The use of a self-expanding metallic stent as a bridge to surgery in acute malignant left colonic obstruction has been suggested as an alternative treatment to emergency surgery. The aim of the present study was to compare the morbi-mortality, cost–benefit and long-term oncological outcomes of both therapeutic options.
MethodsThis is a prospective, comparative, controlled, non-randomized study (2005–2010) performed in a specialized unit. The study included 82 patients with left colon cancer obstruction treated by stent as a bridge to surgery (n=27) or emergency surgery (n=55) operated with local curative intention. The main outcome measures (postoperative morbi-mortaliy, cost–benefit, stoma rate and long-term oncological outcomes) were compared based on an “intention-to-treat” analysis.
ResultsThere were no significant statistical differences between the two groups in terms of preoperative data and tumor characteristics. The technically successful stenting rate was 88.9% (11.1% perforation during stent placement) and clinical success was 81.4%. No difference was observed in postoperative morbi-mortality rates. The primary anastomosis rate was higher in the bridge to surgery group compared to the emergency surgery group (77.8% vs 56.4%; P=.05). The mean costs in the emergency surgery group resulted to be €1391.9 more expensive per patient than in the bridge to surgery group. There was no significant statistical difference in oncological long-term outcomes.
ConclusionsThe use of self-expanding metallic stents as a bridge to surgery is a safe option in the urgent treatment of obstructive left colon cancer, with similar short and long-term results compared to direct surgery, inferior mean costs and a higher rate of primary anastomosis.
El uso de un stent metálico autoexpandible como puente a la cirugía del cáncer de colon izquierdo en oclusión se ha señalado como tratamiento alternativo a la cirugía de urgencia. El objetivo del presente estudio fue comparar la morbimortalidad, el coste-beneficio y los resultados oncológicos a largo plazo de ambas opciones terapéuticas.
MétodosSe trata de un estudio prospectivo, comparativo, controlado y no aleatorizado (2005-2010) realizado en una unidad especializada. El estudio agrupó a 82 pacientes con cáncer de colon izquierdo en oclusión tratados mediante stent como puente a la cirugía (n=27) o cirugía de urgencia (n=55), intervenidos con intención curativa local. Las principales variables del estudio (morbimortalidad postoperatoria, coste-beneficio, tasa de estomas y resultados oncológicos a largo plazo) fueron comparados sobre la base de un análisis «con intención de tratar».
ResultadosNo se encontraron diferencias estadísticamente significativas entre los dos grupos en términos de datos preoperatorios y características tumorales. La tasa de éxito técnico en la colocación de la endoprótesis fue del 88,9% (con un 11,1% de perforaciones derivadas del stent), y el éxito clínico fue del 81,4%. No se observó diferencia alguna en cuanto a los índices de morbimortalidad postoperatoria. La tasa de anastomosis primaria fue superior en el grupo «stent como puente a la cirugía», en comparación al grupo «cirugía de urgencia» (77,8% frente a 56,4%; p=0,05). Los costes medios por paciente en el grupo «cirugía de urgencia» resultaron ser más elevados (+1.391,9€) que en el grupo «stent como puente a la cirugía». No se produjeron diferencias estadísticamente significativas en cuanto a resulados oncológicos a largo plazo.
ConclusionesEl uso de stents metálicos autoexpandibles como puente a la cirugía constituye una opción segura para el tratamiento urgente del cáncer de colon izquierdo en oclusión, con resultados oncológicos similares a largo plazo en comparación a la cirugía de urgencia, con menor coste económico y una tasa superior de anastomosis primarias, evitando numerosos estomas.
Approximately 7–29% of malignant colorectal neoplasms present as a bowel obstruction with 70% of them occurring in the left colon. It is the main reason for emergency surgery in colon cancer.1–3 Despite medical and surgical advances, this emergency surgery continues to have a high morbidity (30–60%) and mortality (10–30%) compared to elective surgery (mortality rate less than 5%).4–8 This difference could be for two reasons: first of all, in emergency surgery the patient is not adequately prepared and optimized in terms of hydration, nutritional status, electrolyte balance, etc. Moreover, emergency surgery is often performed by general surgeons rather than colorectal specialists, with a resulting “surgeon-dependent” negative effect.9 Furthermore, in these cases the colon is often distended and not prepared, meaning primary anastomosis is not possible and a terminal stoma is necessary. Patients will therefore require further surgery to close the stoma and restore bowel continuity. However, in many cases this second intervention is not performed due to the patient preference or the high morbi-mortality and the advanced age of these patients.12 In other cases, the state of the colon is so poor that the patient requires a more extensive resection, such as subtotal or total colectomy, with a subsequent poor quality of life.
Another and very important issue is the increased risk of anastomotic dehiscence in emergency surgery, with an obstructed colon and non-specialist colorectal surgeons. The long-term oncological and quality of life impact are well-established.8–11 However, Frago et al. have shown good results following emergency surgical resection, but only in selected cases performed by specialist colorectal surgeons.13
In 1991, Dohmoto introduced the use of a self-expanding metallic stent (SEMS) as a palliative treatment for malignant obstruction in the left colon.14 The high morbi-mortality in these patients following emergency surgery (ES) has led to the wide use of the stent. Tejero et al. reported using the SEMS in left colon cancer prior to elective surgery.15 Known as a “bridge to surgery” (BS) it gives time for the clinical condition of the patient to improve, to optimization of comorbidities and nutritional status and preoperative studies to be carried out. All of this reduces morbi-mortality and the number of colostomies formed, as reported in recent studies.6,7 Another indication for the SEMS is definitive palliative treatment in advanced metastatic cancer or high surgical risk patients.14
Stent placement is not widely available in all centers and its high cost must also be taken into account.
Although the stent has many advantages, it is not without risk of complication such as perforation, migration or reobstruction.6,16,17 The subsequent negative effect on the survival of these complicated patients has been the subject of much debate.
In fact, three of four main RCTs have been closed prematurely because of unfavorable short-term outcomes in one of the study arms, and not always the same arm. So, the literature does not allow clinicians to determine the best treatment option for those patients.18–21
The hypothesis of the present paper is that in patients with left cancer colon complicated with obstruction the use of SEMS as a “bridge-to-surgery” can improve short-term results without a negative impact in long-term local recurrence or survival. This could be justified by the optimization of the status of the patient, the bowel preparation and the possibility of performing an appropriate tumor staging. Moreover, surgery could be performed by a colorectal specialist.
