The aim of the study was to analyse the impact of locoregional surgery on survival of patients with stage IV breast cancer.
Patients and methodsRetrospective study that included patients with breast cancer and synchronous metastases. Patients with ECOG above 2 and high-risk patients were excluded. The following variables were evaluated: age, tumour size, nodal involvement, histological type, histological grade, hormone receptor status, HER2 overexpression, number of affected organs, location of metastases, and surgical treatment. The impact of surgery and several clinical and pathologic variables on survival was analysed by Cox regression model.
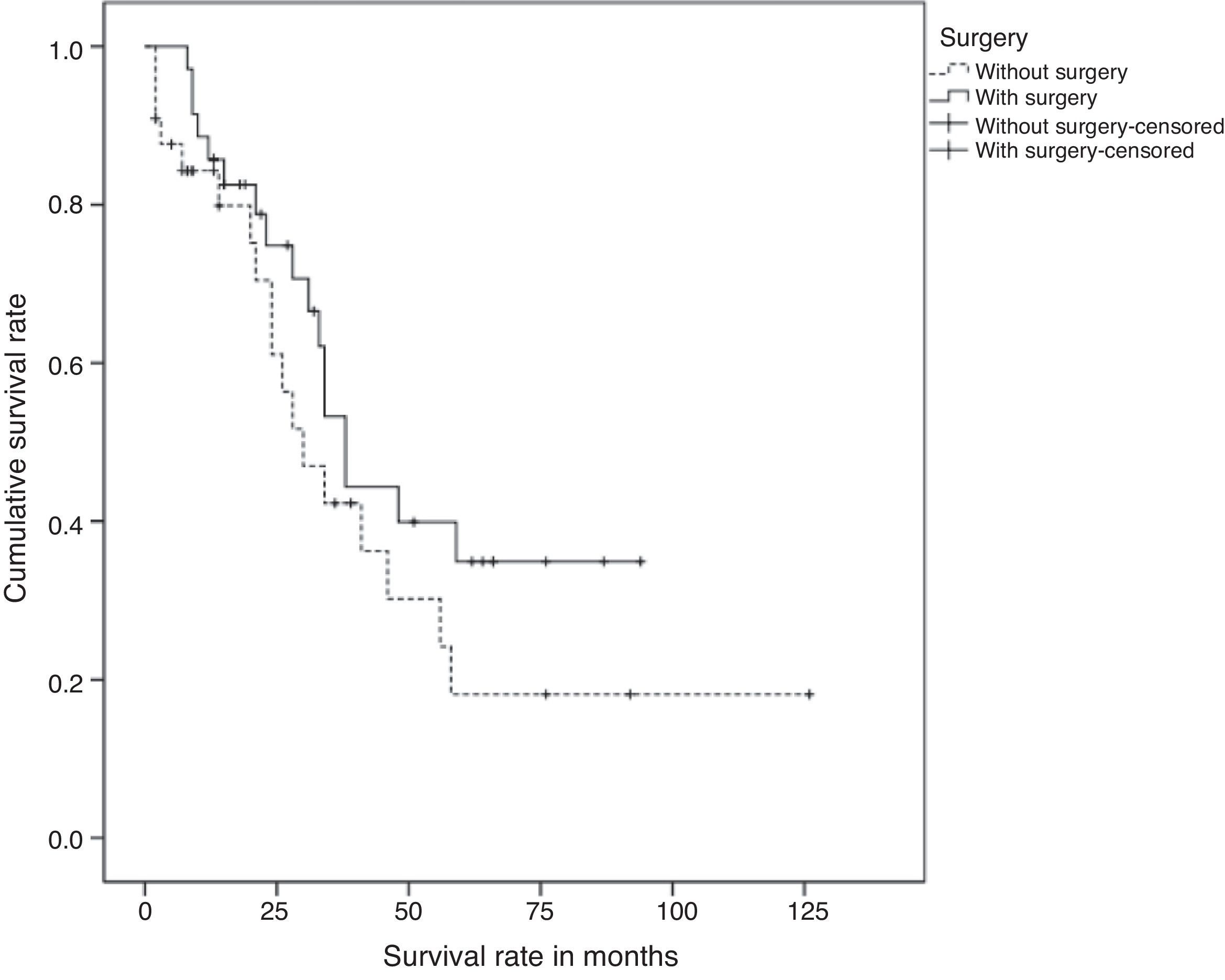
ResultsA total of 69 patients, of whom 36 (52.2%) underwent surgery (study group) were included. After a mean follow-up of 34 months, the median survival of the series was 55 months and no significant differences between the study group and the group of patients without surgery (P=.187) were found. Two factors associated with worse survival were identified: the number of organs with metastases (HR=1.69, CI 95%: 1.05–2.71) and triple negative breast cancer (HR=3.49, CI 95%: 1.39–8.74). Locoregional surgery, however, was not associated with survival.
