The most effective treatment for lung cancer is complete lung resection, although recurrences reach up to 10% and the appearance of second neoplasms, up to 6%. Therefore, the follow-up of these patients will be essential for the early detection and treatment of these events; however there is no definition of the form, time and cadence of these follow-ups. In this consensus document, we try to define them based on the available scientific evidence.
A critical review of the literature is carried out (meta-analysis, systematic reviews, reviews, consensus recommendations of scientific societies, randomized controlled studies, non-randomized controlled studies, observational studies and case series studies) and communications to the main congresses on oncology and thoracic surgery in Spanish, English and French. The evidences found are classified following the GRADE system.
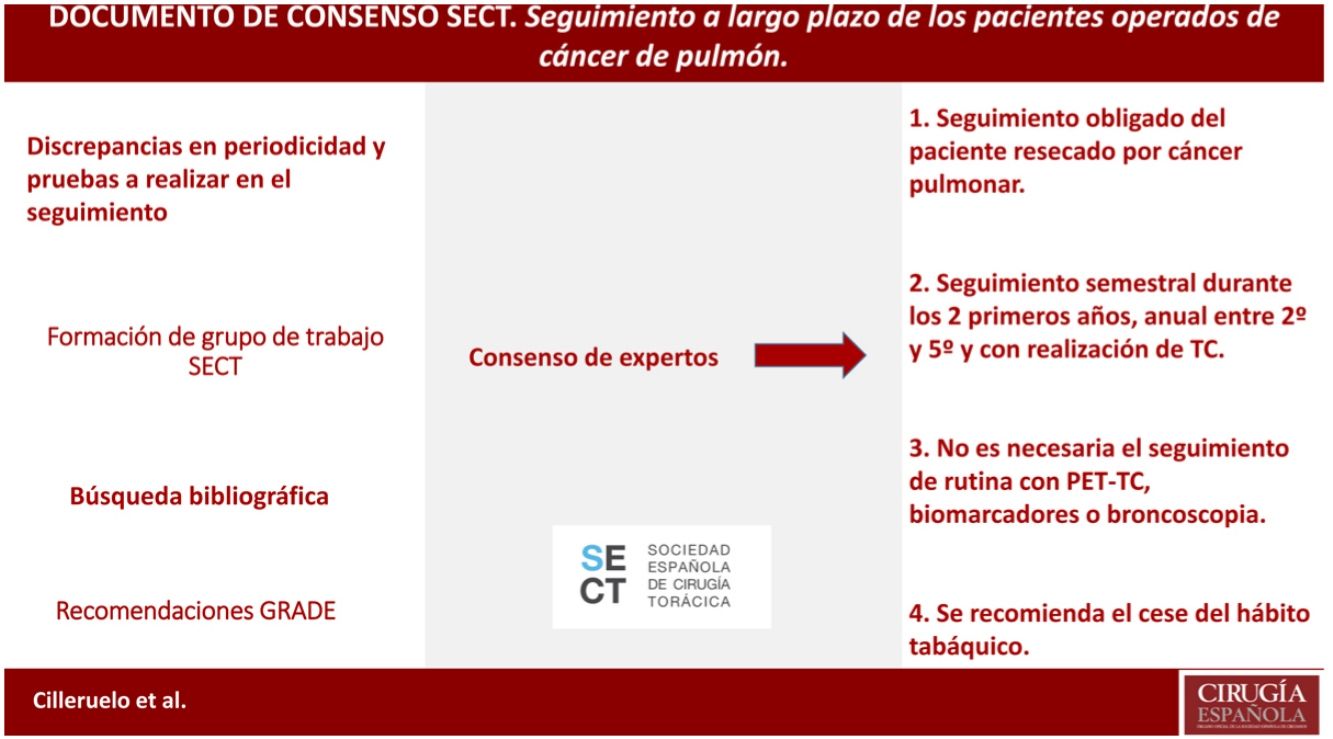
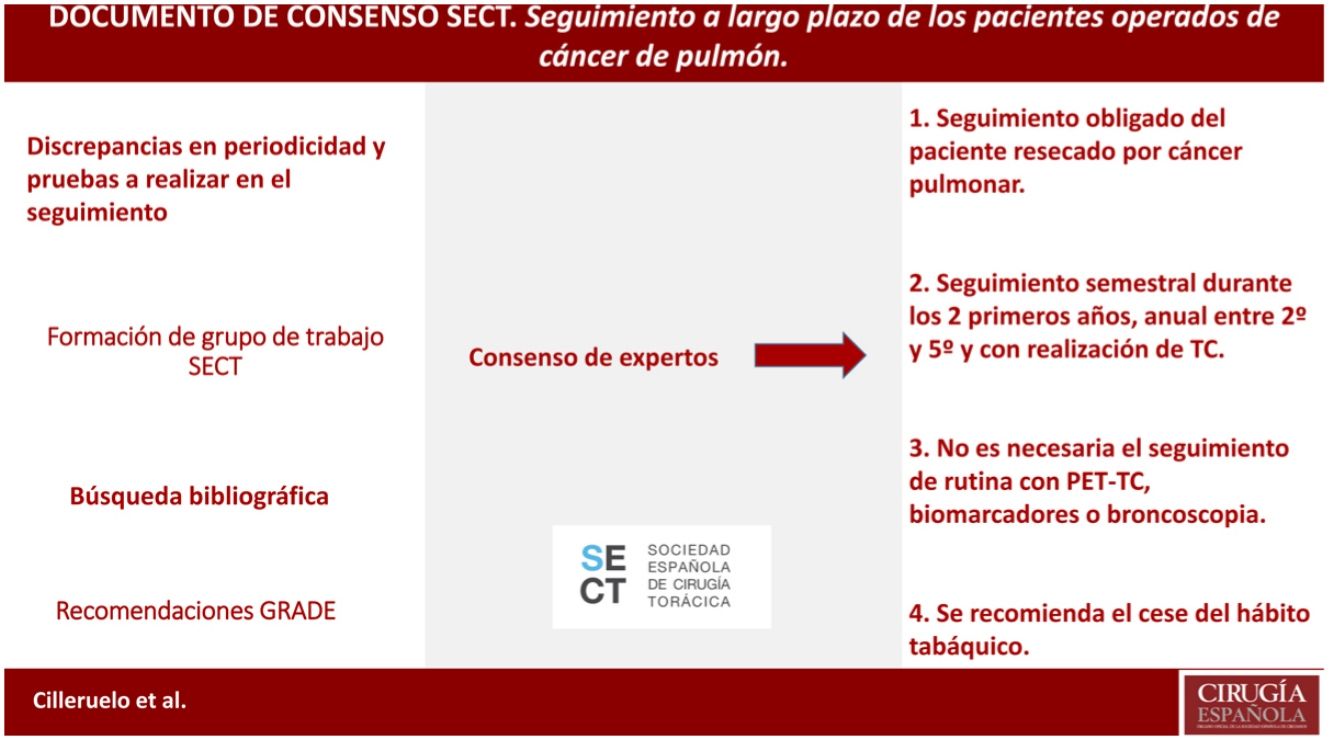
It is defined according to the existing evidence that the patient resected for lung cancer should be followed up, as well as that this follow-up should be close during the first years and with CT (not being necessary to follow up with PET-CT, biomarkers or bronchoscopy). Cessation of smoking is also recommended in this follow-up.