The main objective of the present study is to assess the postoperative morbi-mortality of the SEMS as a “bridge to surgery” and to compare it with emergency surgery in patients with obstructive left colon cancer, treated with curative intention. The secondary objectives were to analyze in each group the costs, the rate of definitive stomas, and the long-term oncological outcomes.
MethodsThis is a prospective, controlled, comparative, non-randomized study performed at the General and Digestive Surgical Department of the University Clinical Hospital, Valencia. Data were collected in a database between January 2005 and December 2010.
All the patients with left colon cancer complicated by acute obstruction, operated at the Unit during the study period were included. “Left colon cancer” was defined as a colon adenocarcinoma located from the splenic flexure to 11cm anal margin as measured with rigid proctoscope. Acute large bowel obstruction diagnosis was confirmed by presenting clinical symptoms of obstruction and abdominal X-ray. Cancer diagnosis was confirmed by flexible colonoscopy and abdomino-pelvic computerized tomography (CT).
In this study we excluded: (1) patients with peritonitis and/or perforation, (2) palliative local resection, (3) definitive SEMS in patients with bad performance status or stage IV disease unsuitable for curative surgery (palliative stent).
For the purpose of the study, patients were divided in two groups: “emergency surgery” (ES) group when emergency surgery was performed and “bridge to surgery” (BS) group when the placement of a SEMS was attempted, followed by delayed planned surgery.
The primary determinant for stent placement and group assignment depended on the availability of an endoscopist with SEMS placement capabilities, irrespective of the characteristics and comorbidity of the patients, the patient choice and the managing surgeon. The local ethics committee approved the study and all patients gave written, informed consent before inclusion.
Colonic StentingIn patients of “bridge to surgery” group the placement of a SEMS was attempted. After rectal enema, a flexible colonoscopy was performed under deep sedation to confirm the diagnosis. Using a double-channel endoscope, a guide wire was introduced across the stenosis and beyond the obstruction. Through the other channel, water-soluble contrast was injected via a catheter to confirm intraluminal placement of the guide wire. The SEMS was inserted over the guide wire and placed within the stenosis. Fluoroscopy and endoscopy then confirmed the correct positioning of the stent. Two types of SEMS were used: Wallstent® and Wallflex®, according to the preferences of the endoscopist.
Technical success was defined when the SEMS was correctly placed with fluoroscopic confirmation. Clinical success was defined as colonic decompression confirmed by X-ray and improved clinical signs with the ability to defer surgery for a minimum of 7 days. In the case of clinical success of SEMS, elective surgery took place within two weeks. During this period the patient remained hospitalized. If an adverse event occurred an emergency surgery were indicated.
Surgical TechniqueThe surgery consisted of oncological resection with curative intention, with or without restoration of bowel continuity, according to the decision of the operative surgeon. A laparoscopic approach was used in selected patients in the last part of the study.
Study DataThe following data were collected: demographic characteristics of patients, location and staging of the tumor, outcomes and complications of stent placement, surgical characteristics and postoperative complications within the first 30 days.
Moreover, direct costs in both groups were evaluated. To calculate the financial cost we used the Alcantara method18 based on the hospital stay, surgery time, and materials used. The required information was taken from the Financial Information System at the Clinic Hospital, Valencia in 2010. The cost for each patient was calculated with the formula: (days in critical care unit)×1518.15€+(duration of the surgery)×11.18€/minute+(days in the ward)×335.07€. In patients that needed multiple admissions (including readmission for complication and admission for restoration of bowel continuity) the total cost was calculated adding up the cost of each admission. In the BS group the stent cost (1500€) was added to the total cost.
Measured oncological outcomes included local and overall recurrence rates at 2 and 5-y follow-up. Local recurrence (LR) was defined as the presence of any anastomotic, pelvic, or perineal tumor documented by proctoscopic, radiologic, or histopathologic examination. Distant recurrence was defined as evidence of recurrent disease in any other location. Calculation of local recurrence rates included both patients affected by local recurrence alone as well as those where both local and distant recurrence had occurred. Overall recurrence was considered when either loco-regional or distant recurrence occurred. Patients were followed-up by serial clinical examination and CEA assessment every 3 months during the first year, every 6 months during the second year and annually thereafter. Thoracic-abdominal CT scanning was performed every six months for the first two years. Colonoscopy was performed after one year and three-to-five years thereafter depending on individual patient risk. If recurrence was suspected, further diagnostic methods were used as required. The oncologist within the Multidisciplinary Team acts as an independent observer to confirm the presence of disease recurrence.
Statistical AnalysisThe analysis was intention-to-treat.
Data were summarized by its mean, standard deviation, median and first and third quartiles in the case of continuous variables and by relative and absolute frequencies in the case of categorical variables. Categorical variables were compared using Chi-square and Fisher's Exact tests. Continuous outcomes were compared using parametric (t-test) and non-parametric (Mann–Whitney U) significance tests as appropriate.
Multivariable logistic regression was used to assess differences between groups regarding postoperative morbidity and surgical data. To account for confounding variables such as age, sex, hypertension, diabetes and tumor location and stage, a propensity score was computed using these variables and was added as covariate to the models. Kaplan–Meier curves and the Log-Rank test were used to investigate prognostic factors on local recurrence (LR), disease free survival (DFS) and cancer specific survival (CSS). Stepwise regression methods (variable inclusion criteria if P<.1) were used for the above model.
Differences in mean costs between both groups were assessed by two methods: a log transformation of the cost values followed by a t-test and the nonparametric bootstrap with 10000 resamples. Costs were also compared using the Wilcoxon test, to assess differences in location.
Statistical significance was set at α=0.05.
The statistical Package for Social Sciences (SPSS version 22.0.0; IBM SPSS statistics, IBM Corporation, Armonk, NY, USA) was used for the descriptive and statistical analyses. Cost analysis and graphs were performed with the R software (version 3.0.2).
