Distance from anal verge of rectal tumours and their anatomical relationships contribute to determine the multidisciplinary therapeutic strategy based on the combination of radio-chemotherapy and radical surgery. Our aims are to investigate which is the most accurate method for the preoperative measuring of the distance from the anal verge in rectal tumours and if the pelvic MRI can substitute the classical instrumental methods.
MethodsProspective study of diagnostic precision between flexible colonoscopy (FC), preoperative rigid rectosigmoidoscopy (pRR) and pelvic MRI in patients scheduled to radical surgery. Rigid intraoperative rectoscopy (iRR) was considered the reference test. The correlations between the different techniques and their determination coefficient as well as the intraclass correlation coefficient and the degree of agreement between the different tests were analyzed.
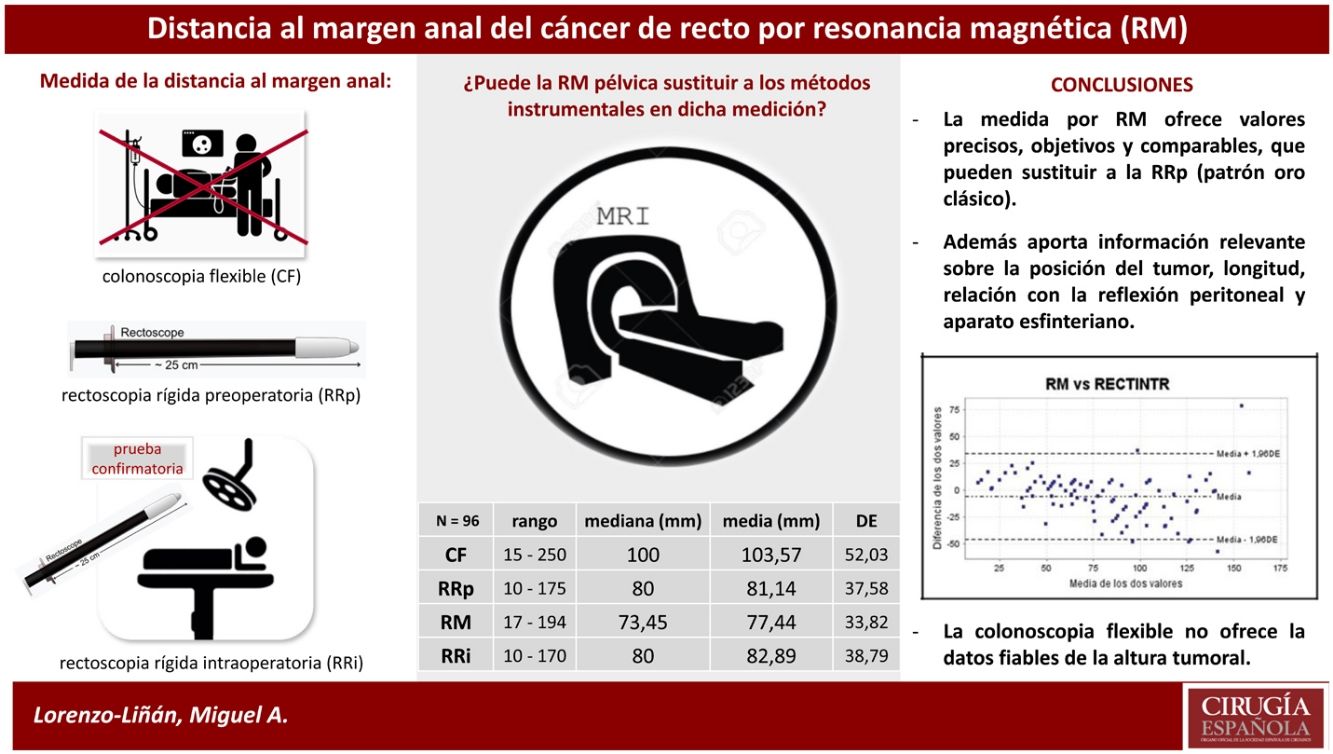
Results96 patients (65% males), mean age (SD): 68 (14.1) years were included. 72% received neoadjuvant treatment. The mean distance to the anal margin measured by FC = 103.5 mm, was significantly greater than others, which had similar values: pRR = 81.1; MRI = 77.4; iRR = 82.9 mm (P < .001). A significant intraclass correlation was observed and there was high agreement between all pre- and intraoperative measurements except for the performed by FC, which overestimated the results. MRI provided more individualized and accurate information.
ConclusionsThere is variability between the measurement methods, being colonoscopy the least reliable. MRI offers objective, comparable, accurate and individualized values that can replace those obtained by pRR for tumours of any location in the rectum.