El tratamiento más efectivo para el cáncer de pulmón es la resección pulmonar completa, si bien las recidivas llegan hasta el 10% y la aparición de segundos primarios, hasta el 6%. Será por tanto indispensable el seguimiento de estos pacientes para la detección y tratamiento precoces de estos eventos; sin embargo no existe una definición de la forma, tiempo y cadencia de estos seguimientos. En el presente documento de consenso, tratamos de definirlos en base a la evidencia científica disponible.
Se realiza una revisión crítica de la bibliografía (metaanálisis, revisiones sistemáticas, revisiones, recomendaciones de consenso de sociedades científicas, estudios controlados aleatorizados, estudios controlados no aleatorizados, estudios observacionales y estudios de series de casos) y comunicaciones a los principales congresos de oncología y cirugía torácica en castellano, inglés y francés. Se clasifican las evidencias halladas siguiendo el sistema GRADE.
Queda definido según la evidencia existente que se debe realizar un seguimiento del paciente resecado por cáncer pulmonar, así como que este seguimiento debe ser estrecho durante los primeros años y con realización de TC (no siendo necesaria el seguimiento con PET-TC, biomarcadores o broncoscopia). Se recomienda también en ese seguimiento el cese del hábito tabáquico
Lung cancer is the foremost malignant neoplasm worldwide, with a high incidence and the highest global mortality rate, causing about 1.9 million deaths annually. Approximately 85% of tumors are classified as non–small-cell lung cancer (NSCLC),1 while small-cell lung cancer (SCLC) accounts for practically the entire remainder.