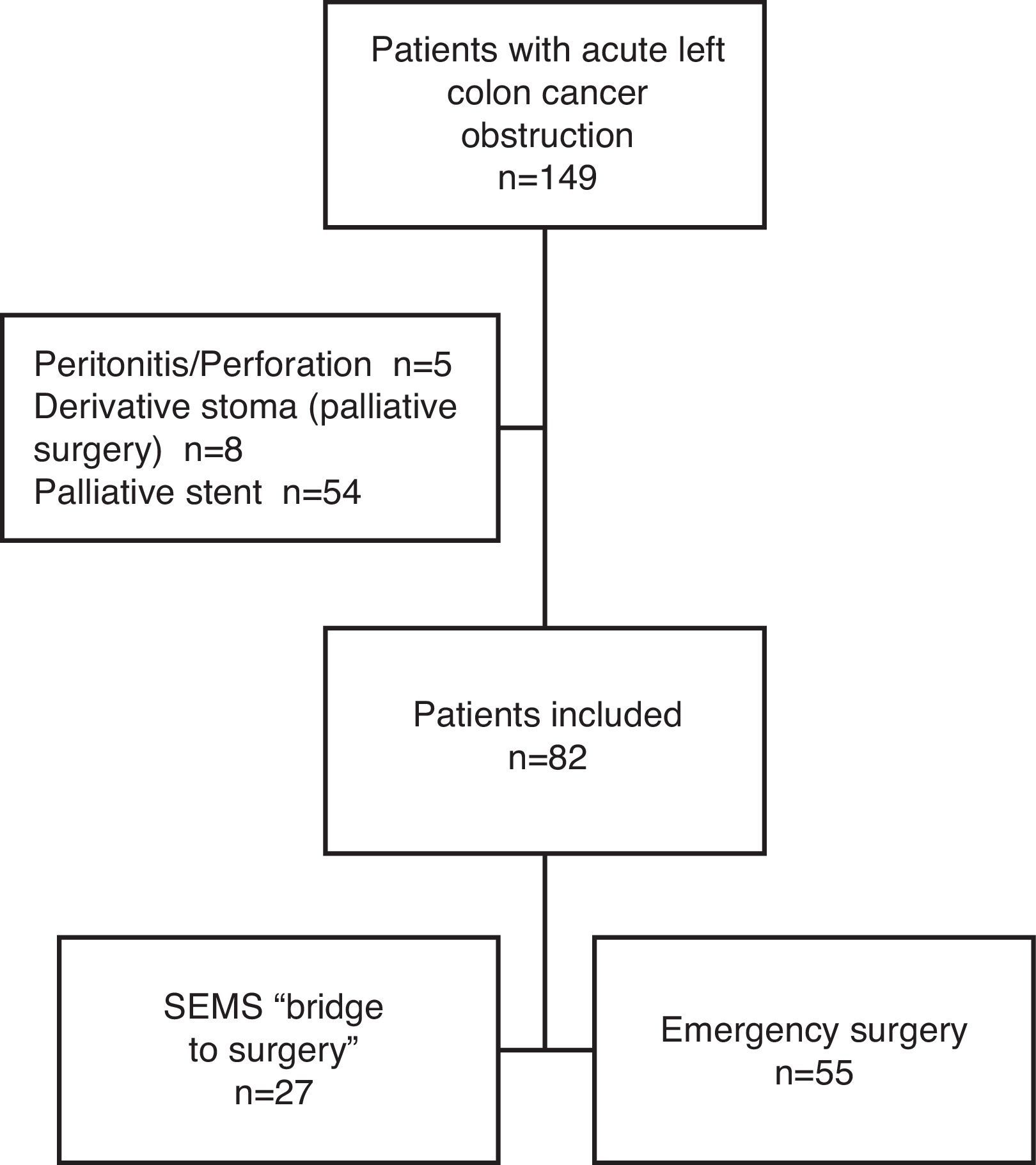
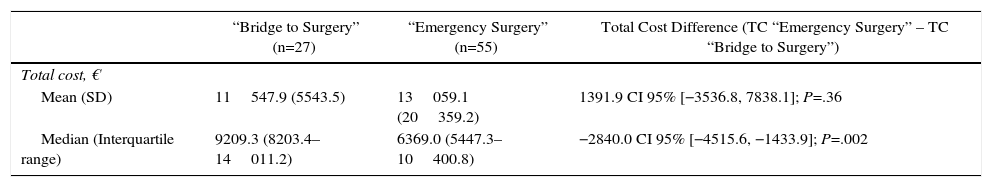
ResultsCharacteristics of PatientsFrom January 2005 until December 2010, a total of 149 patients presented with a left colon cancer obstruction. Of those, 54 were excluded from the study because the stent was palliative, 5 for tumor perforation and peritonitis and 8 for palliative surgery. After inclusion and exclusion criteria were applied, 82 patients were finally included in the study: 27 were initially treated with SEMS (“BS” group) and 55 patients had primary emergency surgery (“ES” group) (Fig. 1).
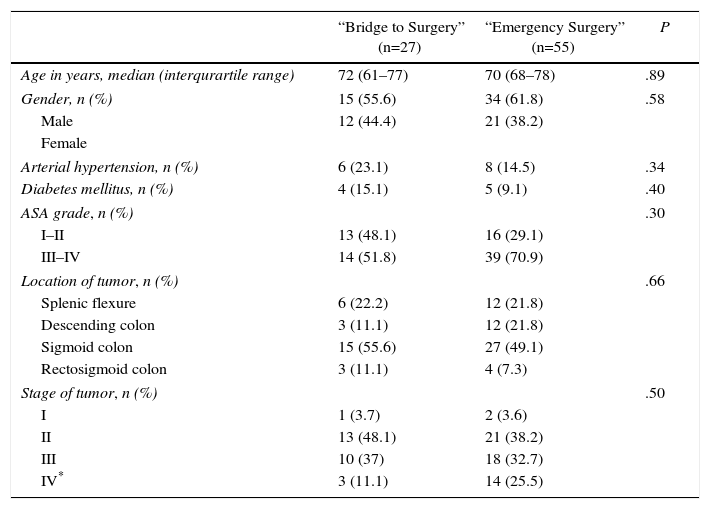
There were no significant statistical differences between the two groups in terms of age, gender, arterial hypertension, diabetes mellitus, ASA grade (American Society of Anesthesiologists), tumor site and tumor staging (minimum P=.30) (Table 1).
