Adenocarcinomas of the gastroesophageal junction represent 27% of all gastric tumors. In recent years, it has been classified as an entity of its own, with specific treatments that are sometimes differentiated from gastric treatments. Treatment can be based on chemotherapy (CTx) or chemoradiotherapy (CRTx) that is administered preoperatively (neoadjuvant), postoperatively (adjuvant) or perioperatively. There are studies that have tested several treatment modalities, but there is currently no single protocolized sequence. The results point to an improvement in survival when we administer preoperative treatment, with evidence in favor of CRTx and CTx. Studies are already underway with targeted treatment that aim to increase the activity of traditional chemotherapy. In the next few years, we should know the role of immunotherapy in this group of patients.
Los adenocarcinomas de la unión esofagogástrica representan un 27% de todos los tumores gástricos. En los últimos años se está clasificando como una entidad propia, con tratamientos específicos y en ocasiones diferenciados de los tratamientos de los adenocarcinomas de cuerpo gástrico. El esquema de tratamiento puede basarse en quimioterapia (QT) o quimiorradioterapia (QTRT), que se administra de forma preoperatoria (neoadyuvante), postoperatoria (adyuvante) o perioperatoria. Existen estudios que han testado las diversas modalidades de tratamiento, pero en estos momentos no se dispone de una única secuencia protocolizada válida. Los resultados apuntan a una mejoría de la supervivencia cuando administramos tratamiento preoperatorio, con evidencia a favor de la QTRT y de la QT. Ya están en marcha estudios con tratamientos dirigidos que pretenden conseguir aumentar la actividad de la QT tradicional y en los próximos años deberemos conocer el papel de la inmunoterapia en este grupo de pacientes.
In 2012, gastric cancer (GC) had an incidence of 951000 cases worldwide, making it the fifth cause of cancer and the third cause of cancer death with 723000 cases.1 In Spain, it is the sixth most frequent type of cancer, with 7810 new cases and 5389 deaths annually.2
GC can be differentiated into 2 topographic categories3: cardia or esophagogastric junction (EGJ),4 and non-cardia or rest of the stomach. Both tumors have differentiated risk factors.
EGJ tumors represent 27% of GC (30% men; 21% women), with an incidence of 3.3/100000 inhabitants (5.3 men; 1.6 women).5
Despite R0 surgery, there is a high incidence of local recurrence and distant recurrence. These results have led to the evaluation of therapies combined with chemotherapy (CTx) and radiotherapy (RTx) as neoadjuvant, perioperative or adjuvant treatment, although the best strategy has not been established.
The aim of this review is to provide a thorough overview of the oncological treatments available for the multimodal treatment of adenocarcinomas of the EGJ.6
Preoperative TreatmentIn EGJ cancer, both CTx and neoadjuvant chemoradiotherapy (CRTx) have been studied (Table 1). The meta-analysis by Gebski et al.7 analyzed neoadjuvant CTx (CTNeo) (8 studies; 1724 patients) and preoperative CRTx (CRTNeo) (10 studies; 1209 patients), showing an absolute benefit in overall 2-year survival (OS) of 7% for CTx and 13% for CRTx. Both treatments improved survival (CRTx [P=.02] and CTx [P=.014]) in the case of adenocarcinoma, but only CRTx showed benefits in patients with squamous carcinoma (P=0.04). The phase III trial CALBG 97818 compared initial surgery with CTx (CDDP+5-FU) and RTx followed by surgery. It recruited 56 of the 475 patients planned, managing to demonstrate a benefit in OS of 4.48 years compared to 1.79 years (P=.002).