ConclusionsLocoregional surgical treatment was not associated with improved survival in patients with stage IV breast cancer. The number of organs with metastases and tumours were triple negative prognostic factors for survival.
El objetivo del estudio fue analizar el impacto de la cirugía locorregional en la supervivencia de pacientes con cáncer de mama estadio IV.
Pacientes y métodosEstudio retrospectivo que incluyó a pacientes con cáncer de mama y metástasis sincrónicas. Se excluyó a pacientes con ECOG superior a 2 y elevado riesgo anestésico-quirúrgico. Se evaluaron las siguientes variables: edad, tamaño tumoral, afectación ganglionar, tipo histológico, grado histológico, receptores hormonales, sobreexpresión de HER2, número de órganos afectos, localización de las metástasis y tratamiento quirúrgico. El impacto de la cirugía y las distintas variables clínico-patológicas sobre la supervivencia se analizó mediante un modelo de regresión de Cox.
ResultadosSe incluyó a 69 pacientes, de los que 36 (52,2%) fueron intervenidos quirúrgicamente (grupo estudio). Tras un seguimiento medio de 34 meses, la supervivencia media de la serie fue de 55 meses y no se encontraron diferencias significativas entre el grupo estudio y el grupo de pacientes sin intervención quirúrgica (p=0,187). Se identificaron 2 factores relacionados con una peor supervivencia: el número de órganos con metástasis (HR=1,69; IC 95%: 1,05- 2,71) y el cáncer triple negativo (HR=3,49; IC 95%: 1,39-8,74). La cirugía locorregional, sin embargo, no se relacionó con la supervivencia.
ConclusionesEl tratamiento quirúrgico locorregional no se asoció con mayor supervivencia en pacientes con cáncer de mama en estadio IV. El número de órganos con metástasis y los tumores triple negativo fueron factores de mal pronóstico de supervivencia.
Systemic therapy is the standard treatment for patients with metastatic breast cancer. Surgical treatment in women with stage IV breast cancer has been generally reserved for palliation of local symptoms of the breast or for avoiding complications of the primary tumour, such as ulceration or pain. However, in recent years, observational studies have showed that 35%–60% of the patients with metastatic disease at the moment of diagnosis receive treatment of the primary tumour.1 In the last 10 years, an increasing number of retrospective studies have been published which have assessed the potential impact on survival of locoregional treatment in cases of metastatic breast cancer. The results of these papers are contradictory: some authors reached the conclusion that resection of the primary tumour improves survival rates,2 whereas other authors observed that locoregional surgery does not improve survival rates in patients with metastatic breast cancer.3 The role of surgical treatment of the primary tumour on the survival rates of patients with breast cancer and synchronous metastases is still controversial.
The objective of this study was to analyse the influence of surgical treatment of the primary tumour in the survival rates of patients with stage IV breast cancer.