La altura exacta del tumor en el recto y sus relaciones anatómicas contribuyen a determinar la estrategia terapéutica multidisciplinar basada en la combinación de radio-quimioterapia y cirugía radical. Nuestro objetivo es valorar cuál es el método diagnóstico más preciso en la medición preoperatoria de la distancia al margen anal, y si la resonancia magnética pélvica (RM) puede sustituir a los métodos instrumentales clásicos.
MétodosEstudio prospectivo de precisión diagnóstica entre colonoscopia (CF), rectoscopia rígida (RRp) y RM en pacientes con indicación de cirugía radical. La RRp intraoperatoria fue considerada la prueba de referencia. Se analizaron las correlaciones entre las distintas técnicas y su coeficiente de determinación, así como el coeficiente de correlación intraclase y el grado de acuerdo entre los distintos test.
ResultadosSe incluyeron 96 pacientes con edad media (DE) de 68 (14,1) años y predominio de varones (65%). Un 72% recibió tratamiento neoadyuvante. La distancia media al margen anal, medida mediante CF = 103,5 mm, fue significativamente mayor al resto, que obtuvieron valores similares: RRp = 81,1, RM = 77,4, RRp intraoperatoria = 82,9 mm (p < 0,001). Se objetivó una significativa correlación intraclase y hubo un elevado acuerdo entre todas las mediciones pre e intraoperatorias a excepción de la realizada mediante CF, que sobreestimó el resultado. La RM aportó información más individualizada y precisa.
ConclusionesExiste variabilidad entre los métodos de medición, siendo la colonoscopia el menos fiable. La RM ofrece valores objetivos, comparables, precisos e individualizados que pueden sustituir a los obtenidos por RR en tumores de cualquier localización del recto.
The use of instrumental methods to determine preoperatively the distance to the anal verge of rectal tumours does not always achieve the desired precision or match the surgeon’s impression during surgery, and can negatively affect the planning of multidisciplinary treatment based on the combination of radiotherapy, chemotherapy, and radical surgery. The inclusion of pelvic magnetic resonance imaging (MRI) in diagnostic protocols for rectal cancer provides essential information for topographical knowledge of the pelvis and locoregional staging1,2. The aims of this study are to determine the most accurate diagnostic method to measure the distance to the anal margin of rectal tumours prior to surgery, and whether MRI can replace classic instrumental methods, both flexible and rigid.
MethodsA prospective study of diagnostic accuracy to determine the reliability and concordance between different methods for preoperative measurement of the distance of the tumour to the anal verge, including patients seen in the Colorectal Surgery Unit of the Consorcio Hospital General Universitario de Valencia with a diagnosis of rectal cancer and candidates for elective surgery with curative intent over 26 consecutive months. Patients undergoing emergency surgery, those selected for local resection (because complete release of the rectum was not achieved) and those who could not undergo MRI due to medical intolerance or contraindication were excluded.
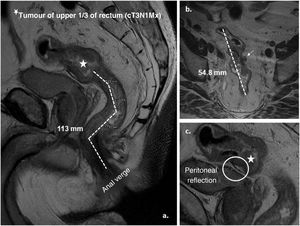
After giving their informed consent, validated by the centre’s clinical research committee, all patients underwent preoperative video colonoscopy with a flexible instrument (FC) of 13.2 mm Ø and 2 working channels, Olympus® model (Olympus Europa SE&CO Hamburg. Germany), rigid rectosigmoidoscopy (pRR) (Welch-Allyn®, Skaneateles Falls, NY, USA) and staging pelvic magnetic resonance imaging (MRI) by a single radiologist specially trained in rectal cancer staging. The MRI model used was GE Signa LX 1.5 T version 9.1, (Healthcare™, Milwaukee, Wisconsin, USA). The measurement technique used3 sets the proximal limit at the lower end of the tumour, and the distal or anal verge is marked by the lower edge of the external anal sphincter. Straight lines are drawn in a sagittal plane following the axes of the anal canal and rectum, and the final result is the sum of the partial measurements (Fig. 1). We did not routinely use intrarectal gel to avoid overdistension of the rectum, nor intravenous contrast, but phased-array coils were used to increase accuracy. Subsequently, during surgery, after mobilisation and release of the rectum from the pelvic fixations, rigid rectosigmoidoscopy (iRR) was repeated to redetermine the tumour height, considering the measurement obtained as the reference for the study. Rigid instrument measurements (pRR and iRR) were performed by accredited colorectal surgeons (European Board). For any one of the tests, tumours of the lower third of the rectum (lR) were considered those whose distal edge was 5 cm or less from the anal verge, of the middle rectum (mR) between 5.1 and 10 cm, and tumours of the upper rectum (uR) above 10.1 cm (up to a maximum height of 15 cm), although a rectal tumour was considered as defined by rigid proctoscopy (classic gold standard).
