Accurate quantification of the inflammatory activity in Crohn’s Disease is essential to decide the adequate treatment for each patient. The aim of the present study is to assess the correlation between the pre-operative Magnetic Resonance Index of Activity (MaRIA) and the histologic degree of inflammation from surgically resected intestinal Crohn’s Disease lesions.
MethodsThis is a prospective study including a consecutive case series of patients with small bowel Crohn’s Disease, who underwent surgical resection. A Magnetic Resonance Enterography was performed during the three months prior to surgery, applying a pre-established protocol. Relative contrast enhancements, wall thickness, presence of edema or ulcerations were the parameters used to calculate the MaRIA Index. All patients underwent surgery and every specimen was analysed. The modified Chiorean classification was applied for the histological analysis and an ordinal regression analysis was used in order to correlate MaRIA and the grade of inflammation for each lesion.
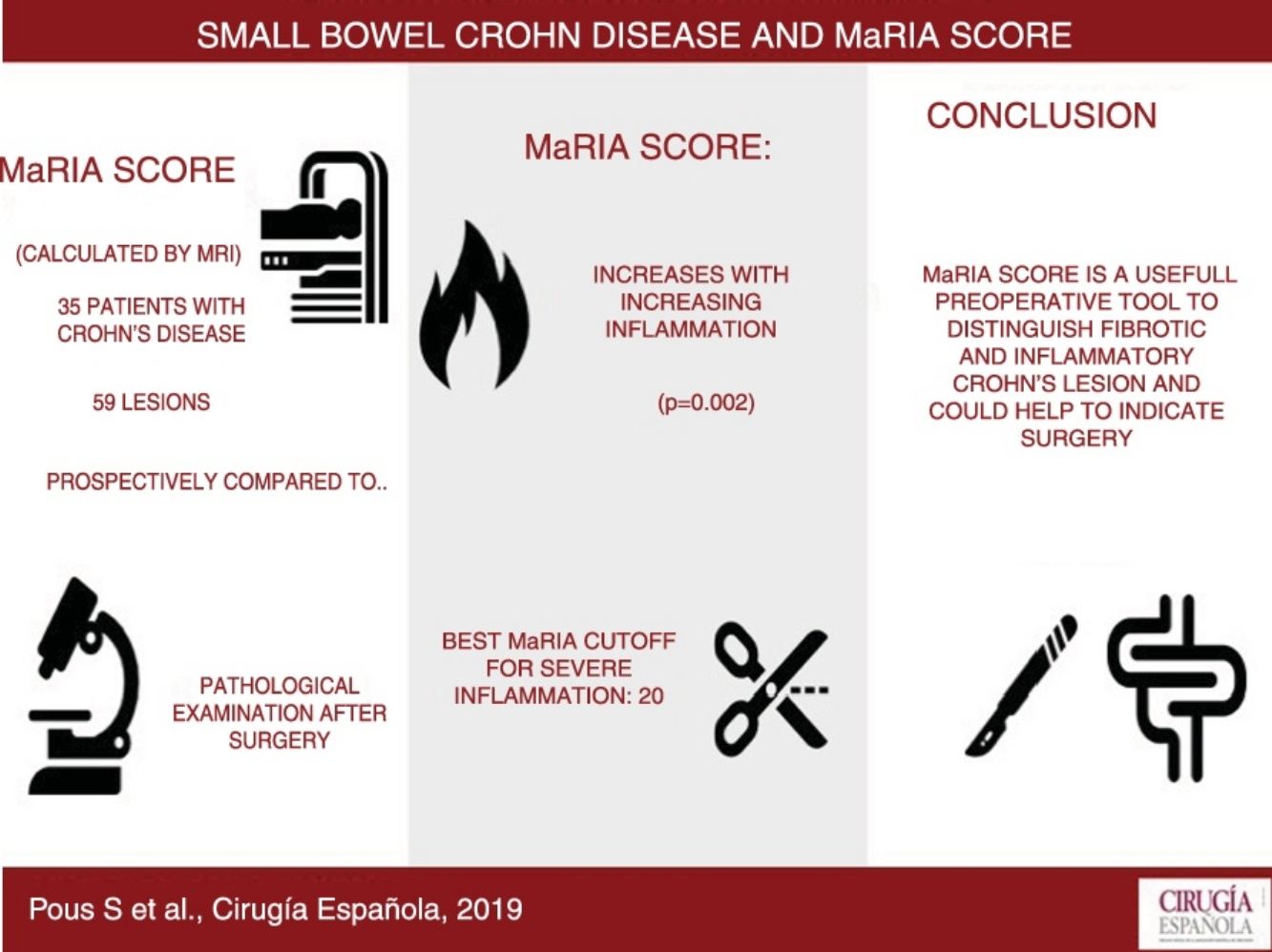
Results59 lesions from 35 different patients were analysed. The degree of inflammation of the lesions was statistically correlated to the MaRIA values (P = .002). The MaRIA index was significantly different (P < .001) between the different histological type of the Crohn’s Disease lesions (inflammatory/ fibrotic). The best cut-off for detecting severe inflammation using MaRIA was 20 (AUC: 0.741, 74.1% sensitivity and 78.1% specificity).