Patients and MethodRetrospective study of patients of both genders, with stage IV breast cancer treated in the Breast Pathology Unit of the Hospital Médico-Quirúrgico de Jaén between January 2004 and April 2013. Inclusion criteria are: patients diagnosed with breast cancer and synchronous metastasis. Exclusion criteria: value over 2 in the Eastern Cooperative Oncology Group (ECOG) scale and high anaesthetic-surgical risk. Patients with grade 3 of the ECOG scale were excluded from the study, i.e., those who were bedridden over half a day due to the presence of symptoms and needed help for most of their daily activities. Patients who were bedridden all day long and needed help for all daily activities were also excluded (patients grade 4 of the ECOG scale). The existence of comorbidity in patients that would imply high anaesthetic-surgical risk was also an exclusion criterion. Demographic data (gender, age, menopausal state), clinical characteristics (clinical tumour size, clinical tumour involvement of regional lymph nodes), histopathological characteristics of the tumour (histological type, histological grade, hormone receptors, HER2 over-expression determined with herceptest), number of organs with metastasis and location of metastases, surgical treatment of the primary tumour, and survival rate were recorded for every patient. TNM classification was based on the sixth edition of AJCC Cancer Staging Manual. The general condition was assessed by means of the ECOG scale. The location of metastases was classified as only osseous, only visceral and osseous plus visceral. The indications for surgery were locoregional control in patients who had good response of metastases to systemic treatment, unknown metastatic disease at the moment of surgery and treatment of local complications. In all the cases with unknown metastases at the moment of surgery, the diagnosis of systemic disease was performed 2 months after surgical treatment.
A comparative study of the clinical and pathological characteristics between the study group (patients who underwent a surgical procedure) and the control group (patients who had no surgery) was performed, the global survival rate of the sample was determined, the survival curves of the groups were compared and an analysis of prognostic factors related to survival was performed.
Statistical AnalysisIn the case of quantitative variables, the hypothesis of normality was firstly studied and, according to the results, Student's t-test was used for variables with normal distribution and the non-parametric Mann–Whitney U test was used in the case normality was not found. In order to contrast the qualitative variables, Fisher's exact test was used for 2×2 tables, and chi-square test was used for the rest of the cases.
Global survival was defined as the elapsed time between the histopathological diagnosis date and the death or last consultation date.