Demographics and Oncological Characteristics of Patients.
| “Bridge to Surgery” (n=27) | “Emergency Surgery” (n=55) | P | |
|---|---|---|---|
| Age in years, median (interqurartile range) | 72 (61–77) | 70 (68–78) | .89 |
| Gender, n (%) | 15 (55.6) | 34 (61.8) | .58 |
| Male | 12 (44.4) | 21 (38.2) | |
| Female | |||
| Arterial hypertension, n (%) | 6 (23.1) | 8 (14.5) | .34 |
| Diabetes mellitus, n (%) | 4 (15.1) | 5 (9.1) | .40 |
| ASA grade, n (%) | .30 | ||
| I–II | 13 (48.1) | 16 (29.1) | |
| III–IV | 14 (51.8) | 39 (70.9) | |
| Location of tumor, n (%) | .66 | ||
| Splenic flexure | 6 (22.2) | 12 (21.8) | |
| Descending colon | 3 (11.1) | 12 (21.8) | |
| Sigmoid colon | 15 (55.6) | 27 (49.1) | |
| Rectosigmoid colon | 3 (11.1) | 4 (7.3) | |
| Stage of tumor, n (%) | .50 | ||
| I | 1 (3.7) | 2 (3.6) | |
| II | 13 (48.1) | 21 (38.2) | |
| III | 10 (37) | 18 (32.7) | |
| IV* | 3 (11.1) | 14 (25.5) | |
ASA, American Society of Anesthesiologist.
Twenty-four patients (88.9%) had technically successful stenting. In the remaining 3 patients (11.1%) a perforation occurred during stent placement, needing emergency surgery (2 Hartmann's procedures and 1 anterior resections with anastomosis and loop ileostomy). Clinical success was demonstrated in 21 (of 27) patients (77.8%), within 48h of stenting in 19 patients, and in the other 2 patients within 72h. In one patient with initial clinical success, stent migration occurred after 7 days causing intestinal occlusion that needed emergency surgery (anterior resection with anastomosis and loop ileostomy). No bleeding or re-obstruction complications occurred in the BS group.
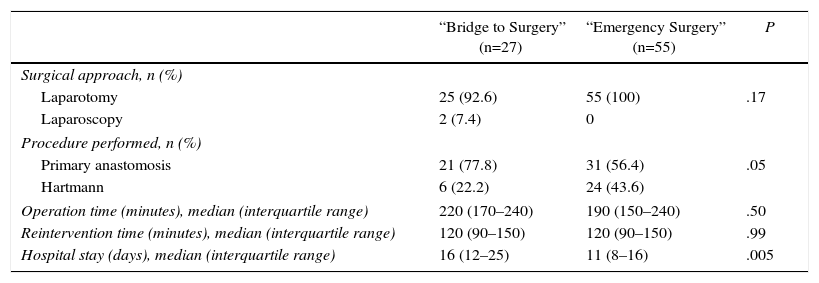
Surgical DataWe did not observe any significant statistical difference in the surgical approach (laparotomy vs laparoscopy) and operation time (Table 3). Median length of hospital stay was longer in the BS group (16 vs 11; P=.005).
A marginally significant difference was found in the procedure performed with a greater primary anastomosis in the BS group compared to ES group (77.8% vs 56.4%; P=.05).
Only in 4 out of the 30 patients (13.3%) that underwent Hartmann's procedure, bowel continuity was restored: 0/6 patients in the BS group and 4/24 patients in the ES group (P=.24). The reasons for non-reconstruction in the BS group were disease progression (n=5) and postoperative death (n=1). In the ES group the reasons were disease progression (n=2), postoperative death (n=2) and patient comorbidity (n=16). Definitive stoma rate was 22.2% in BS group and 36.4% in ES group (P=.21). Surgical data are detailed in Table 2.
Surgical Data.
| “Bridge to Surgery” (n=27) | “Emergency Surgery” (n=55) | P | |
|---|---|---|---|
| Surgical approach, n (%) | |||
| Laparotomy | 25 (92.6) | 55 (100) | .17 |
| Laparoscopy | 2 (7.4) | 0 | |
| Procedure performed, n (%) | |||
| Primary anastomosis | 21 (77.8) | 31 (56.4) | .05 |
| Hartmann | 6 (22.2) | 24 (43.6) | |
| Operation time (minutes), median (interquartile range) | 220 (170–240) | 190 (150–240) | .50 |
| Reintervention time (minutes), median (interquartile range) | 120 (90–150) | 120 (90–150) | .99 |
| Hospital stay (days), median (interquartile range) | 16 (12–25) | 11 (8–16) | .005 |
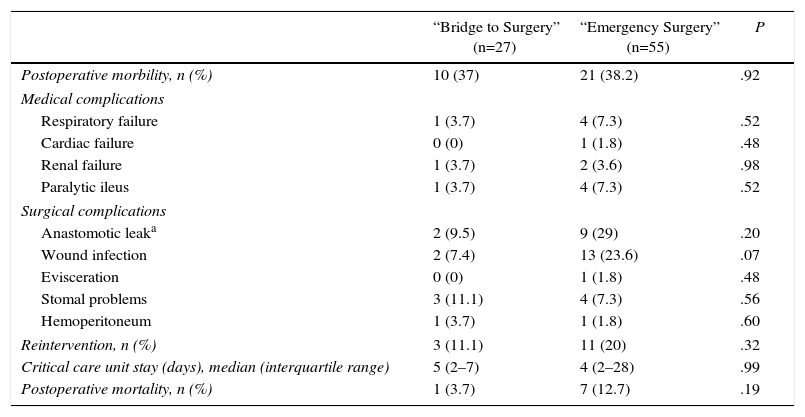
Postoperative morbidity rate was not different between the two groups (37% BS group vs 38.2% ES group; P=.92). When detailing between individual complications, there was a trend toward more wound infections (7.4% BS group vs 23.6% ES group; P=.10) and anastomosis leak (9.5% BS group vs 29% ES group; P=.20) in the ES group, although differences were not statistically significant. 3 patients (11.1%) were re-operated in the BS group (2 anastomosis leaks and 1 colostomy detachment) and 11 (20%) in the ES group (9 anastomosis leaks, 1 evisceration and 1 hemoperitoneum) (P=.32).
The postoperative mortality rate in the BS group was 3.7% (n= pneumonia) and the ES group 12.7% (n=7: 2 pneumonia, 3 septic shock following leakage, 1 subcutaneous fistula and 1 colon ischemia) (P=.19) (Table 3).