ConclusionMaRIA is a reliable tool to distinguish inflammatory from fibrotic lesions; therefore, it could be considered crucial to determine the most appropriate Crohn’s Disease treatment for each patient.
La correcta cuantificación de la actividad inflamatoria de las lesiones de la enfermedad de Crohn es necesaria para establecer cuál es el manejo más adecuado para cada paciente. El objetivo del presente estudio es valorar la relación entre el Índice de Actividad Inflamatorio Radiológico de la Resonancia Magnética (MaRIA) preoperatorio y el grado de inflamación histológico de las lesiones obtenidas en la cirugía.
MétodosEstudio prospectivo observacional consecutivo que incluye una serie de pacientes con Enfermedad de Crohn ileal. Se realizó una resonancia magnética mediante enterografía, con protocolo y secuencias pre-establecidas, en los tres meses previos a la cirugía y se calculó el índice MaRIA. Todos los pacientes fueron intervenidos quirúrgicamente y se remitieron muestras de cada lesión de pared completa a estudio anatomopatologico. En el análisis histológico se empleó la clasificación de Chiorean. Se realizó un ánálisis de regresión ordinal e intergrupos.
ResultadosSe incluyen 35 pacientes con 59 lesiones. A medida que aumenta el grado de inflamación se obtienen, de forma significativa (p = 0.002), valores mayores del MaRIA. El índice de MaRIA fue significativamente diferente (p < 0.001) en los diferentes tipo de lesiones por Enfermedad de Crohn (inflamatorias/fibrotica). El mejor punto de corte del índice MaRIA para determinar la presencia de inflamación severa en una lesión ha resultado ser 20 (ABC 0,741, sensibilidad 74.1%, especificidad 78.1%).
ConclusionesEn el estudio de la Enfermedad de Crohn ileal, la resonancia y el índice MaRIA son unas herramientas de gran utilidad para diferenciar lesiones inflamatorias vs. fibrosas y por lo tanto imprescindible para decidir el tratamiento más adecuado.
Even though the need for surgery in patients with Crohn’s Disease (CD) has decreased in the last few decades, a third of them will still need a surgical intervention during the first 5 years after diagnosis, and about 50% during the first 10 years.1
Many healthcare resources are utilized in the management of CD patients. The elevated costs develop accomplish accurate diagnostic tests. Biomarkers are required to assess the degree of inflammation and to estimate the extent of disease, since this will allow the most effective treatment in each case, decreasing side effects, post-surgical complications and costs.2,3 Predominantly inflammatory lesions may improve with medical therapy as opposed to lesions with large fibrotic component were a more aggressive approach such as endoscopic dilatation and/or surgery may be warranted. There is a need for a tool that allows us to accurately identify the inflammatory or fibrotic component of a lesion. There are numerous studies that try to correlate MRI findings to the activity and severity of CD. Variables that are evaluated in studies include: wall thickness, pattern of enhancement, presence of luminal stenosis, ulcerations, edema (target sign), hyper-vascularization (comb sign), lymphadenopathy, abscesses, fistulas, fibro-adipose proliferation, upstream dilatation, diffusion studies. Several radiological indexes based on these findings have been described in order to assess the activity and severity of CD.4–19 One of the most renowned, is the Magnetic Resonance Index of Activity (MaRIA), created by Rimola et al., and externally validated by themselves in 2011, showing an optimal correlation with the Crohn's Disease Endoscopic Index of Severity (CDEIS).10,11 However, MaRIA is still in need of external validation, since correlation with pathological findings is still uncertain.
The goal of this study is to assess the accuracy of MaRIA to reliably determine the degree of inflammation small bowel Crohn’s disease lesions, by using pathological examination of surgical specimens.