The most effective method for its control continues to be complete resection of NSCLC in early stages (I, II and in some stages III of the American Joint Committee on Cancer TNM). In the case of SCLC, resection with curative intent can also be achieved in stage I.2
Despite radical treatment, cumulative 5-year patient survival after surgery with curative intent is 50%–60%, although these rates show a significant and relevant decrease as the pathological tumor stage increases.2 This drop in survival may be due to complications of surgical treatment, present comorbidities, tumor recurrence (treatable/untreatable) and the appearance of secondary tumors.
Immediate postoperative complications are not uncommon. In an analysis of the Surveillance, Epidemiology and End Results (SEER) database, up to 12.8% of patients required readmission in the month after pulmonary surgery due to cardiorespiratory complications. This was related with identified factors, such as resection type, age, previous radiotherapy and comorbidities. The risk of 90-day mortality in these patients was 6 times higher than in patients who did not present these complications.3 Furthermore, patients who presented higher long-term excess mortality due to causes other than cancer also presented respiratory or cardiovascular comorbidity.4
In a large surgical series, the individual risk of tumor recurrence ranged from 6% to 10% per year in the first 4 years after surgery, falling to 2% per year thereafter. The risk of a second pulmonary neoplasm did not decrease over time and remained constant at 3%–6%.5
In the first 2 years of follow-up, the recurrence pattern is usually local and regional. Metastatic recurrence becomes more significant between the second and fourth years, with a subsequent gradual decrease over time.6 The risk of second tumors based on previous smoking habit has still not been clarified.7
For a follow-up strategy after treatment with curative intent to be successful, the detection of a possible local or distant recurrence or a second metachronous tumor should be able to be treated with some type of radical treatment to prolong survival.
Curative treatments after local recurrence are rarely possible, and 5-year survival rates after recurrence are 15%.8 In the case of a second primary tumor, the figures are better, with 5-year survival of up to 60%.9 This possibility of increasing survival after recurrence or a second neoplasm makes it especially indicated to develop a follow-up strategy for this potential benefit in survival.
Even in the case of recurrences in the form of metastatic disease, where the possibility of cure is scarcely achievable, there are prospective data that suggest that management with ablative therapies may have a positive impact on survival.10
For all these reasons, a standardized and protocolized follow-up methodology should be developed, based on periodic anamnesis, physical examination and a rational use of complementary tests in patients treated with curative resection for lung cancer, given the potential beneficial impact on survival of these patients in cases of tumor recurrence.
The objective of this consensus document is to propose a follow-up protocol for patients treated surgically for lung cancer that would be applicable and reproducible in different healthcare settings, based on currently available scientific evidence.
MethodsIn January 2019, the working group for the “Long-term follow-up study of patients operated on for lung cancer” was created within the Spanish Society of Thoracic Surgery (SECT), consisting of thoracic surgeons and oncologists.
Initially, a literature search was carried out in PubMed, Cochrane Library, UpToDate, Embase databases, using the following search terms: “lung cancer”, “thoracic surgery”, “follow-up” AND “lung resection surgery” OR “lung cancer surgery”. Eligible publications included meta-analyses, systematic reviews, reviews, consensus recommendations from societies other than those involved in these guidelines, randomized controlled trials, non-randomized controlled trials, observational studies, case series studies. The search included studies where full access to content was available, published from January to May 2010|2020, that were written in English, French, or Spanish. Additionally, some older references were added due to their relevance. The total number of papers reviewed was, 75, 26 of which were excluded because they did not meet the minimum number of patients included in the study (10), or because there was some subsequent updated bibliographic reference. Similarly, we reviewed the follow-up recommendations of the main international scientific societies, well as|as, the most relevant communications in international congresses of thoracic surgery, medical oncology. The bibliographical analysis was carried out by all the members of the group, who, based on their findings, decided which aspects of the follow-up deserved to be, carefully assessed, each expert analyzed each assigned area in depth. Each author provided recommendations, the discrepancies among these were resolved in a team meeting, sharing all arguments, reaching a consensus decision of the entire working group electronically.