Postoperative Complications.
| “Bridge to Surgery” (n=27) | “Emergency Surgery” (n=55) | P | |
|---|---|---|---|
| Postoperative morbility, n (%) | 10 (37) | 21 (38.2) | .92 |
| Medical complications | |||
| Respiratory failure | 1 (3.7) | 4 (7.3) | .52 |
| Cardiac failure | 0 (0) | 1 (1.8) | .48 |
| Renal failure | 1 (3.7) | 2 (3.6) | .98 |
| Paralytic ileus | 1 (3.7) | 4 (7.3) | .52 |
| Surgical complications | |||
| Anastomotic leaka | 2 (9.5) | 9 (29) | .20 |
| Wound infection | 2 (7.4) | 13 (23.6) | .07 |
| Evisceration | 0 (0) | 1 (1.8) | .48 |
| Stomal problems | 3 (11.1) | 4 (7.3) | .56 |
| Hemoperitoneum | 1 (3.7) | 1 (1.8) | .60 |
| Reintervention, n (%) | 3 (11.1) | 11 (20) | .32 |
| Critical care unit stay (days), median (interquartile range) | 5 (2–7) | 4 (2–28) | .99 |
| Postoperative mortality, n (%) | 1 (3.7) | 7 (12.7) | .19 |
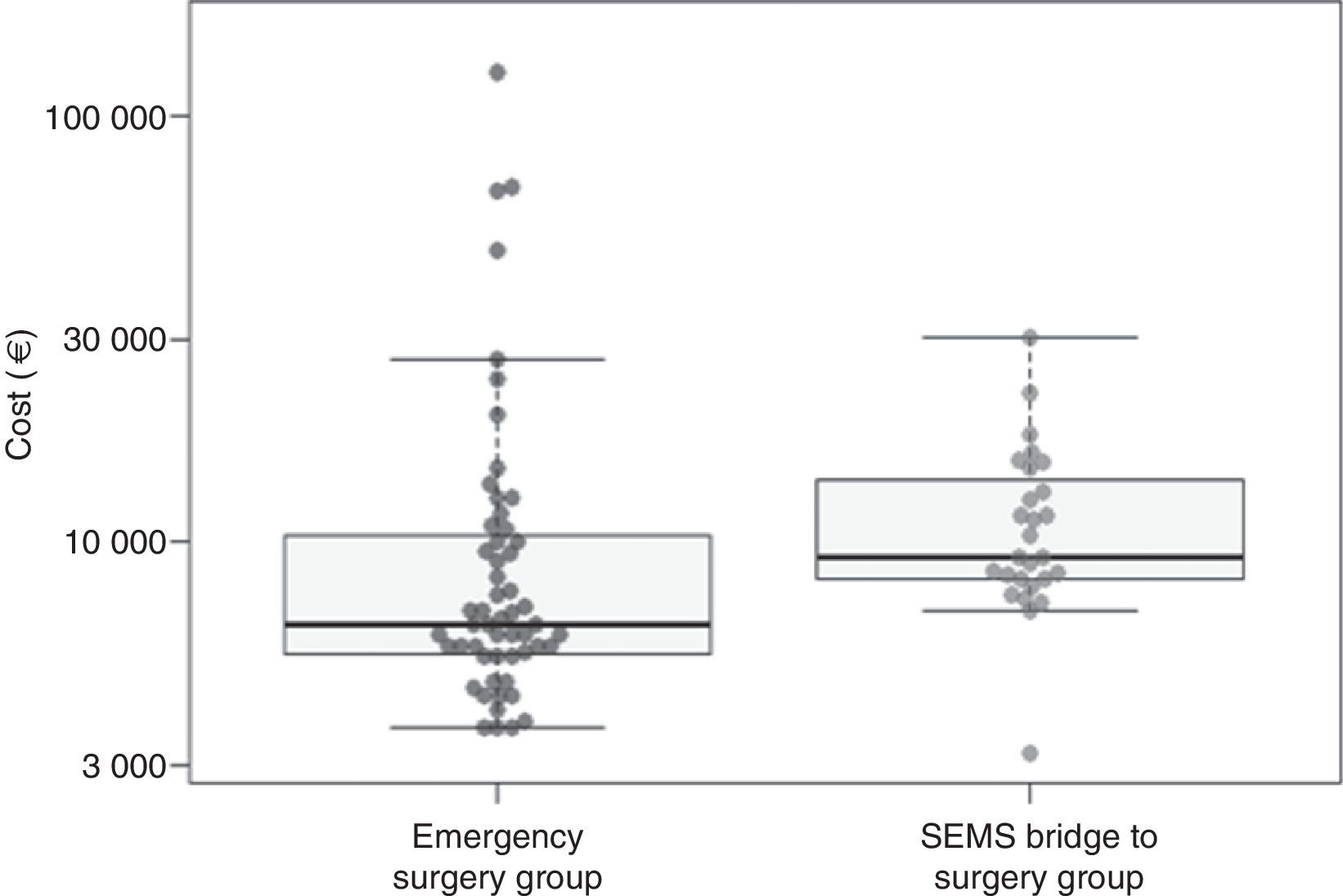
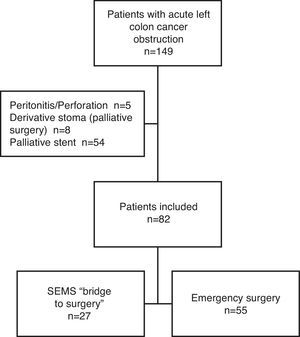
When considering the mean of costs, treatment in the ES group had a mean cost of 13059.1€ (SD=20359.2€) and the BS group had a mean cost of 11547.9€ (SD=5543.5€); therefore, ES group turned out to be 1391.9€ more expensive than in the BS group. This difference was not statistically significant though (P=.11 with log-transformation and t test and P=.36 with nonparametric bootstrap) (Table 4, Fig. 2).
Costs.
| “Bridge to Surgery” (n=27) | “Emergency Surgery” (n=55) | Total Cost Difference (TC “Emergency Surgery” – TC “Bridge to Surgery”) | |
|---|---|---|---|
| Total cost, € | |||
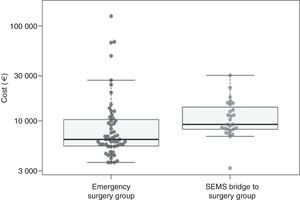
| Mean (SD) | 11547.9 (5543.5) | 13059.1 (20359.2) | 1391.9 CI 95% [−3536.8, 7838.1]; P=.36 |
| Median (Interquartile range) | 9209.3 (8203.4–14011.2) | 6369.0 (5447.3–10400.8) | −2840.0 CI 95% [−4515.6, −1433.9]; P=.002 |
TC, total cost.