MethodsThis is a prospective case series including 35 consecutive patients with small bowel Crohn’s disease, who needed surgery due to medical treatment failure or complications. MRI was performed in all patients within the 3 months prior to surgery (1 month if the patient had received treatment with anti-tumor necrosis factor biological drugs). A sub-analysis of the present case series comparing MRI and surgical findings have been already published.20
The Hospital Ethics Committee approved the study protocol. All patients accepted to participate in the study by signing an informed consent.
CD was diagnosed according to the Lennard-Jones criteria, after excluding infectious, ischemic or vascular, neoplastic or actinic causes.21
Patients were classified by age at the time of diagnosis, disease location and disease behavior, according to the Montreal classification.22,23 The Harvey-Bradshaw index was used to describe the clinical activity of the CD.24
A preoperative colonoscopy was performed in all patients in order to exclude colonic involvement and, if possible, take a biopsy of the terminal ileum. All the decisions regarding the therapeutic strategy were taken at a multidisciplinary team meeting.
MRE examinations were performed according to a standardized clinical protocol on a 3T scanner (GE Medical Systems, Milwaukee, Wisconsin, USA). Patients fasted for at least 6 h before the procedure and then ingested 1500 ml of a 5% mannitol solution over 45 min immediately before the exploration in order to distend the small bowel. To reduce bowel peristalsis, 10 mg of intravenous (i.v.) hyoscine butylbromide (Buscopan; Boehringer Ingelheim, Ingelheim, Germany) was administered before initiating the study and another 10 mg was given before administering the contrast bolus.
Multi-phase dynamic contrast-enhanced scans (LAVA-XV sequence) were performed on coronal plane before and at 35, 70, 120, and 420 s after intravenous administration of gadobenate dimeglumine (Multihance; Bracco Diagnostics Inc., Milan, Italy). 0.2 ml/kg of gadobenate dimeglumine was administered at a rate of 2 ml/s using a power injector. Images were obtained in the prone position. The sequences and parameters of the MRE protocol are described in the supplementary material (Supl. 1).
Contrast enhancement was analyzed in each affected small bowel segment. Three regions of interest (ROIs) were placed on bowel wall thickness with the highest contrast enhancement for 70 s after contrast injection and other three ROIs were placed on sequence before contrast (at the same place) to calculate relative contrast enhancement. ROIs diameters were always the same size, including at least mucosal/submucosal and muscularis bowel layers (Supl 2). The value of the MaRIA index was calculated applying the formula published by Rimola et al.10 (MaRIA = 1.5*wall thickness + 0.02*RCE + 5* edema + 10*ulceration), which includes the following parameters: relative contrast enhancement (RCE), wall thickness and presence of edema and ulcerations.
The quantitative assessment of bowel thickening was measured on 3D T1-weighted (LAVA-XV) sequences after contrast injection. The following qualitative parameters were also obtained and recorded: presence of mural edema (hyperintesity on T2-weighted sequences of the bowel wall relative to the signal of the psoas muscle) and presence of deep ulcers (depressions in the mucosal surface).
All images were evaluated by two radiologists with experience in abdominal imaging. In case of discrepancies on the parameters used to calculate MaRIA, a final evaluation of the image was made by consensus. Both radiologists were blinded to the clinical and laboratory data.
All resected bowel segments were submitted for pathological examination indicating the number and location of the lesions and, specifying their distance from the ileocecal valve. During surgery, the lesions previously identified by MRE, were located. The ones that were not confirmed during surgery (n = 2) and/or not identified by MRE (n = 7), were excluded from the analysis. When performing the strictureplasty, complete wall samples were obtained for a histological study. Members of the Colorectal Surgery Unit, operated on all of the patients following standardized surgical criteria.
Two experienced pathologists, who specialize in digestive diseases and are part of the multidisciplinary team for management of inflammatory bowel diseases, were blinded to the MRE findings when assessing all the specimens. In case of discrepancies, data evaluation was made by consensus.
Fibrosis and inflammation of the lesions were classified according to Chiorean criteria.25 Lesions presenting a moderate or severe inflammation component and, an absent, mild or moderate fibrosis were defined as “Inflammatory lesions”. In case of presenting both: a severe fibrotic component and absent or mild inflammation, they were defined “Fibrotic lesions”. All the other cases were defined as “Mixed lesions” (Supl. 3).
In this study, we modified the Chiorean classification by subdividing the mixed group into mild mixed (absent or mild inflammation and mild or moderate fibrosis) and severe mixed (moderate or severe inflammation and severe fibrosis) (Supl. 3).