To evaluate the quality of the evidence and to develop the recommendations, the GRADE guidelines were followed. A strong recommendation implies that the majority of patients should receive the intervention, and a weak one recognizes that different options are valid, so patient values and preferences should be taken into consideration. The level of evidence on which the recommendation is based can be high, moderate, low or very low; the classification is based on the design and methodological quality of the studies analyzed11 (Table 1).
Summary of the GRADE working group guidelines.
| Recommendation grade/description | Benefits vs risks and burdens | Methodological quality of the evidence | Implications |
|---|---|---|---|
| 1A Strong recommendation, high-quality evidence | The benefits clearly exceed the risks and burdens, or vice-versa. | RCT without important limitation or overwhelming evidence from observational studies. | Strong recommendation. This may be applied to most patients, under most circumstances, without reservations. |
| 1B Strong recommendation, moderate-quality evidence | The benefits clearly outweigh the risks and burdens, or vice-versa. | RCT with significant limitations (inconsistent results, methodological defects, indirect or imprecise) or exceptionally strong evidence from observational studies. | Strong recommendation. This may be applied to most patients, under most circumstances, without reservations. |
| 1C Strong recommendation, low or very low-quality evidence | The benefits clearly outweigh the risks and burdens, or vice-versa. | Observational studies or case series | Strong recommendation, which may change when higher quality evidence becomes available. |
| 2A Weak recommendation, high-quality evidence | The benefits are balanced the risks and burdens. | RCT without significant limitations, or overwhelming evidence of observational studies | Weak recommendation. The best action may change depending on the patient circumstances or social values. |
| 2B Weak recommendation, moderate-quality evidence | The benefits are similar to the risks and burdens. | RCT with significant limitations (inconsistent results, methodological defects, indirect or imprecise) or exceptionally strong evidence from observational studies. | Weak recommendation. The best action may change depending on the patient circumstances or their social values. |
| 2C Weak recommendation, low or very low-quality evidence | Uncertain estimation of benefits, risks and burden; benefits, risks and burden may be balanced. | Observational studies or case series | Very weak recommendations. Other alternatives can be equally reasonable. |
RCT, randomized controlled trial.
Routine follow-up of patients after radical-intent surgery for localized-stage lung cancer is recommended by all the guidelines of the main scientific societies, both nationally and internationally. However, given the scarcity of scientific evidence, there is no consensus on the optimal patient follow-up schedule or modalities. The main recommendations of other scientific societies are summarized below:
Spanish Society of Medical Oncology (SEOM)12Year of publication: 2019.
- 1
Recommendation: Close follow-up after curative treatment to identify treatment-related complications, detection of treatable relapse, or development of a second primary lung cancer.
- 2
Modality: follow-up visit including clinical history and physical examination, as well as thoracic computed tomography (CT) scan 6–12 months during the first 2 years and annually thereafter.
- 3
Other recommendations:
- -
Smoking cessation.
- -
Routine follow-up with blood tests, positron emission tomography/computed tomography (PET/CT) or other radiological evaluation is not recommended.
Year of publication: 2016.
- 1
Recommendation: follow-up by a multidisciplinary team, considering complications related to therapy and detecting tumor recurrence and/or the appearance of any second primary tumor.
- 2
Modality: initial monitoring every 3 or 6 months and then once a year. A period of five years is not enough to consider a patient cured, particularly in cases of vascular or lymph node involvement.
Year of publication: 2020.
- 1
Recommendation: routine follow-up with scheduled screening tests for the detection of local recurrence or a second primary lesion.
- 2
Modality: Surveillance every 6 months for 2 years (at least at 12 and 24 months), and then annually, with an office visit that includes history, physical examination, and chest CT, preferably with contrast, to detect second primary tumors.
- 3
Other recommendations:
- -
Smoking cessation, preferably combining behavioral techniques with medical treatment.
- -
In the event of a new finding, the case should be discussed in a multidisciplinary team to assess whether it is a complication of the treatment, a metastasis, or a new primary tumor.
Year of publication: 2017.
- 1
Recommendation: periodic follow-up recommended after radical surgery, considering the risk of recurrence.
- 2
Modality: periodic visit (no defined frequency) including medical history, physical examination, and chest CT with/without contrast for the first 2–5 years, followed by an annual visit with medical history, physical examination and low-dose CT.
- 3
Other recommendations:
- -
Smoking cessation, including counseling and available medical treatments.
- -
PET/CT or magnetic resonance imaging (MRI) of the brain are not recommended as routine tests.
Year of publication: 2013.