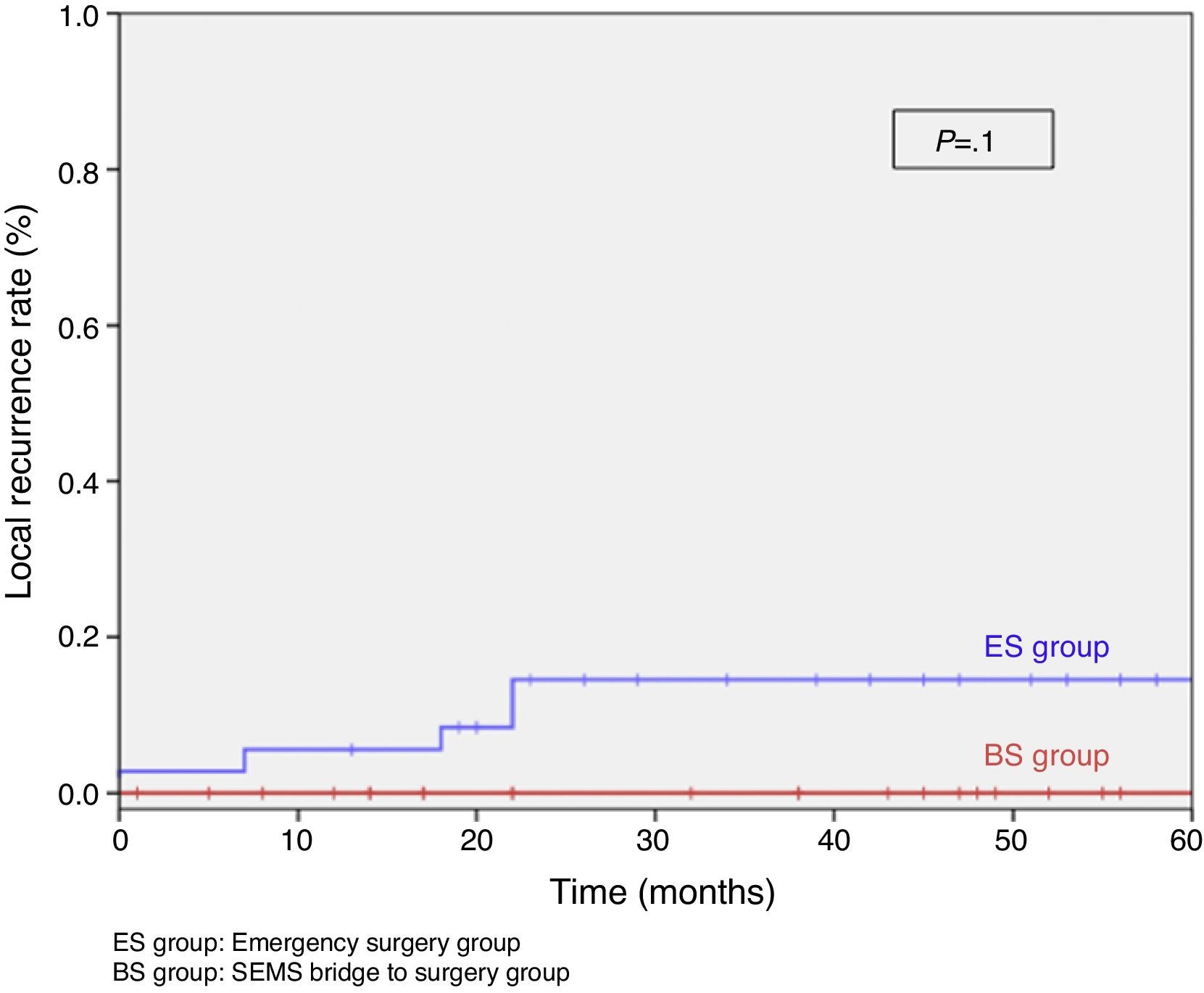
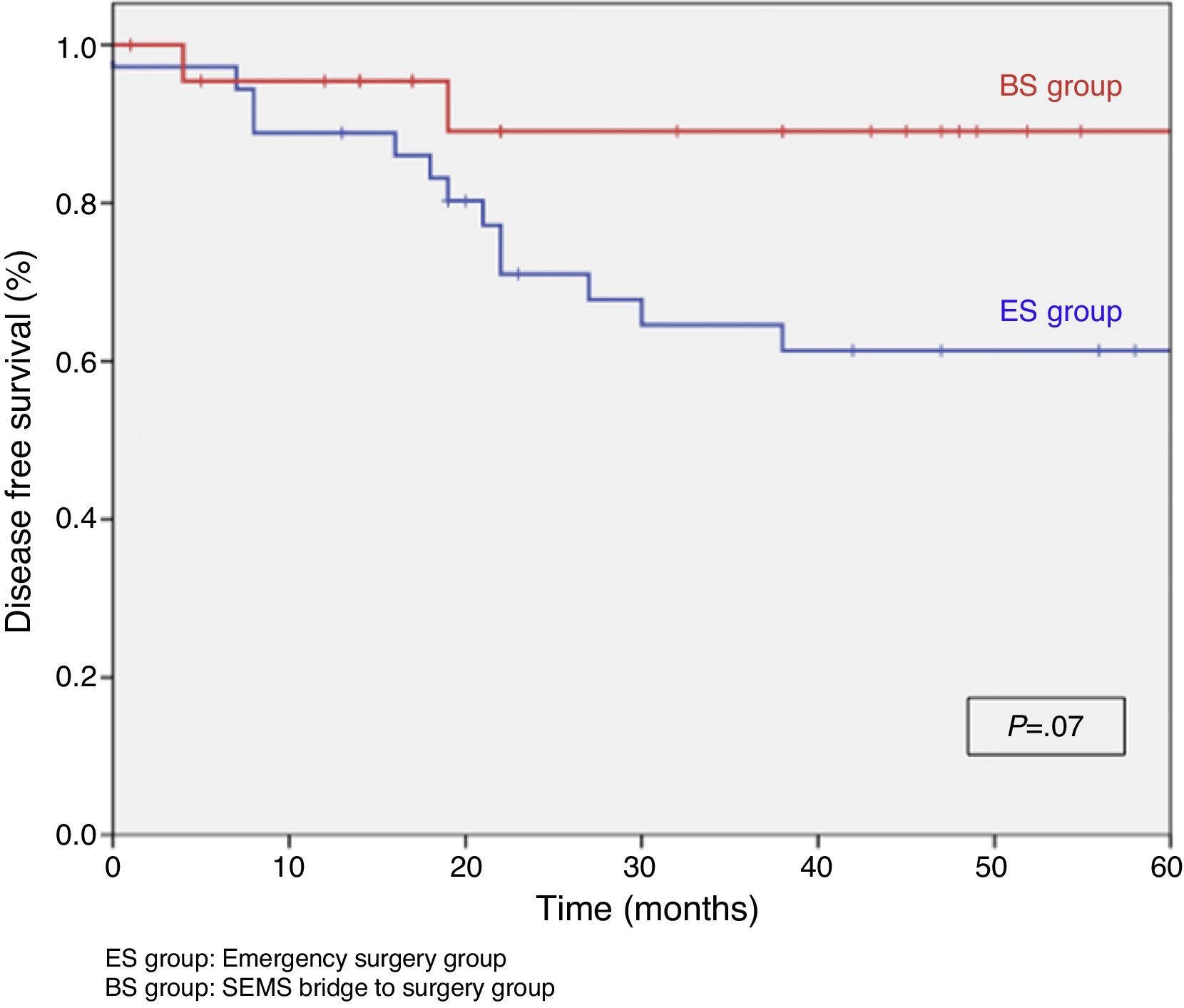
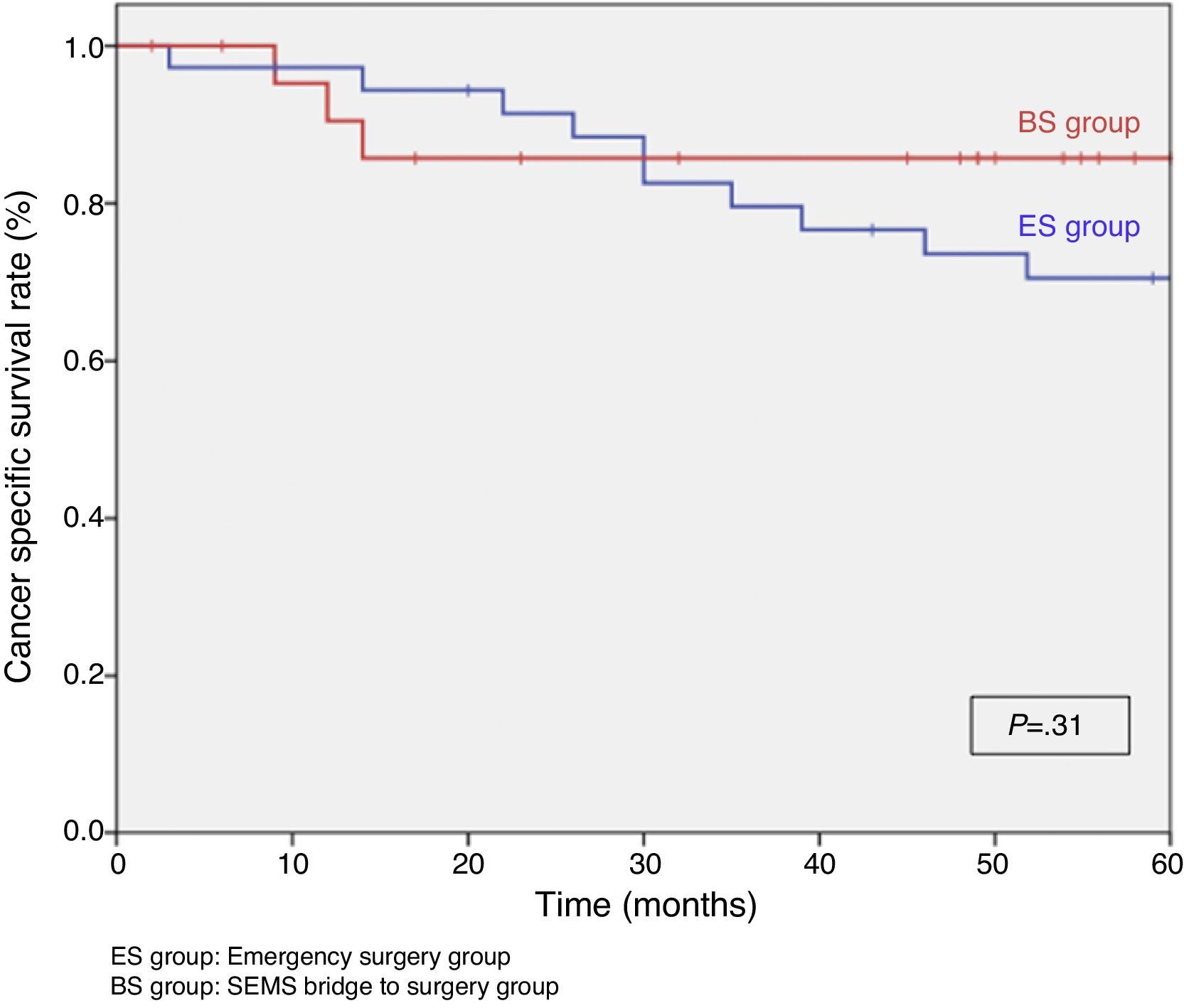
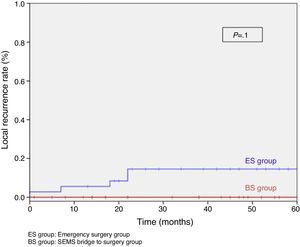
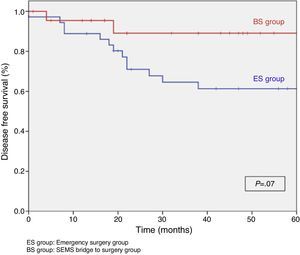
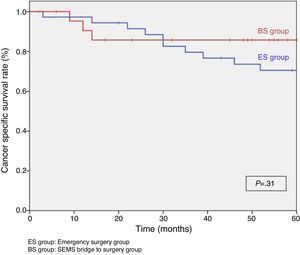
When excluding metastatic patients (n=17) and postoperative deaths (n=8) median follow-up was 58 (30–75) months. There were five local recurrences (LR) out of 59 patients (8.5%). We did not observe any statistically significant difference in LR, disease free survival (DFS) or cancer specific survival (CSS). At 5 years follow-up, actuarial LR rate in BS group was 0% and 14.5% in ES group (P=.10) (Fig. 3). Disease free survival in BS group was 89.1% and 61.3% in ES group (P=.07) (Fig. 4). Cancer specific survival was 85.7% in BS group and 70.5% in ES group (P=.31) (Fig. 5).