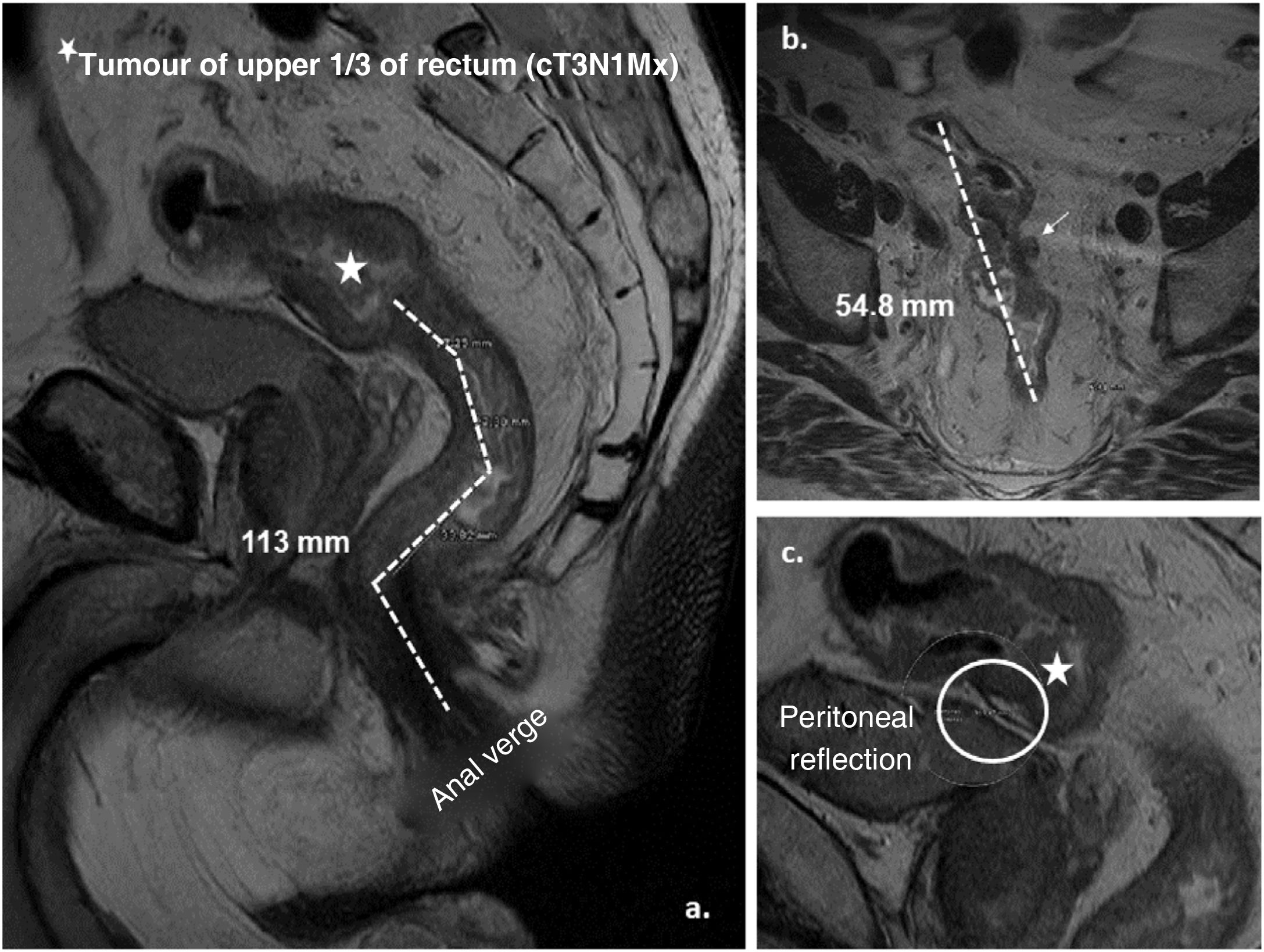
Graphical representation of the measurement by MRI of a tumour of the upper third of the rectum (cT3a N1 Mx). A. Sagittal plane: distance from the tumour to the anal verge (lower limit of the external anal sphincter) (113 mm) by adding the dashed lines. B. Axial plane: craniocaudal length of the tumour (54.8 mm) and presence of adenopathy in mesorectal fat (cN1). C. Sagittal plane: location of the anterior peritoneal reflection and relationship with the tumour.
