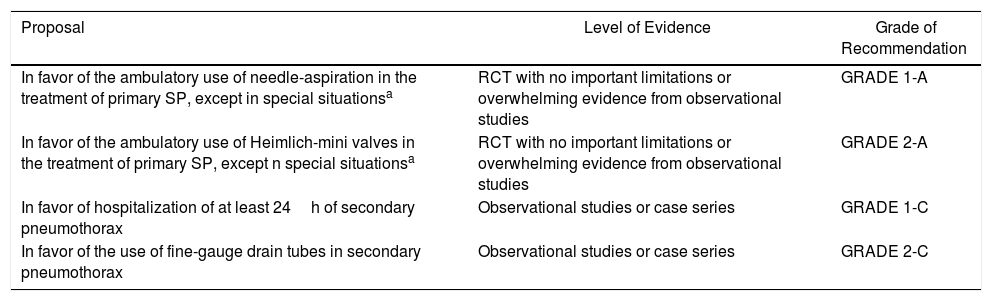
Summary of the RecommendationsEtiopathogenesisDiagnosis| Proposal | Level of Evidence | Grade of Recommendation |
|---|---|---|
| Against the systematic use of ECG, arterial blood gas and lung function tests | Observational studies or case series | GRADE 1-C |
| Against the systematic use of chest radiograph in expiration | RCT with important limitations or exceptionally strong evidence from observational studies | GRADE 1-B |
| In favor of ultrasound as a complementary diagnostic test of conventional radiography or for the follow-up of pneumothorax by radiologists or clinicians with experience | Observational studies or case series | GRADE 2-C |
| Against the systematic use of chest CT | RCT with important limitations or exceptionally strong evidence from observational studies | GRADE 1-B |
| In favor of the use of thoracic CT to determine the actual size of the pneumothorax | RCT without important limitations or overwhelming evidence from observational studies | GRADE 2-A |
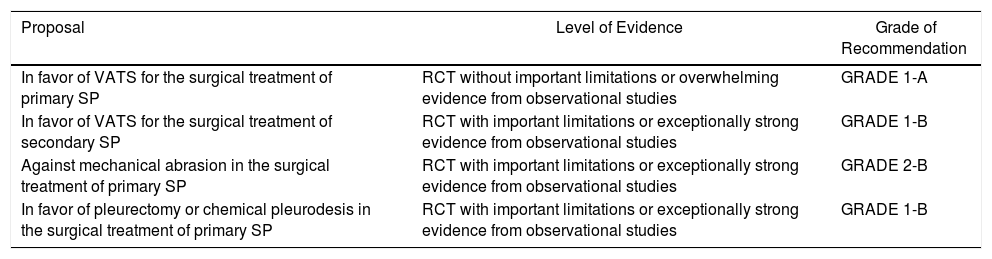
| Proposal | Level of Evidence | Grade of Recommendation |
|---|---|---|
| In favor of the ambulatory use of needle-aspiration in the treatment of primary SP, except in special situationsa | RCT with no important limitations or overwhelming evidence from observational studies | GRADE 1-A |
| In favor of the ambulatory use of Heimlich-mini valves in the treatment of primary SP, except n special situationsa | RCT with no important limitations or overwhelming evidence from observational studies | GRADE 2-A |
| In favor of hospitalization of at least 24h of secondary pneumothorax | Observational studies or case series | GRADE 1-C |
| In favor of the use of fine-gauge drain tubes in secondary pneumothorax | Observational studies or case series | GRADE 2-C |
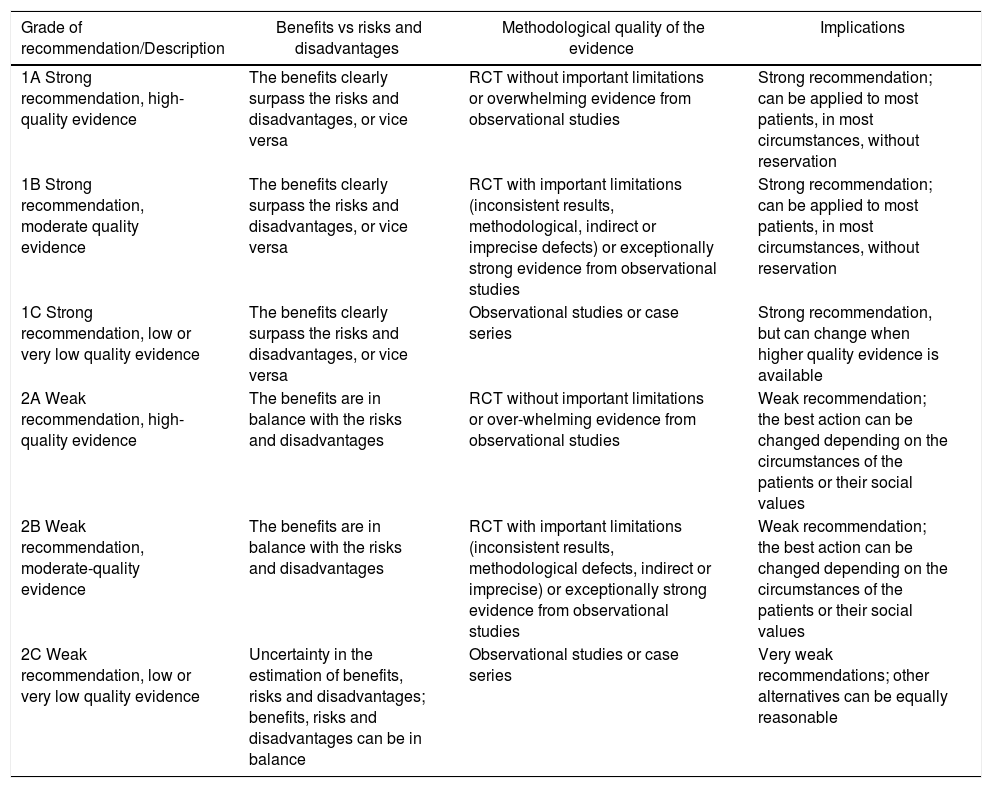
| Proposal | Level of Evidence | Grade of Recommendation |
|---|---|---|
| In favor of VATS for the surgical treatment of primary SP | RCT without important limitations or overwhelming evidence from observational studies | GRADE 1-A |
| In favor of VATS for the surgical treatment of secondary SP | RCT with important limitations or exceptionally strong evidence from observational studies | GRADE 1-B |
| Against mechanical abrasion in the surgical treatment of primary SP | RCT with important limitations or exceptionally strong evidence from observational studies | GRADE 2-B |
| In favor of pleurectomy or chemical pleurodesis in the surgical treatment of primary SP | RCT with important limitations or exceptionally strong evidence from observational studies | GRADE 1-B |