Statistical AnalysisData from patients and lesions were analysed. Continuous variables were expressed by means and standard deviations, while categorical variables were expressed by absolute frequencies and percentages. In the bivariate analysis, the analysis of the variance or ANOVA was used as a parametric test of comparison of means between several groups. A P < .05 was considered statistically significant.
An ordinal regression study was performed to correlate MaRIA values with qualitative ordinal or quasi-quantitative variables as, for example, the degree of histological inflammation. In order to assess the reliability of radiological variables (MaRIA) as possible diagnostic tests in determining the inflammation degree, the receiver´s operating characteristic (ROC) curves were analysed and the area under the curve (AUC) for each of the these variables was calculated.26 The Youden index (sensitivity + specificity −1)27 was used to select the most appropriate value as a cut-off point.
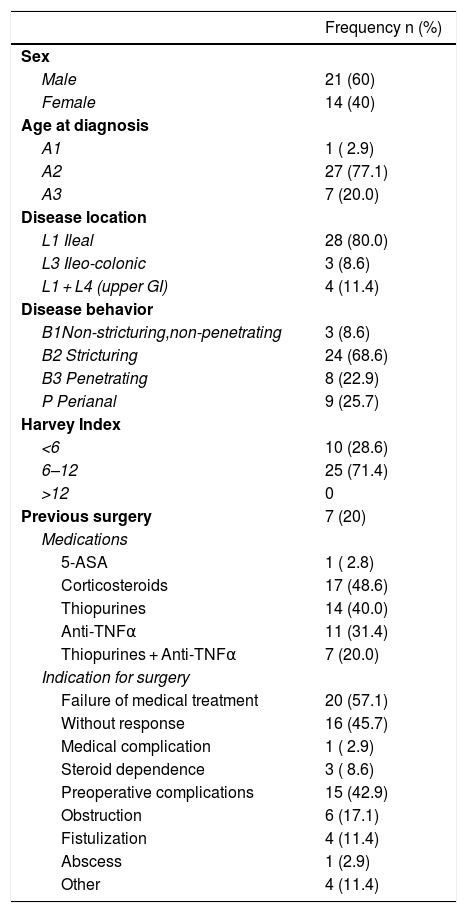
ResultsA total of 59 small bowel CD lesions in 35 patients (mean age 39.2 years; range 17–64 years; 14 female and 21 male) were included in the present analysis. Demographic and clinical baseline data of the study population are summarized in Table 1.
Demographic and Clinical Data of Patients (N = 35). Age at Diagnosis, Disease Location and Behavior Were Defined According to the Montreal Classification.22,23
| Frequency n (%) | |
|---|---|
| Sex | |
| Male | 21 (60) |
| Female | 14 (40) |
| Age at diagnosis | |