Global survival was determined according to the Kaplan–Meier method, and survival curves between the groups were compared with the log-rank test. In the survival curves, those patients from both cohorts (study group and control group) who reached the end of their follow-up and did not show the event of interest, i.e., they had not died, were considered as censored data. A Cox proportional hazards model was used for the study of the prognostic factors. In the bivariate analysis of prognostic factors associated with the survival rate, the net hazard ratio (HR) and the 95% confidence interval (CI) from each of the variables were calculated. In light of the results of the bivariate study, a multivariate model was suggested with those variables that turned out to be individually significant and the variables that are considered clinically relevant to determine the survival rate.
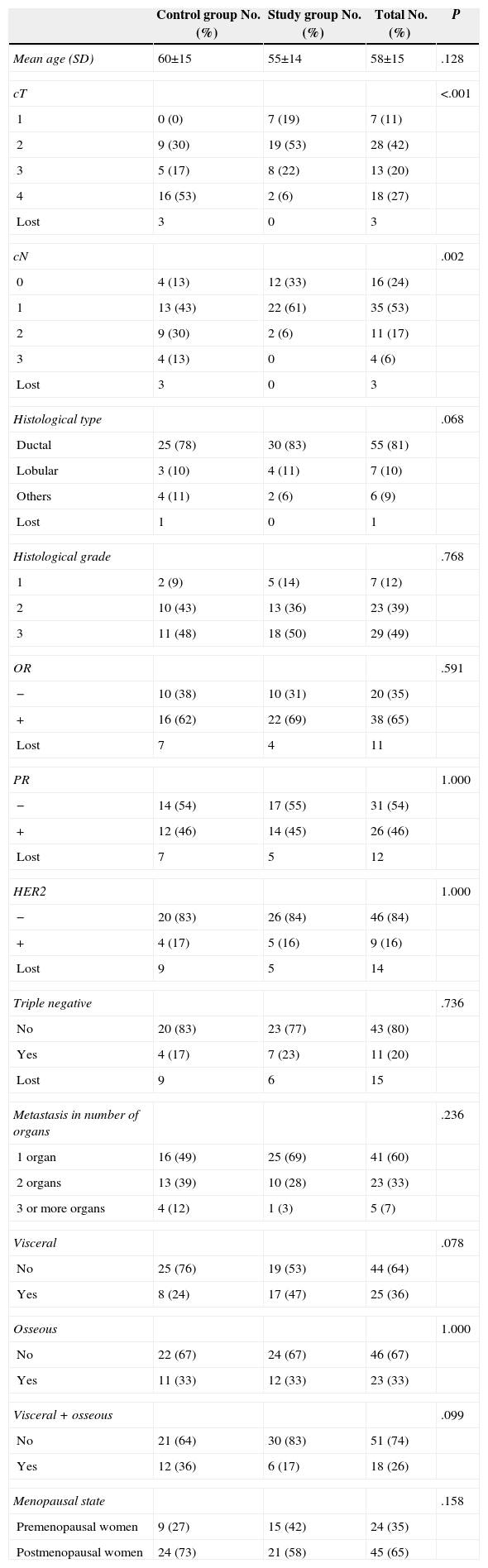
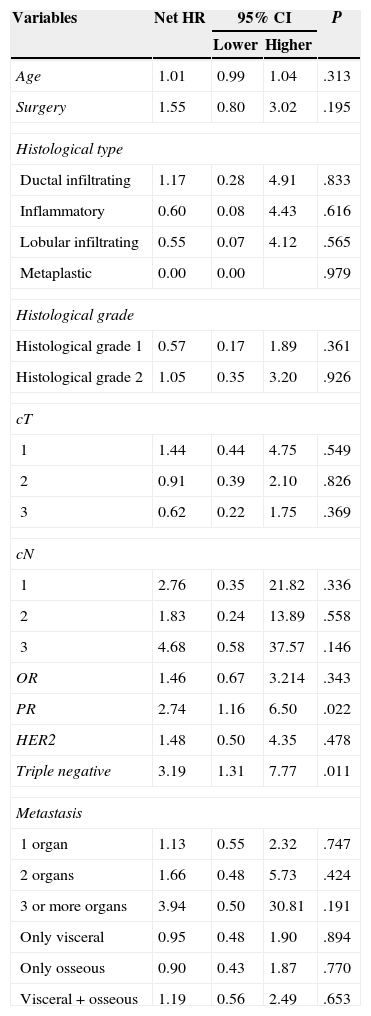
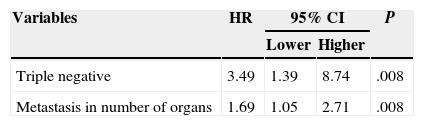
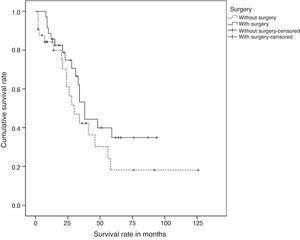
ResultsSixty nine patients were included in the study: 66 women and 3 men. Surgery of the primary tumour (study group) was performed in 36 patients (52% of the total sample), and 33 patients, who did not undergo a surgical procedure, were included in the control group. In 21 out of 36 operated patients (58%), surgical treatment of the primary tumour and axila was performed. In the rest of the cases, only the primary tumour was surgically treated. The mean time of follow-up was 34 months (CI: 26.91–41.08). Clinical and histological characteristics of patients in the study group and control group, as well as in the total sample, are shown in Table 1. When contrasting the variables in the 2 groups of patients, it was observed that the patients from the study group showed a smaller tumour size and less lymph node involvement in comparison with the control group. These differences were statistically significant. There were no significant differences found in the rest of the studied variables. During the follow-up period, 35 patients (50%) died. The global mean survival rate of the sample was of 55 months (CI: 41.81–68.25). In the study of the survival curves, it was proved that there were no statistically significant differences between the survival rate of the study group and that of the control group (P=.187) (Fig. 1). In the bivariate study of possible prognostic factors of survival, a statistically significant association was found with the progesterone receptors (P=.022) and with negative oestrogen receptors+negative progesterone receptors+negative HER2 (triple negative) (P=.011) (Table 2). In the multivariate study, 2 factors of worse prognosis were identified, which significantly influenced the survival time: number of organs with metastasis (HR=1.69; 95% CI: 1.05–2.71) and the triple negative tumours (HR=3.48; 95% CI: 1.39–8.74) (Table 3).