- 1
Recommendation: routine follow-up is recommended after resection
- 2
Modality: follow-up visit with chest CT every six months during the first two years and every year thereafter.
- 3
Other recommendations:
- -
Referring physicians should participate in decision-making during follow-up.
- -
Use of validated health-related quality of life instruments at initial visits and during follow-up.
- -
PET/CT, somatostatin receptor scintigraphy, and abdominal ultrasound are not recommended as routine examinations.
- -
Follow-up analysis of biomarkers is not recommended (outside clinical trials).
Year of publication: 2019.
- 1
Recommendation: periodic follow-up recommended to detect recurrences and new primary lung tumors after the first 2 years.
- 2
Modality: follow-up every six months for two years, and then annually, with chest CT being the optimal imaging test for follow-up. After 2 years, a low-dose chest CT should be performed.
- 3
Other recommendations:
- -
PET/CT and brain magnetic resonance imaging (MRI) should not be used for follow-up.
- -
The analysis of circulating biomarkers for the detection of recurrence is not recommended.
- -
Age should not be considered a restriction for follow-up. It is recommended to consider general health status, chronic medical conditions, and patient preferences.
- -
In the case of stages I-III SCLC undergoing treatment with curative intent, brain MRI can be used every 3 months during the first year and every 6 months during the second year.
Taking into account the above, one of the factors that most influences the survival of patients operated on for lung cancer (50 %–60 % at 5 years) is the development of recurrences.5 The dynamics of these recurrences shows a peak in the first 9 months after treatment, as well as at the end of the second and fourth years.6 For this reason, post-treatment follow-up is recommended to detect the appearance of recurrences or second neoplasms early before symptoms appear and initiate potential treatment without delay, thereby improving survival and quality of life.18–20
Today, we have several clinical guidelines for the staging, treatment and follow-up of patients with lung cancer. Most are based on observational studies and systematic reviews, and there is no consensus on which is the best method or the ideal frequency of postoperative follow-up. In other neoplasms, check-ups are carried out every 3–6 months, so that most clinicians consider this follow-up schedule reasonable in the case of lung cancer.21,22 In contrast, a recent study has shown that close follow-up every 3 months after lung resection is not associated with improved overall survival or survival after recurrence, compared with biannual or annual follow-up.23
The main guidelines mentioned all recommend a closer follow-up during the first 2 years, coinciding with the maximum risk of recurrence. However, they differ both in the modality and in the recommended time intervals.
In conclusion, there is no consensus regarding the ideal frequency and duration of patient follow-up after surgery for lung cancer. Based on the available evidence regarding other solid tumors, it seems reasonable to establish clinical controls every 6 months during the first 2 years, including imaging tests (chest CT). (Strong recommendation; low level of evidence).
On the other hand, follow-up beyond the first 2 years after curative treatment is recommended, especially due to the risk of developing new lung neoplasms within 5 years, and this follow-up can be extended to 10 years. (Weak recommendation; low level of evidence).
Interview, examination and complementary tests during follow-upa) Clinical interview and physical examinationThe clinical interview and physical examination can be decisive in the early diagnosis of postoperative complications, since up to 12.8% of operated patients can be readmitted after pulmonary resection, the main reasons being: respiratory failure, pneumonia, pneumothorax and cardiac complications.3 Taking into account the current times in which we find ourselves, the use of office automation tools that provide the ability to monitor patient status without the need for patient travel is especially interesting. In 2017, a study was published that compared the traditionally accepted follow-up with one based on web tools, and longer survival was observed in the intervention group (median overall survival [OS] 19.0 months vs 12.0 months; hazard ratio [HR] 0.32, 95% confidence interval [CI], 0.15−0.67).24 Later, the same group showed that follow-up via internet is more cost-effective than traditional follow-up (difference of Є362 per patient per year, in France).25 The online questionnaire contained 12 signs or symptoms that are recommended to be assessed at each visit, both physical and virtual: weight loss, decreased appetite, pain, cough, dyspnea, depression, fever, facial sweating, voice changes, hemoptysis and appearance of lumps under the skin.24,25
During these patient visits, it is also essential to emphasize the need for the patient to stop smoking, as better results can be achieved with treatment.26 (Strong recommendation; high level of evidence).