| A1 | 1 ( 2.9) |
| A2 | 27 (77.1) |
| A3 | 7 (20.0) |
| Disease location | |
| L1 Ileal | 28 (80.0) |
| L3 Ileo-colonic | 3 (8.6) |
| L1 + L4 (upper GI) | 4 (11.4) |
| Disease behavior | |
| B1Non-stricturing,non-penetrating | 3 (8.6) |
| B2 Stricturing | 24 (68.6) |
| B3 Penetrating | 8 (22.9) |
| P Perianal | 9 (25.7) |
| Harvey Index | |
| <6 | 10 (28.6) |
| 6–12 | 25 (71.4) |
| >12 | 0 |
| Previous surgery | 7 (20) |
| Medications | |
| 5-ASA | 1 ( 2.8) |
| Corticosteroids | 17 (48.6) |
| Thiopurines | 14 (40.0) |
| Anti-TNFα | 11 (31.4) |
| Thiopurines + Anti-TNFα | 7 (20.0) |
| Indication for surgery | |
| Failure of medical treatment | 20 (57.1) |
| Without response | 16 (45.7) |
| Medical complication | 1 ( 2.9) |
| Steroid dependence | 3 ( 8.6) |
| Preoperative complications | 15 (42.9) |
| Obstruction | 6 (17.1) |
| Fistulization | 4 (11.4) |
| Abscess | 1 (2.9) |
| Other | 4 (11.4) |
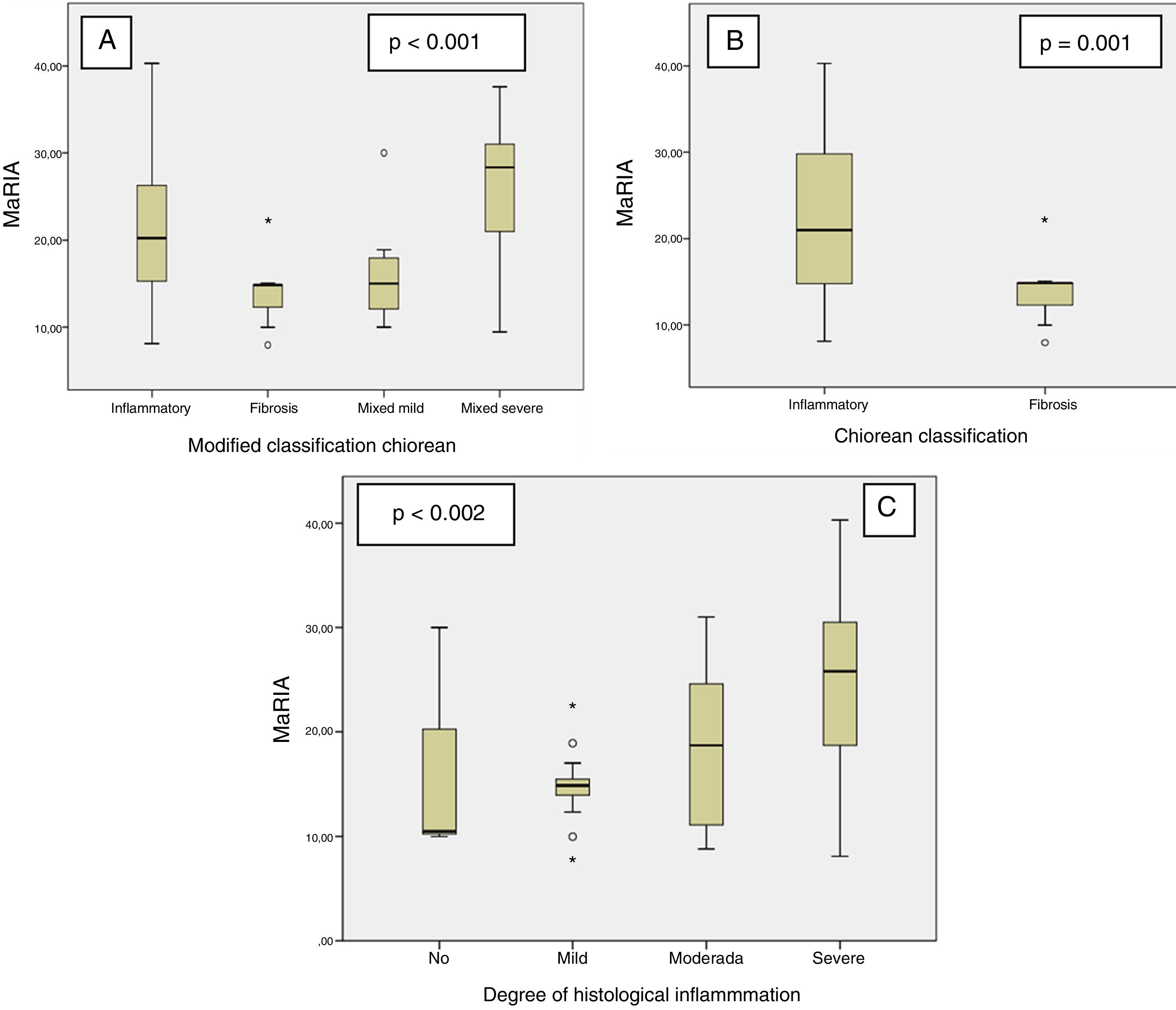
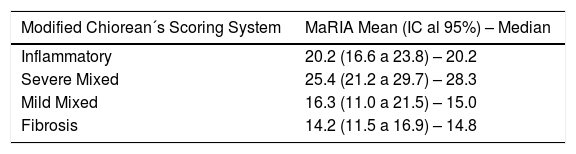
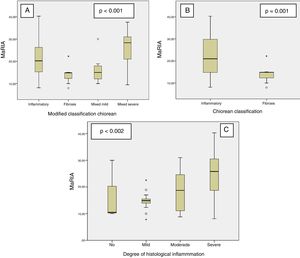
MaRIA index was significantly different (P < .001) between the different histological type of the CD lesions (inflammatory, fibrotic, mixed with mild inflammation, mixed with severe inflammation) (Fig. 1A and Table 2). Inflammatory lesions and mixed lesions with severe or moderate inflammation showed greater MaRIA values than fibrotic or mixed lesions with absent or mild inflammation (median value 21.1 vs. 14.8, P = .002). Fibrotic lesions and mixed lesions with mild inflammation had similar MaRIA values (P = .6).