Clinical and Histological Characteristics of the Patients.
| Control group No. (%) | Study group No. (%) | Total No. (%) | P | |
|---|---|---|---|---|
| Mean age (SD) | 60±15 | 55±14 | 58±15 | .128 |
| cT | <.001 | |||
| 1 | 0 (0) | 7 (19) | 7 (11) | |
| 2 | 9 (30) | 19 (53) | 28 (42) | |
| 3 | 5 (17) | 8 (22) | 13 (20) | |
| 4 | 16 (53) | 2 (6) | 18 (27) | |
| Lost | 3 | 0 | 3 | |
| cN | .002 | |||
| 0 | 4 (13) | 12 (33) | 16 (24) | |
| 1 | 13 (43) | 22 (61) | 35 (53) | |
| 2 | 9 (30) | 2 (6) | 11 (17) | |
| 3 | 4 (13) | 0 | 4 (6) | |
| Lost | 3 | 0 | 3 | |
| Histological type | .068 | |||
| Ductal | 25 (78) | 30 (83) | 55 (81) | |
| Lobular | 3 (10) | 4 (11) | 7 (10) | |
| Others | 4 (11) | 2 (6) | 6 (9) | |
| Lost | 1 | 0 | 1 | |
| Histological grade | .768 | |||
| 1 | 2 (9) | 5 (14) | 7 (12) | |
| 2 | 10 (43) | 13 (36) | 23 (39) | |
| 3 | 11 (48) | 18 (50) | 29 (49) | |
| OR | .591 | |||
| − | 10 (38) | 10 (31) | 20 (35) | |
| + | 16 (62) | 22 (69) | 38 (65) | |
| Lost | 7 | 4 | 11 | |
| PR | 1.000 | |||
| − | 14 (54) | 17 (55) | 31 (54) | |
| + | 12 (46) | 14 (45) | 26 (46) | |
| Lost | 7 | 5 | 12 | |
| HER2 | 1.000 | |||
| − | 20 (83) | 26 (84) | 46 (84) | |
| + | 4 (17) | 5 (16) | 9 (16) | |
| Lost | 9 | 5 | 14 | |
| Triple negative | .736 | |||
| No | 20 (83) | 23 (77) | 43 (80) | |
| Yes | 4 (17) | 7 (23) | 11 (20) | |
| Lost | 9 | 6 | 15 | |
| Metastasis in number of organs | .236 | |||
| 1 organ | 16 (49) | 25 (69) | 41 (60) | |
| 2 organs | 13 (39) | 10 (28) | 23 (33) | |
| 3 or more organs | 4 (12) | 1 (3) | 5 (7) | |
| Visceral | .078 | |||
| No | 25 (76) | 19 (53) | 44 (64) | |
| Yes | 8 (24) | 17 (47) | 25 (36) | |
| Osseous | 1.000 | |||
| No | 22 (67) | 24 (67) | 46 (67) | |
| Yes | 11 (33) | 12 (33) | 23 (33) | |
| Visceral+osseous | .099 | |||
| No | 21 (64) | 30 (83) | 51 (74) | |
| Yes | 12 (36) | 6 (17) | 18 (26) | |
| Menopausal state | .158 | |||
| Premenopausal women | 9 (27) | 15 (42) | 24 (35) | |
| Postmenopausal women | 24 (73) | 21 (58) | 45 (65) | |
cN: clinical tumour involvement of regional lymph nodes; cT: clinical tumour size; SD: standard deviation; Lost: no information available; OR: oestrogen receptors; PR: progesterone receptors.