DiscussionAcute malignant colon obstruction is a common clinical problem and requires emergency treatment, whether it is emergency surgery or SEMS placement as a bridge to elective surgery.15 The main indication for SEMS placement is in distal tumors from the splenic flexure up to 11cm anal margin. Stenting in the proximal colon at the splenic flexure is possible but not widely used.18,22,23 Furthermore, emergency right colon surgery has a similar morbimortality as elective surgery.24
SEMS placement with elective surgery attempts to reduce the postoperative morbimortality and stoma formation. Martinez-Santos et al.6 demonstrated that stenting prior to elective surgery, compared to emergency surgical resection, was associated with a higher rate of primary anastomosis, fewer complications, shorter critical care unit stay and shorter hospital stay. Moreover, similar oncological results in terms of overall survival have been shown.7 A recent meta-analysis showed SEMS placement as a bridge to surgery in a colon cancer obstruction increased the primary anastomosis rate and decreased stoma formation rate. Also, there is evidence to suggest fewer complications than emergency surgery, although postoperative mortality remains unaffected.25
In our study, we observed a clinical success rate following stent placement in 21 out of 27 patients (77.8%) similar to results published in other reports.19,20,26–28 This treatment allowed the clinical optimization of these patients and pre-operative staging studies before elective surgical intervention. At the same time, we noted a technical success rate of 88.9% as seen in other studies.21–26 Migration and re-occlusion are two of the main complications associated with stenting, but the most serious is colon perforation with a variable incidence up to 16%.19–21,26–28 In our study we saw a perforation rate of 11.1%. Several studies report that perforations could have oncological significance, potentially resulting in tumor cell seeding and disemination.20,21,26,28 New studies are necessary to show the adverse effect of perforation and patient survival.
Our results are comparable to other prospective, randomized studies and systematic reviews, in which they conclude that stent placement as BS is safe and effective and is associated with lower morbimortality.16,22,29–32 In contrast, Pirlet et al. reported in a controlled, randomized multicenter study that stent placement, as a bridge to surgery in colon cancer obstruction, is neither safer nor more effective than ES. They cannot demonstrate that stenting significantly reduces the need for stomas. They had to interrupt the trial because of the high rate of technical failures (bowel perforation) occurring in the SEMS group.25 Similar findings were reported as causes for discontinuation of a randomized study due a substantial morbidity and mortality occurring in the stenting group.26 The high rate of technical failures may reflect endoscopist experience. Volume and experience correlate with outcome and colonic stenting is not an exception.33 This could explain the negative results with SEMS in the above studies.
We not observed significant differences in post-operative complication rate between both groups (37% vs 38.2%), similarly to other studies.22 Regarding post-operative mortality both groups show no significant statistical difference (3.7% vs 12.7%). The death in the “bridge to surgery” group was caused by post-operative medical problems and not complications following stenting. However, the deaths within 30 days following emergency surgery were due to surgical problems, probably because these patients were not adequately prepared pre-operatively.
The use of SEMS in colon cancer obstruction allows programming the surgery that, therefore, can be performed by a colorectal specialist surgeon. It has been previously shown that this fact implies a more extensive use of laparoscopy, with better short terms results and faster postoperative recovery.20,22,28
The presence of a colorectal surgeon, along with the better patient status, may justify the higher primary anastomosis rate found in the BS group in the present study.
In the present study, if we take into account the mean cost, we have a higher cost in the ES group, even though there is no significant statistical difference. This difference in mean cost is due to higher surgical and medical complication rates in ES group. On the other hand, the higher hospital stay cost in BS patients is due to inclusion of the time between the SEMS insertion and elective surgery and not due to a longer post-operative stay. A different hospitalization strategy, with patient discharge between SEMS insertion and surgery, could reduce BS group costs.
We must also consider that in ES group there is a higher stoma without closure rate, which increases the use of medical and financial resources. Alcantara et al., in a similar analysis, showed similar results when evaluating surgical cost and length of stay, founding no difference between both groups.16 However, they found a difference when comparing the cost including materials used, the higher cost being BS group. This contradicts other studies that conclude that stenting is a cost-effective procedure with reduced hospital stay and lower costs compared to ES.34–36
If we focus on oncology outcomes, we do not see significant differences in LR rate, DFS or CSS in either group. This may be due to the small patient number in the study. Another important fact is that no LR was seen in the 3 patients where the stent perforated the colon. These findings are similar to those of Kavanagh et al.35 Although, Sabbagh et al.35 report that global survival and survival at the 5 year follow-up is significantly lower in the BS group than ES group. They also show the cancer specific mortality is significantly higher in BS group than ES group (48% vs 21%). They found significant statistical difference in DFS, recurrence rate and the mean time before recurrence, these being higher in ES group.
The limitations of the present study are a single-center study, the small sample size and it is no-randomized nature. There is also a risk of selection bias, because the decision of the treatment is influenced by surgeon preference and stenting availability. However, the long-term follow-up of the study is very useful to demonstrate that SEMS do not have an adverse oncological outcome.
In conclusion, the present study shows that SEMS placement as a bridge to surgery is a safe option in the urgent treatment of obstructive left colon cancer, with similar short- and long-term results compared to direct surgery, inferior mean costs and allowing an higher rate of primary anastomoses. Further prospective studies with a larger sample size are required to demonstrate the beneficial effect of SEMS placement as a bridge to surgery.
FundingThe authors declare no funding has been received for the present study.
Authors’ ContributionStudy design: Flor-Lorente, Frasson, E. García-Granero.
Acquisition of data: Baguena, A. García-Granero, Sanchiz, Peña, Espí
Analysis and interpretation of results: Flor-Lorente, Frasson, E. García-Granero, Cervantes, Esclapez.
Manuscript preparation: Flor-Lorente, Baguena.
Critical revision and approval of the manuscript: all authors.
Conflicts of InterestThe authors declare no conflict of interest.
The financial Department of the Hospital Universitario Cliníco Valencia (Mrs Marisa Román) to facilitate data Económicos, as the calculation of the costs.
Please cite this article as: Flor-Lorente B, Báguena G, Frasson M, García-Granero A, Cervantes A, Sanchiz V, et al. Stents metálicos autoexpandibles como puente a la cirugía en el tratamiento del cáncer de colon izquierdo en oclusión. Análisis coste-beneficio y resultados oncológicos. Cir Esp. 2017;95:143–151.