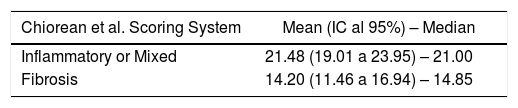
All lesions with some inflammatory component (inflammatory and mixed lesions) showed higher MaRIA value than lesions without an inflammatory component (fibrotic lesions) with statistically significant value (P = .012) (Table 3 and Fig. 1B). Furthermore, as the degree of pathological inflammation increased, significantly higher (P = .002) MaRIA values were obtained (Fig. 1C).
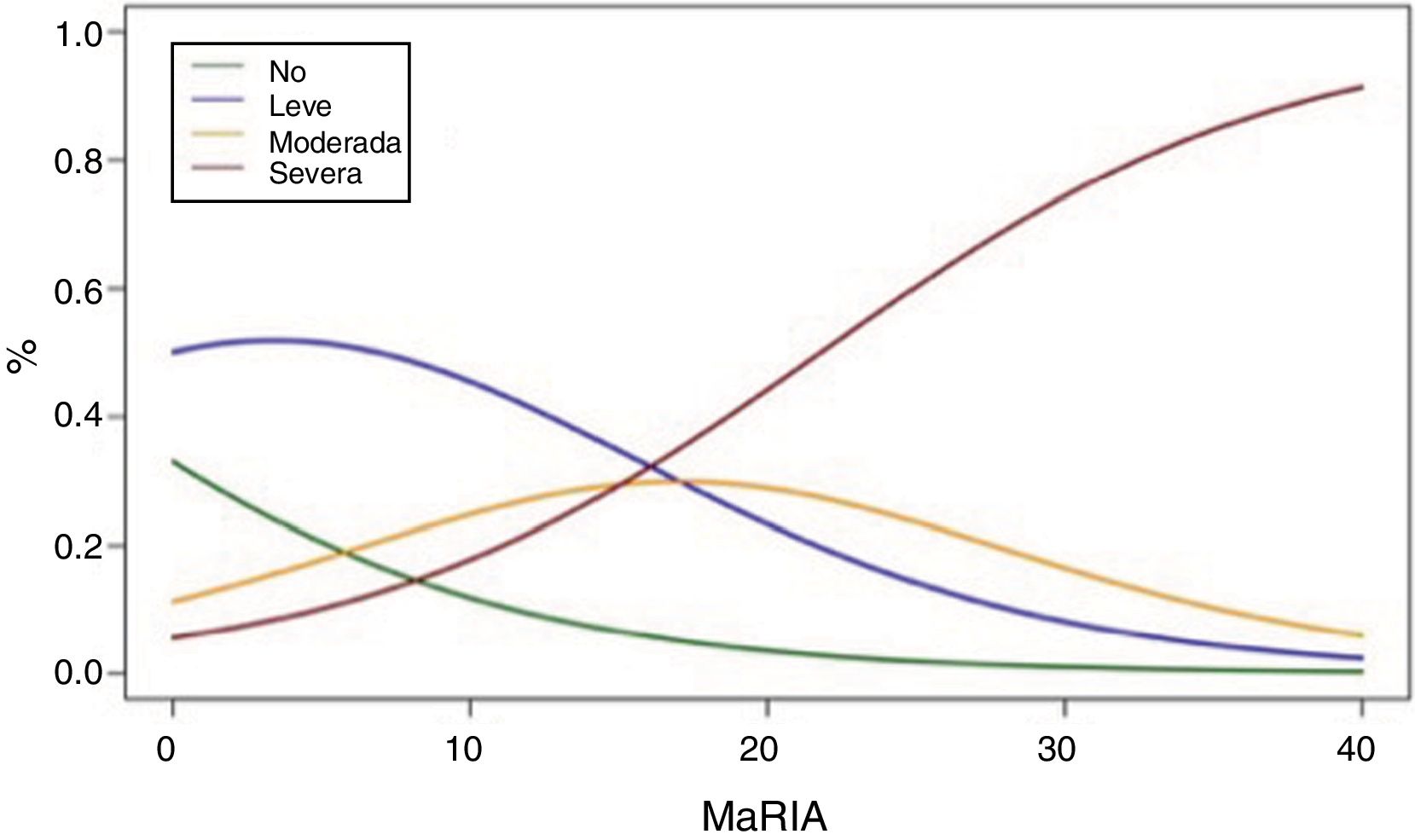
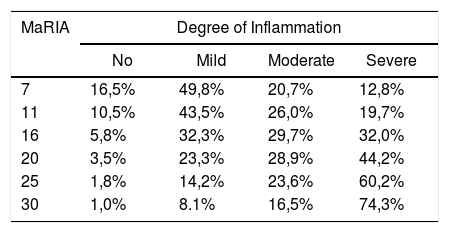
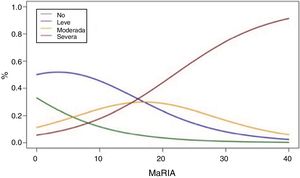
Fig. 2 shows the probability of obtaining different degrees of pathological inflammation with a given MaRIA value. Higher MaRIA values are more likely to show a greater and more severe inflammation of the lesion (Table 4). The area under the ROC curve (AUC) for the MaRIA, as a diagnosis biomarker of severe inflammation was 0.741 (95% confidence interval (CI) 0.607 to 0.875), with a 74.1% sensitivity and a 78.1% specificity considering 20 as a cut-off point. For the diagnosis of moderate or severe inflammation, the AUC was 0.756 (95% CI 0.629 to 0.883), with the best cut-off point at 16 (sensitivity 73.0%, specificity of 77.8%).
Probabilities of Each Degree of Inflammation for Different Examples of MaRIA’s Values.
| MaRIA | Degree of Inflammation | |||
|---|---|---|---|---|
| No | Mild | Moderate | Severe | |
| 7 | 16,5% | 49,8% | 20,7% | 12,8% |
| 11 | 10,5% | 43,5% | 26,0% | 19,7% |
| 16 | 5,8% | 32,3% | 29,7% | 32,0% |
| 20 | 3,5% | 23,3% | 28,9% | 44,2% |
| 25 | 1,8% | 14,2% | 23,6% | 60,2% |
| 30 | 1,0% | 8.1% | 16,5% | 74,3% |
Correlation between MaRIA values greater than 16 and the diagnosis of moderate or severe inflammation provided a diagnostic accuracy of 74.6% and a high association [OR = 9.9 (95% CI) 2.6–35.3)]. The same reliability was obtained with MaRIA greater than 20 for histologically proved severe inflammation with a significant odds ratio [OR = 8.6 (95% CI 2.6–27.8)].