Bivariate Analysis of Prognostic Factors Associated With Survival Rate.
| Variables | Net HR | 95% CI | P | |
|---|---|---|---|---|
| Lower | Higher | |||
| Age | 1.01 | 0.99 | 1.04 | .313 |
| Surgery | 1.55 | 0.80 | 3.02 | .195 |
| Histological type | ||||
| Ductal infiltrating | 1.17 | 0.28 | 4.91 | .833 |
| Inflammatory | 0.60 | 0.08 | 4.43 | .616 |
| Lobular infiltrating | 0.55 | 0.07 | 4.12 | .565 |
| Metaplastic | 0.00 | 0.00 | .979 | |
| Histological grade | ||||
| Histological grade 1 | 0.57 | 0.17 | 1.89 | .361 |
| Histological grade 2 | 1.05 | 0.35 | 3.20 | .926 |
| cT | ||||
| 1 | 1.44 | 0.44 | 4.75 | .549 |
| 2 | 0.91 | 0.39 | 2.10 | .826 |
| 3 | 0.62 | 0.22 | 1.75 | .369 |
| cN | ||||
| 1 | 2.76 | 0.35 | 21.82 | .336 |
| 2 | 1.83 | 0.24 | 13.89 | .558 |
| 3 | 4.68 | 0.58 | 37.57 | .146 |
| OR | 1.46 | 0.67 | 3.214 | .343 |
| PR | 2.74 | 1.16 | 6.50 | .022 |
| HER2 | 1.48 | 0.50 | 4.35 | .478 |
| Triple negative | 3.19 | 1.31 | 7.77 | .011 |
| Metastasis | ||||
| 1 organ | 1.13 | 0.55 | 2.32 | .747 |
| 2 organs | 1.66 | 0.48 | 5.73 | .424 |
| 3 or more organs | 3.94 | 0.50 | 30.81 | .191 |
| Only visceral | 0.95 | 0.48 | 1.90 | .894 |
| Only osseous | 0.90 | 0.43 | 1.87 | .770 |
| Visceral+osseous | 1.19 | 0.56 | 2.49 | .653 |
The results are provided as hazard ratio and 95% confidence interval.
cN: clinical tumour involvement of regional lymph nodes; cT: clinical size of primary tumour; HR: hazard ratio; CI: confidence interval; OR: oestrogen receptors; PR: progesterone receptors.
Multivariate Analysis of Prognostic Factors Associated With Survival Rate.
| Variables | HR | 95% CI | P | |
|---|---|---|---|---|
| Lower | Higher | |||
| Triple negative | 3.49 | 1.39 | 8.74 | .008 |
| Metastasis in number of organs | 1.69 | 1.05 | 2.71 | .008 |
The results are provided as hazard ratio and 95% confidence interval.
HR: hazard ratio; CI: confidence interval.
The studies that analyse the role of primary tumour surgery in the survival rate of patients with stage IV breast cancer lead to contradictory results. The retrospective nature of the studies, the absence of inclusion criteria in many of the series and the detection of selection biases in some of them make their conclusions questionable, and the controversy remains on the effect of surgery on the survival rate.
In our study, we have considered as exclusion criteria the presence of added baseline disease which would imply an increase of the anaesthetic-surgical risk and ECOG index higher than 2. Patients with these characteristics were not included in the study, since we considered that, due to their bad general condition, high surgical risk or low life expectancy, they were not eligible for a possible surgery. The studies of Pérez-Fidalgo et al.2 and Blanchard et al.4 have considered as exclusion criteria the existence of serious comorbidity, age over 80 years or an ECOG index higher than 2. However, the fact that, in the other series we have consulted, the inclusion criteria are not described attracted our attention, which could suppose a bias because the patients with bad general condition or low life expectancy have less chances of surgical treatment.