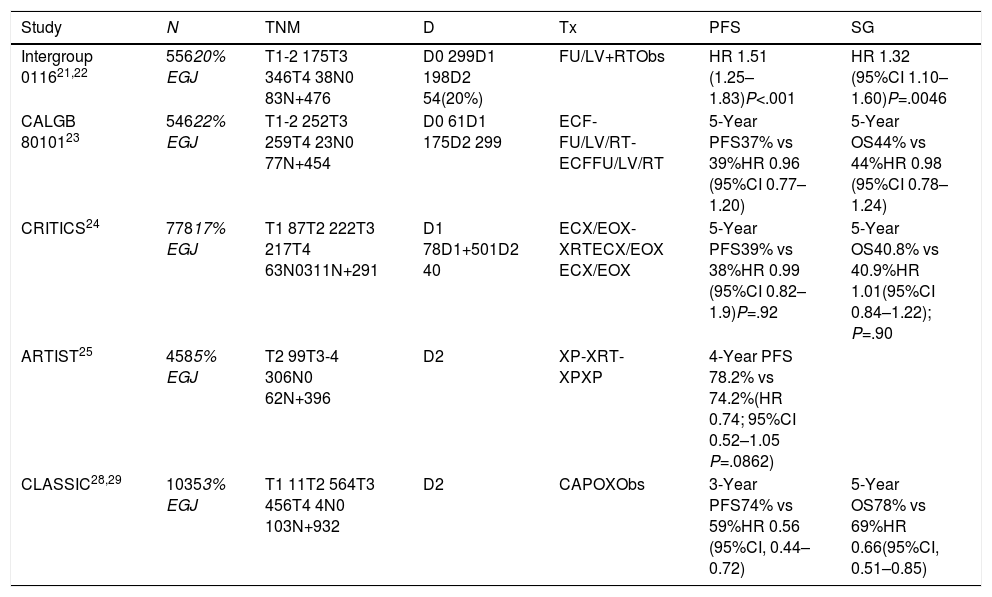
Clinical Trials Analyzing Preoperative and Perioperative Treatment With Chemotherapy and Radiochemotherapy Including Patients With Adenocarcinoma of the EGJ.
| Study | N | TNM | D | Tx | PFS | SG |
|---|---|---|---|---|---|---|
| Intergroup 011621,22 | 55620% EGJ | T1-2 175T3 346T4 38N0 83N+476 | D0 299D1 198D2 54(20%) | FU/LV+RTObs | HR 1.51 (1.25–1.83)P<.001 | HR 1.32 (95%CI 1.10–1.60)P=.0046 |
| CALGB 8010123 | 54622% EGJ | T1-2 252T3 259T4 23N0 77N+454 | D0 61D1 175D2 299 | ECF-FU/LV/RT-ECFFU/LV/RT | 5-Year PFS37% vs 39%HR 0.96 (95%CI 0.77–1.20) | 5-Year OS44% vs 44%HR 0.98 (95%CI 0.78–1.24) |
| CRITICS24 | 77817% EGJ | T1 87T2 222T3 217T4 63N0311N+291 | D1 78D1+501D2 40 | ECX/EOX-XRTECX/EOX ECX/EOX | 5-Year PFS39% vs 38%HR 0.99 (95%CI 0.82–1.9)P=.92 | 5-Year OS40.8% vs 40.9%HR 1.01(95%CI 0.84–1.22); P=.90 |
| ARTIST25 | 4585% EGJ | T2 99T3-4 306N0 62N+396 | D2 | XP-XRT-XPXP | 4-Year PFS 78.2% vs 74.2%(HR 0.74; 95%CI 0.52–1.05 P=.0862) | |
| CLASSIC28,29 | 10353% EGJ | T1 11T2 564T3 456T4 4N0 103N+932 | D2 | CAPOXObs | 3-Year PFS74% vs 59%HR 0.56 (95%CI, 0.44–0.72) | 5-Year OS78% vs 69%HR 0.66(95%CI, 0.51–0.85) |
In subsequent studies and meta-analyses,9 the hypothesis of neoadjuvant treatment being beneficial was maintained, and in 2012 the CROSS10 phase III study validated the CRTNeo strategy. The study included 368 patients (24% EGJ); CTNeo (CBCA-paclitaxel) and concomitant RTx followed by surgery were compared with surgery alone. The OS was 49 months versus 24 months in favor of CRTNeo treatment (P=.003). R0 resections were 92% versus 69% in favor of CRTNeo (P<.001), and the morbidity and mortality rates were the same in both treatment arms.