b) Tumor markersAlthough tumor markers may play a relevant role in the follow-up of some solid tumors, their clinical utility in non–small-cell lung cancer has not been demonstrated.16
Currently, there are many biomarkers under investigation in lung cancer, an example of which is the postoperative follow-up of minimal residual disease (MRD) by fluid biopsy, but at the moment they are under investigation. Therefore, the use of tumor markers in follow-up outside of clinical trials is not recommended.13,16,26 (Weak recommendation; low level of evidence).
c) Simple chest X-rayAlthough radiography has traditionally been used for the follow-up of lung cancer after surgery, the latest updates of the main guidelines preferably recommend follow-up by CT scan.13,16,26 For this reason, it seems that the role of radiography in follow-up will depend mainly on the frequency of check-ups, the presence of postoperative complications, and findings in the exploration or anamnesis of patients. A chest X-ray could be considered when, for any clinical reason, a closer follow-up is preferred between the periods in which the CT is performed, as well as when a complication is suspected. Follow-up with plain radiography is not recommended in the absence of suspected pleuropulmonary complications. (Strong recommendation; moderate level of evidence).
d) CT: standard, low and minimum dosesToday, CT is one of the most widely used postoperative surveillance strategies in these patients (Table 2). However, most of the evidence available to justify its routine use derives from retrospective observational studies, thus having a low level of evidence.
Summary of complementary tests recommended by scientific societies.
| 6 months | 12 months | 18 months | 24 months | Up to 5 years | After 5 years | |
|---|---|---|---|---|---|---|
| SEOM (12) | CT | CT | CT | Not defined | ||
| ESMO (14) | CT | CT | CT | CT | CT (preferably with contrast) | Not defined |
| NCCN (15) | CT without defined frequency for 2−5 years | Annual low-dose CT | ||||
| ACCP (16) | CT | CT | CT | CT | Annual CT | Not defined |
| ASCO (17) | CT | CT | CT | CT | Low-dose CT | Not defined |
SEOM, Spanish Society of Medical Oncology; ESMO, European Society of Medical Oncology; NCCN, National Comprehensive Cancer Network; ACCP, American College of Chest Physicians; ASCO, American Society of Clinical Oncology; CT, computed tomography.
A meta-analysis including data from 1669 patients operated on for NSCLC showed that CT follow-up was associated with a significant improvement in survival when asymptomatic recurrence was diagnosed (odds ratio [OR]: 0.61, 95% CI: 0.5−0.7).27 However, the authors themselves warn of the heterogeneity in the design and potential limitations of the studies included.
The only evidence of follow-up from a prospective randomized trial is the multicenter IFCT-0302 study,28 which evaluated the follow-up strategy with clinical examination and chest radiography, or follow-up with thoracoabdominal CT and bronchoscopy (optional for adenocarcinomas). We included 1775 patients with resected stage I-II-IIIA NSCLC who completed follow-up visits every 6 months for the first 2 years, and annually for up to 5 years. Their preliminary results were presented at the European Congress of ESMO 2017 in Madrid, with a median follow-up of 8 years and 10 months. No differences in OS were observed between the groups (HR 0.95, 95%CI: 0.82–1.09), with a median of 99.7 months in the control arm and 123.6 months in the experimental arm. Three-year disease-free survival rates were also similar (63.3% and 60.2%, respectively), as were 8-year OS rates (51.7% and 54.6%, respectively). The final results of this study have not yet been published.
Since the main objectives of follow-up are the detection of locoregional recurrence and the early diagnosis of metachronous lung cancer, follow-up with thoracic CT is recommended. There is no evidence to justify the inclusion of the cerebral or abdominopelvic fields for the detection of asymptomatic extrathoracic metastases.
We also do not have conclusive evidence regarding the optimal frequency of CT follow-up. Most scientific societies recommend closer surveillance during the first 2 years, as specified in the corresponding section of this document.
One of the main arguments against follow-up with CT is the continuous exposure to ionizing radiation, with the potential iatrogenic risk that this entails.29 This has led to a growing interest in new technological modalities of low- and minimal-dose tomography. Table 3 summarizes the effective radiation doses of the most commonly used imaging tests in this context.