The authors have no conflict of interests to declare.
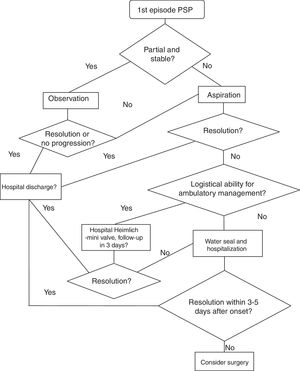
| Grade of recommendation/Description | Benefits vs risks and disadvantages | Methodological quality of the evidence | Implications |
|---|---|---|---|
| 1A Strong recommendation, high-quality evidence | The benefits clearly surpass the risks and disadvantages, or vice versa | RCT without important limitations or overwhelming evidence from observational studies | Strong recommendation; can be applied to most patients, in most circumstances, without reservation |
| 1B Strong recommendation, moderate quality evidence | The benefits clearly surpass the risks and disadvantages, or vice versa | RCT with important limitations (inconsistent results, methodological, indirect or imprecise defects) or exceptionally strong evidence from observational studies | Strong recommendation; can be applied to most patients, in most circumstances, without reservation |
| 1C Strong recommendation, low or very low quality evidence | The benefits clearly surpass the risks and disadvantages, or vice versa | Observational studies or case series | Strong recommendation, but can change when higher quality evidence is available |
| 2A Weak recommendation, high-quality evidence | The benefits are in balance with the risks and disadvantages | RCT without important limitations or over-whelming evidence from observational studies | Weak recommendation; the best action can be changed depending on the circumstances of the patients or their social values |
| 2B Weak recommendation, moderate-quality evidence | The benefits are in balance with the risks and disadvantages | RCT with important limitations (inconsistent results, methodological defects, indirect or imprecise) or exceptionally strong evidence from observational studies | Weak recommendation; the best action can be changed depending on the circumstances of the patients or their social values |
| 2C Weak recommendation, low or very low quality evidence | Uncertainty in the estimation of benefits, risks and disadvantages; benefits, risks and disadvantages can be in balance | Observational studies or case series | Very weak recommendations; other alternatives can be equally reasonable |
The authors declare that they have no conflict of interest.
Please cite this article as: Aguinagalde B, Aranda JL, Busca P, Martínez I, Royo I, Zabaleta J, et al. Guía de práctica clínica de la SECT sobre el manejo de pacientes con neumotórax espontáneo. Cir Esp. 2018;96:3–11.
These guidelines have been approved by the scientific committee of the Spanish Association of Thoracic Surgery (Sociedad Española de Cirugía Torácica).