Neoadjuvant ChemotherapyThe phase III OE02 trial11 recruited 802 patients with esophageal and EGJ cancer (10%) and compared direct surgery with CTNeo (cisplatin-5-FU) followed by surgery. The trial demonstrated a benefit in 5-year OS of 23% versus 17% in favor of neoadjuvant therapy (P=.03). The phase III OE05 trial compared cisplatin-5-FU with the neoadjuvant epirubicin-cisplatin-capecitabine (ECF) triplet followed by surgery and showed no benefit for triple therapy. A meta-analysis published by Sjoquist et al.9 confirmed the benefit in OS for CTNeo of 5.1% after 2 years (P=.005), which was shown in patients with adenocarcinoma (P=.01) but not in the squamous carcinoma group (P=.18).
Chemoradiotherapy Versus Preoperative ChemotherapyIn a study specifically aimed at patients with adenocarcinoma of the EGJ,12 126 patients out of 354 were included. CTNeo (cisplatin+5-FU) and subsequent surgery were compared to CRTNeo followed by surgery. The 3-year OS (47.4% vs 27.7%; P=.07) and 5-year OS13 (P=.055), although higher in the CRTx group, did not reach statistically significant differences. Perioperative mortality was also higher in the CRTx group (10.2% vs 3.8%). A second study14 included 181 patients (18% EGJ), comparing CTNeo (cisplatin-5-FU) versus CRTNeo. The CRTNeo arm showed a higher rate of pathological complete responses (pRC) (28% vs 9%; P=.002) and R0 resections (87% vs 74%; P=.004), although OS was similar in both groups.
Therefore, the benefit of neoadjuvant treatment has consistent evidence, although there is still controversy about the most beneficial strategy.
Due to the benefit of trastuzumab treatment in patients who overexpress HER2 in the metastatic stage,15 the phase II-III INNOVATION study (NCT02205047) (CTx+trastuzumab±neoadjuvant pertuzumab) and the phase II trial (NCT01196390) (RTx-carboplatin-taxol±trastuzumab) are underway to demonstrate the potential effect of these therapies in the treatment of these tumors.
Cetuximab (anti-EGFR monoclonal antibody) added to CRTNeo showed no benefit in OS in the phase II-III SCOPE116 trial or in the phase III RTOG 0436 trial.17 Bevacizumab (anti-VEGF monoclonal antibody) has also demonstrated no activity in a phase II study18 comparing bevacizumab and erlotinib associated with CRTNeo (CBDCA-paclitaxel-5-FU).
Current trials are evaluating the effectiveness of other immunotherapeutic drugs in neoadjuvant therapy, such as nivolumab and ipilimumab (NCT03604991), and pembrolizumab (NCT02730546).
Adjuvant TreatmentOS after the resection of EGJ tumors ranges between 18 and 50%,19 with a recurrence rate of more than 50%. Therefore, the use of adjuvant treatment is often necessary.20
There are studies that evaluate adjuvant treatment with CTx (CTAdj) or CRTx (CRTAdj) (Table 2).
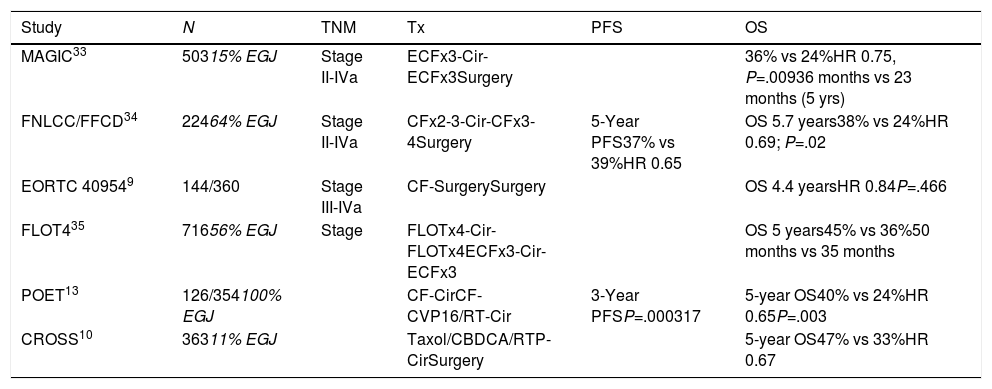
Clinical Trials Analyzing Postoperative Adjuvant Treatment With Chemotherapy and Radiochemotherapy Including Patients With Adenocarcinoma of the EGJ.