DiscussionAmong the MRE indexes described for CD assessment, MaRIA is probably the most frequently mentioned in literature; however, it is not commonly used in clinical practice. The present study establishes the MaRIA score as a reliable tool to determine the degree of inflammatory activity of CD small bowel lesions.
Other scores such as Clermont score, London score, and Crohn disease MRI Index have been previously described. A previous study28 comparing them along with MaRIA concluded that all scoring systems were comparable in terms of interobserver agreement, correlation to the endoscopic and histopathologic reference standard, and diagnostic accuracy; however, the London score, MaRIA, and Clermont score have the additional benefit of having validated cutoff values for both active and ulcerating endoscopic disease.
MRI has recently acquired greater relevance in the diagnosis, classification of the phenotype and especially in the follow-up of patients with CD. MRI enterography has proven high reliability without the inconvenience of the ionising radiation of the computed tomography and with the advantage over the ultrasound to be less dependent on the radiologist experience and the location of the bowel loops.2
In recent years, there is a tendency to reverse the therapeutic pyramid in CD patients by early introduction of immunosuppressants and anti-tumor necrosis factor biological (anti-TNF) drugs. This implies an increased incidence of side effects and a significant increase in treatment’s costs (the approximate cost of treatment with anti-TNF is 10,000 € per year/ patient).29,30 Therefore, it is essential to assess the inflammatory grade of each CD lesion in order to decide whether medical treatment is indicated or surgical resection is necessary.22,23
It is well known that CD is a dynamic disorder whose phenotype may evolve over time. Chronic and continuous inflammation may consequently lead to the fibrosis of the bowel wall. In these cases, escalating therapy could lead to worsening of the patient's quality of life, with potential complications, medication side effects and higher costs.