In the study we present, the surgical treatment of the primary tumour was not associated with a higher survival rate. Other authors agree with our results and conclude that locoregional surgery of the primary tumour does not improve the survival rate in patients with metastatic breast cancer, and they suggest that the response to chemotherapy is the only factor associated with a better survival rate.3,5–7
In the Breast Pathology Unit of the Hospital Médico-Quirúrgico de Jaén, the locoregional control in patients who had a good response of the metastases to systemic treatment was considered an indication for surgery. We recognise that this circumstance supposes a selection bias, since women with a favourable response to systemic therapy have a higher survival rate than those whose metastases do not respond to chemotherapy, regardless if they receive surgical treatment or not. In spite of this, we did not find significant differences in the survival rate between the study group and the control group in our results. Other authors have pointed out that the response to systemic therapy can affect the decision to proceed to surgery. It should be noted that surgical treatment is not offered to women who do not have a significant response to systemic treatment, while patients with good clinical response are usually prescribed a more aggressive surgical treatment.8
Recent studies have investigated the role of the moment of surgery in survival rates. For that, they have selected a subgroup of patients who received surgical treatment before receiving systemic chemotherapy, aiming to prevent that the surgical decision be conditioned by the response to the systemic therapy. These studies led to the result that the moment of surgery has no influence on the survival.2,3
There are numerous studies that reached the conclusion that the resection of the primary tumour improves the survival rate in the group of the studied sample or in groups of selected patients.2,9–15 Neuman et al.12 observed a trend towards a better survival rate with surgery in the total population of their study, although it was more evident in the subgroup of patients with positive hormone receptors and positive HER2, which suggests that the impact of local control on the survival rate is more evident when an efficient targeted therapy was followed. Rashaan et al.13 conclude in a study of retrospective cohorts that women with more favourable prognostic factors (young patients, without comorbidity, with a small tumour, with positive hormone receptors, and with only visceral metastases) were more often operated on. In this group of patients, an association between surgery and a better survival rate was proven. Similar results were obtained by Rapiti et al.14 in a retrospective study of 300 patients in which they showed that women treated by surgery were younger, had tumours of smaller clinical size (cT), less clinical involvement of the axillary lymph nodes (cN), and only one metastatic location. In our study, by comparing the clinical and histological characteristics between the study group and the control group, we detected the existence of a selection, since patients who underwent a resection of the primary tumour showed smaller tumours and less involvement of the regional lymph nodes than the patients who did not receive surgical treatment. As shown in Table 1, the differences observed had statistical significance. In the rest of the studied variables, there were no significant differences between both groups.
In a recently published meta-analysis, Harris et al.15 reached the conclusion that patients with stage IV disease who were treated by the surgical removal of the primary tumour reached a survival rate higher than patients who did not undergo a surgical procedure. These authors emphasise that the patient screening for surgery favoured those women with smaller primary tumours and less metastatic load.
We highlight 2 randomised clinical trials that were showed in the 36th Annual San Antonio Breast Cancer Symposium celebrated in December 2013. Badwe et al.16 included in a prospective and randomised study 350 women with metastatic breast cancer who had an objective response of the tumour after treatment with anthracycline. Out of 350 women, 173 patients were randomly selected for locoregional treatment and 177 women were selected for the group without locoregional treatment. These authors concluded that the locoregional treatment of the primary tumour and the axillary lymph nodes had no impact on the survival rate of patients diagnosed with metastatic breast cancer who responded to first-line chemotherapy. In this study, no subgroup of patients susceptible to benefit from locoregional treatment was identified. According to the authors, surgical treatment must be reserved for women who need it for palliative reasons. In the second study, Soran et al.17 achieved similar results. These authors, studied 278 women with stage IV breast cancer in a phase III randomised trial, 140 of them were assigned to the surgery group and 138 to the non-surgery group. After a mean follow-up of 21.1±14.5 months, there were no significant differences observed in the survival rate between both groups. In this study, a subgroup of patients with solitary bone metastasis was identified, in which locoregional surgery had a statistically significant benefit in the survival rate in comparison with women who did not undergo surgery.
In the study we present, the number of organs with metastasis and triple negative patients behaved as bad prognosis factors that significantly influenced the survival time. In the analysis of prognostic factors, the surgical treatment of the primary tumour was not significantly related to the survival rate.
We agree with the reference authors in that the retrospective nature of the studies represents an important limitation and, besides, the selection bias of cases in the group of patients treated with resection of the primary tumour can largely explain the apparent advantage of survival of surgery. The randomised studies we have mentioned avoid the biases described in the retrospective studies, and the results obtained in both studies help to clarify the existing controversy about the real impact of locoregional surgery on the survival rate of patients with stage IV breast cancer.
Conflict of InterestThe authors declare that there are no conflicts of interest.
Please cite this article as: Jiménez Anula J, Sánchez Andújar B, Machuca Chiriboga P, Navarro Cecilia J, Dueñas Rodríguez B. Tratamiento quirúrgico del tumor primario en pacientes con cáncer de mama en estadio IV. Cir Esp. 2015;93:375–380.