| Study | N | TNM | Tx | PFS | OS |
|---|---|---|---|---|---|
| MAGIC33 | 50315% EGJ | Stage II-IVa | ECFx3-Cir-ECFx3Surgery | 36% vs 24%HR 0.75, P=.00936 months vs 23 months (5 yrs) | |
| FNLCC/FFCD34 | 22464% EGJ | Stage II-IVa | CFx2-3-Cir-CFx3-4Surgery | 5-Year PFS37% vs 39%HR 0.65 | OS 5.7 years38% vs 24%HR 0.69; P=.02 |
| EORTC 409549 | 144/360 | Stage III-IVa | CF-SurgerySurgery | OS 4.4 yearsHR 0.84P=.466 | |
| FLOT435 | 71656% EGJ | Stage | FLOTx4-Cir-FLOTx4ECFx3-Cir-ECFx3 | OS 5 years45% vs 36%50 months vs 35 months | |
| POET13 | 126/354100% EGJ | CF-CirCF-CVP16/RT-Cir | 3-Year PFSP=.000317 | 5-year OS40% vs 24%HR 0.65P=.003 | |
| CROSS10 | 36311% EGJ | Taxol/CBDCA/RTP-CirSurgery | 5-year OS47% vs 33%HR 0.67 |
The SWOG 9008/INT-0116 trial evaluated the efficacy of surgery followed by CRTAdj in the OS of patients with resectable adenocarcinoma of the stomach or UGE (20%).21 The 10-year OS was beneficial for CRTAdj (27 months vs 36 months; P=.0046). Local recurrence (19% vs 27%) benefited from CRTAdj.22 These results have standardized CRTAdj in GC or completely resected EGJ without preoperative treatment.
The CALGB 80101 study evaluated 546 patients (22% EGJ)23 and compared 5 FU to the ECF scheme associated with RTx. No differences were found in OS after 6.5 years (44% vs 44%; HR 0.98) or in progression-free survival (PFS) (37% vs 39%; HR 0.96).
The CRITICS study included 17% EGJ tumors.24 It compared the ECX/EOX regimen with adjuvant CRTx, with no differences in 5-year OS (40.8% for CTx vs 40.9% for CRTx).
The ARTIST study compared CTx with cisplatin and capecitabine (XP) with CRTx (XP-XRT-XP),25 including only 3% of EGJ tumors. There were no differences in OS (HR: 1.13) or recurrences (P=.0862). Patients with positive lymph nodes presented a 3-year PFS that favored CRTAdj (76% vs 72%; P=.0365). Currently, the ARTIST-2 study is testing the benefit in patients with positive lymph nodes.
While the addition of CRTAdj has been associated with benefits in survival in patients with EGJ cancer with positive lymph nodes,26,27 the efficacy of postoperative CRTx compared to surgery alone has not been demonstrated in a randomized trial.
Adjuvant ChemotherapyThere are more than 30 randomized studies that compare adjuvant CTx with surgery alone in resected GC. The results of the majority were negative for improving OS and do not provide data for EGJ tumors.
In the CLASSIC Asian study of patients with gastric adenocarcinoma (3% EGJ) with D2 lymphadenectomy,28 CTAdj (capecitabine+oxaliplatin) was compared with surgery alone. The 3-year PFS (74% vs 59%; HR: 0.56) and 5-year OS benefited the CTAdj group (78% vs 69%), and the patients with the greatest benefit were those with lymph node involvement.29
Meta-analyses30,31 give a better prognosis to Asian patients, with a 15% lower risk of death when they were treated with CTAdj (HR: 0.85).32
Perioperative ChemotherapyThere are 4 relevant studies about perioperative chemotherapy treatment versus surgery alone in patients diagnosed with GC (Table 1).
The MAGIC33 study included patients with potentially resectable adenocarcinoma of the stomach (74%), distal esophagus (15%) and EGJ (11%). CTx (perioperative ECFx6) was compared with surgery alone and achieved greater R0 surgery (79% vs 70%), T1/2 tumors (52% vs 37%), N0 disease (84% vs 71%) and 5-year OS of 36% versus 23% of the surgery alone group.
The French FNCLCC/FFCD study34 (144 EGJ) studied perioperative CTx with cisplatin-5-FU versus surgery alone. The 5-year PFS (34% vs 19%) and OS (38% vs 24%) favored CTx.