However, as MRI Enterography has become a decisive tool for therapeutic decision-making,31 with time and experience certain doubts about its use in the ileal locations of CD arose. In a study published by Rimola et al.,10 50 patients (11 with ileal and 24 with ileocolic location) were included, and the findings of the MRE were correlated with the endoscopic activity evaluated by CDEIS (Crohn´s Disease Endoscopic Index of Severity). The lesions were classified as absent, mild (inflammation without ulcers) and severe (presence of ulceration). The MaRIA index proved to be highly reliable in determining the disease´s activity (sensitivity 0.81 and specificity 0.89) and predicting the presence of endoscopic ulcers (sensitivity 0.95 and specificity 0.91).10 Later, the authors validated the index and concluded that a MaRIA value equal to or greater than 7 indicated an active disease; and greater than or equal to 11 indicated severe disease.11 However, authors specified that one of the limitations of the study is the lack of an independent cohort of results for validation, since colonoscopy exploration required for CDEIS (endoscopic index) is often very difficult or even impossible to perform in patients with stenotic (inflammatory or fibrotic) lesions.
Our prospective study, correlated for the first time the MaRIA index to the pathological findings of the resected bowel segments. The demographic characteristics of the patients analysed in our study are similar to those in larger series of patients who underwent surgery.32
The pathological classification published by Chiorean et al. in 200725 was used as a score for inflammatory and fibro-stenotic features. This classification is the easiest and one of the most practical tools to classify lesions as predominantly inflammatory or predominantly fibro-stenotic, or mixed. Nevertheless, the mixed group quite frequently includes lesions with very different characteristics. For this reason, we subdivided the mixed group into a mild and a severe, in order to analyse differences in the MaRIA values. The severe mixed group (segments with moderate or severe inflammation) showed similar results to segments with inflammatory predominance, while the lesions of the mild mixed group (segments with mild or absent inflammation) showed values that were similar to the ones found in fibrotic lesions. The severe mixed subgroup shows the highest values of the MaRIA series, probably due to the adding effect of the two pathological characteristics.
Alike previous publications, our study reveals that, as the value of the MaRIA index increases, the severity of lesion’s inflammation is more likely to increase. In an ordinal regression analysis, using MaRIA values, it is possible to calculate the different probabilities of having an inflammatory lesion and calculating its degree of inflammation, which renders this study of significant practical interest. In contrast to other studies, in our series, the mean value of the MaRIA obtained in cases of pure fibrosis was 14.2, while values equal to or greater than 16 indicated lesions with moderate to severe inflammation and values equal to or greater than 20 indicated severe inflammation with an accuracy of 74.6%. Other authors reported that values equal to or higher than 7 indicate active disease and greater than or equal to 11 indicate severe disease.10,11 In their study, the inflammatory activity was measured by endoscopy (CDEIS) and there were a high percentage of colic or ileocolic lesions. In contrast, in our study inflammatory activity was measured by the histology of the complete wall of the affected bowel segment and we evaluated jejuno-ileal lesions that would not allow neither endoscopic specimen sampling nor CDEIS, in most of the cases. These facts could probably explain the difference in the results of our study, probably indicating the necessity of modifying the MaRIA cut-off points when studying small bowel lesions.
As recently suggested by Rimola et al.33 severe fibrosis could also be evaluated pre-operatively by MRE on the basis of the enhancement gain between early and late phases. It has also been recently proposed that MaRIA reliability could be improved by modification with diffusion weighted MRI secuences.34
Finally, MaRIA accuracy in the evaluation of the response to medical treatment should be further studied.
Conflict of InterestThere is no conflict to declare
FundingNo specific funding has been received for this project.
Author ContributionsStudy Design: Salvador Pous-Serrano, Matteo Frasson, Jose Pamies-Guilabert, Eduardo Garcia-Granero, Pilar Nos Mateu.
Data Collection: Salvador Pous-Serrano, Jose Pamies-Guilabert, Icíar Puchades-Roman, Belén Beltrán.
Data Analysis and Interpretation: Salvador Pous-Serrano, Jose Pamies-Guilabert, Matteo Frasson.
Manuscript Writing: Salvador Pous-Serrano, Matteo Frasson, Jose Pamies-Guilabert, Polina Rudenko.
Please cite this article as: Pous-Serrano S, Frasson M, Pàmies-Guilabert J, Rudenko P, Puchades-Roman I, Beltrán B, et al. La resonancia magnética y el índice MaRIA en la valoración preoperatoria de la enfermedad de Crohn ileal. Cir Esp. 2019;97:582–589.