Compared to the approximate 7−8 mSv dose of a standard CT scan (equivalent to some 70–80 plain chest X-rays), low-dose CT emits around 1.5 mSv.30 Although there are no specific studies that analyze this technology for follow-up, the good results obtained in the field of lung cancer screening may perhaps be extrapolated to this high-risk subpopulation.18,31,32
Minimal-dose CT delivers a mean dose of 0.2 mSv, which is comparable to 0.1 mSv from a plain chest radiograph. In a prospective study comparing these two imaging tests in 231 patients after resection of lung carcinoma, minimal-dose CT had a higher negative predictive value than radiography for the diagnosis of recurrence or a second neoplasm.33 Another prospective study confirmed a sensitivity of 91% for the detection of pulmonary nodules, using standard-dose CT as the reference.34 Some publications warn of the possible limitations of the technique in obese patients or for the evaluation of ground-glass opacities.34,35
Therefore, CT follow-up is recommended as a diagnostic tool. (Strong recommendation; low level of evidence).
e) PET/CTPET/CT is not considered a test of choice in the follow-up of asymptomatic patients who have undergone surgery. In the postoperative context, false positives derived from inflammation can be obtained, especially in thoracotomy scars.36 Some studies have demonstrated its usefulness in diagnosing recurrences,37,38 which is even better than standard radiological examinations,39,40 but up to 10% of lesions identified on CT may also go unnoticed due to the impossibility of performing the technique with controlled breathing.41 In addition, it involves a high radiation dose (around 25 mSv), which is not easily accessible in all clinical settings, and there is controversy about its cost-effectiveness.
In any case, PET/CT can be useful in cases of high clinical-radiological suspicion or confirmed recurrence to determine the best therapeutic strategy and prognosis.42,43
In conclusion, PET/CT is considered appropriate in patients operated on for lung cancer with suspected recurrence or metastasis on CT, and it is not recommended for routine follow-up. (Weak recommendation; low level of evidence).
f) fiberoptic bronchoscopyIn a classic study of intensive postoperative surveillance with CT and bronchoscopy on 192 patients, recurrence was diagnosed exclusively thanks to the endoscopic technique in 7% of the patients.44 The results of the IFCT-0302 study show that there are no significant differences in overall survival when comparing follow-up with CT plus bronchoscopy versus chest x-ray.28
In contrast, endoscopic follow-up is of vital importance after selected surgeries with narrow resection margins, such as partial or complete bronchoplasty (sleeve resection), as well as in the case of endoscopic resection of carcinoid tumors45 or in the event of suspected central recurrence on imaging tests. In this context, endobronchial ultrasound (EBUS) has acquired special prominence due to its versatility, additionally allowing for the biopsy of lesions without an endobronchial component or hilar-mediastinal lymphadenopathies.46,47
There is no solid evidence about the recommended frequency and duration of follow-up in these selected contexts. Given that most recurrences after surgery for lung carcinoma are diagnosed in the first 2 years,48 this data is usually extrapolated to the close follow-up of a bronchoplasty. In the case of endoscopic resection of carcinoid tumors, it seems prudent to maintain follow-up for at least 10 years.49
Therefore, it is recommended not to perform bronchoscopy as a systemic follow-up strategy. (Weak recommendation; low level of evidence).
Summary of SECT recommendations- 1
It is recommended to emphasize the need to stop smoking in follow-up consultations. (Strong recommendation; high level of evidence).
- 2
Simple x-ray follow-up is recommended when pleuropulmonary complications are suspected, but not for routine follow-up. (Strong recommendation; moderate level of evidence).
- 3
Follow-up with CT scan every six months during the first 2 years (Strong recommendation; low level of evidence).
- 4
Follow-up with CT is recommended every 12 months, from the second to the fifth year, which can be extended up to 10 years due to the possibility of developing second neoplasms. (Weak recommendation; low level of evidence).
- 5
The routine use of tumor markers during follow-up is not recommended outside of clinical trials (Weak recommendation; low level of evidence).
- 6
PET/CT is considered appropriate in patients operated on for lung cancer who present suspected recurrence or metastasis on CT, but it is not recommended for routine follow-up. (Weak recommendation; low level of evidence).
- 7
Bronchoscopy follow-up is not recommended in patients operated on for lung cancer. (Weak recommendation; low level of evidence).
The authors have received no funding to conduct this study.
Conflict of interestsThe authors have no conflicts of interests to declare.
Please cite this article as: Cilleruelo Ramos Á, Figueroa Almánzar S, López Castro R, Martínez Hernández NJ, Mezquita Pérez L, Moreno Casado P, et al. Documento de consenso de la Sociedad Española de Cirugía Torácica (SECT). Seguimiento a largo plazo de los pacientes operados de cáncer de pulmón. Cir Esp. 2022;100:320–328.