A meta-analysis9 concluded that CTNeo increased OS (HR: 1.32) and PFS (HR: 1.85).
The phase III study FLOT4-AIO35 compared FLOT perioperative FLOT treatment (docetaxel+oxaliplatin+5-FU) with ECF/ECX and randomized 716 patients with gastric (44%) and EGJ (56%) cancer. FLOT was significantly better in pRC (16% vs 8%), T0/T1 (25% vs 15%), N0 (49% vs 41%) and 5-year OS (45% vs 36%). Postoperative complications were similar. These results have made the FLOT regimen a standard in perioperative treatment.
Currently, immunotherapy treatment is being tested in perioperative (NCT03221426, NCT03421288) and adjuvant (NCT2743494, NCT03006705) strategies.
Stage iv Treatment (Metastasis)EGJ tumors have been included in studies exploring the treatment of stage IV gastric adenocarcinoma. A Cochrane review36 demonstrated the benefit in OS of treatment with CTx compared to the best support treatment (4.3 vs 11 months).
The drugs with proven efficacy in metastatic gastric/EGJ cancer are fluoropyrimidines (5-FU, capecitabine), platinum salts (oxaliplatin and cisplatin), taxanes (paclitaxel, docetaxel), irinotecan, TAS 102, monoclonal antibodies trastuzumab and ramucirumab, as well as immunotherapy (pembrolizumab and nivolumab), especially in tumors with microsatellite instability or overexpression of PDL1.
The choice of treatment depends on the general condition and comorbidity of the patient as well as HER2 overexpression in the tumor tissue. Combination treatments with 2 drugs (fluoropyrimidines-platinum) are standard,37 but combinations with 3 drugs (5-FU+platinum+docetaxel, ECF) in patients with excellent general condition, or monotherapy (fluoropyrimidine, irinotecan, taxane weekly) in patients with poor general condition, could be the choice.37
First Line of TreatmentThe phase III ToGA trial randomized patients with HER2 overexpression38 (20% EGJ) to cisplatin+5-FU±trastuzumab.16 Both the OS (13.8 vs 11.1 months; P=.0046) and the objective response rate (47% vs 35%; P=.0017) were favorable to the use of trastuzumab. In tumors without HER2 overexpression, the main combined regimens are FOLFOX and CAPOX. The use of triplets39 (cisplatin+docetaxel+5-FU) achieves more responses and PFS with greater toxicity. The contribution of anthracyclines and irinotecan does not show a clear benefit in the PFS or OS.40 Anti-EGFR monoclonal antibodies (cetuximab, panitumumab) or anti-VEGF/VEGF-R (bevacizumab, ramucirumab, aflibercept) have not demonstrated activity.
Second Line of TreatmentOther treatments are weekly paclitaxel, docetaxel or irinotecan.41 The paclitaxel+ramucirumab combination (RAINBOW42 phase III study with 20% of EGJ tumors) presented a PFS of 4.4 versus 2.9 months and an OS of 9.6 months versus 7.4 months (P=.017) in favor of adding ramucirumab to taxol, becoming the second-line treatment of choice.37
ImmunotherapyThe utility of immunotherapy has been demonstrated in the metastatic stage,43 with pembrolizumab and nivolumab in the KEYNOTE-59,44 KEYNOTE-6145 and ATTRACTION-2 studies.46
ConclusionsAdenocarcinoma of the EGJ is an entity that is increasing in incidence, due, in part, to eating habits and behaviors. Localized EGJ tumors can be treated, achieving cure rates higher than tumors of the gastric body. Studies have analyzed surgery and CTx or RTx administered as neoadjuvant, adjuvant or perioperative treatment. However, no strategy has been defined as standard to date. For this reason, the treatment of EGJ adenocarcinoma continues to be a therapeutic challenge for multidisciplinary tumor committees.
Conflict of InterestsThe authors have no conflict of interests to declare regarding this manuscript.
Please cite this article as: Pericay C, Macías-Declara I, Arrazubi V, Vilà L, Marín M. Tratamientos oncológicos en el cáncer de unión esofagogástrica: pasado, presente y futuro. Cir Esp. 2019;97:459–464.