A single investigator collected and analysed the study variables using SPSS Statistics 18.0 software (SPSS Inc.™, Chicago IL, USA). Descriptive analysis of the quantitative variable "tumour distance to anal verge" was performed for the 4 diagnostic tests to be compared (FC, pRR, iRR and MRI), verifying the normal distribution of the sample. The linear relationship and variability between measurements was established using Pearson’s correlation coefficient (r) and the coefficient of determination (r2) — or the ability of one test to predict the result of another. The intraclass correlation coefficient (ICC) measures the agreement between tests, taking the value 1 if the variability is due to differences between subjects (and not between measurement methods) and 0 when it is due to chance. Similarly, we use the Kappa index (k) to determine the degree of agreement between tests, which is interpreted using the Landis and Koch scale4. We used the Bland-Altman method for the graphical representation of the consistency and agreement observed between tests5.
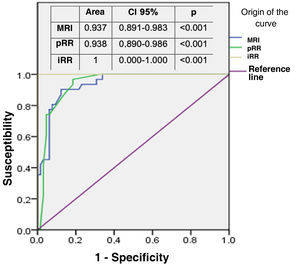
Firstly, the preoperative test measurements (FC, pRR and MRI) were compared with each other, and then agreement with the intraoperative reference measurement (iRR) was assessed. The accuracy of the aspirant test (MRI) with respect to the gold standard (pRR) was confirmed by means of a ROC curve, establishing a cut-off point at 10 cm from the anal verge (theoretical location of the peritoneal reflection) and taking the intraoperative measurement (iRR) as accurate. Finally, the complementary topographic information provided by MRI was analysed in relation to the other tests.
ResultsFrom a total of 118 patients, 18 were excluded because transanal surgery was indicated and 4 could not undergo MRI because they felt claustrophobic or were pacemaker carriers. The final sample comprised 96 patients with a mean (SD) age of 67.7 (14) years, and a predominance of males n = 62 (65%). MRI staging was performed using the TNM-7.6th ed. system6, according to which 22 (22.8%) patients were early stage I and II, 66 (68.6%) were stage III, and 8 (8.3%) were stage IV (Table 1). After histological confirmation and evaluation by the multidisciplinary committee, neoadjuvant treatment was administered to 69 (72%) of the patients, the most commonly used regimen was long-course radiochemotherapy followed by radical surgery at 6 weeks. The most commonly performed intervention was low anterior resection with bypass ileostomy in 35 (36.5%) patients, performing a total mesorectal excision (TME) in a total of 65 (68%) (Table 1).
Preoperative staging of the sample by MRI and type of surgery and mesorectal excision performed.
| Stage | Substage | TNM | N | % per stage |
|---|---|---|---|---|
| I | I | T1 N0 M0 | 0 | |
| T2 N0 M0 | 12 | 12.5 | ||
| IIA | T3 N0 M0 | 8 | ||
| II | IIB | T4a N0 M0 | 2 | |
| IIC | T4b N0 M0 | 0 | 10.3 | |
| IIIA | T1-2 N1-1c M0 | 5 | ||
| T1 N2a M0 | 0 | 5.2 | ||
| IIIB | T3-4a N1-1c M0 | 18 | ||
| III | T2-3 N2a M0 | 8 | ||
| T1-2 N2b M0 | 0 | 27 | ||
| IIIC | T4a N2a M0 | 6 | ||
| T3-4a N2b M0 | 9 | |||
| T4b N1-2 M0 | 20 | 36.4 | ||
| IV | IVA | any T and N M1a | 5 | |
| IVB | any T and N M1b | 3 | 8.3 | |
| Total N | 96 | 100 | ||
| Type of intervention | N | (%) | ||
| Low AR + ileostomy | 35 | 36.5 | ||
| High AR (without ileostomy) | 21 | 21.9 | ||
| Hartmann’s procedure | 16 | 16.7 | ||
| APR | 14 | 14.6 | ||
| ULAR + ileostomy | 8 | 8.3 | ||
| Proctocolectomy + ileostomy | 2 | 2.1 | ||
| Mesorectal excision | ||||
| TME | 65 | 68 | ||
| STME | 31 | 32 | ||
APR: abdominoperineal resection; AR: anterior resection; M: distant metastasis; N: lymph node involvement; STME: subtotal mesorectal excision; TME: total mesorectal excision; T: tumour penetration into the wall; ULAR: ultra-low anterior resection.
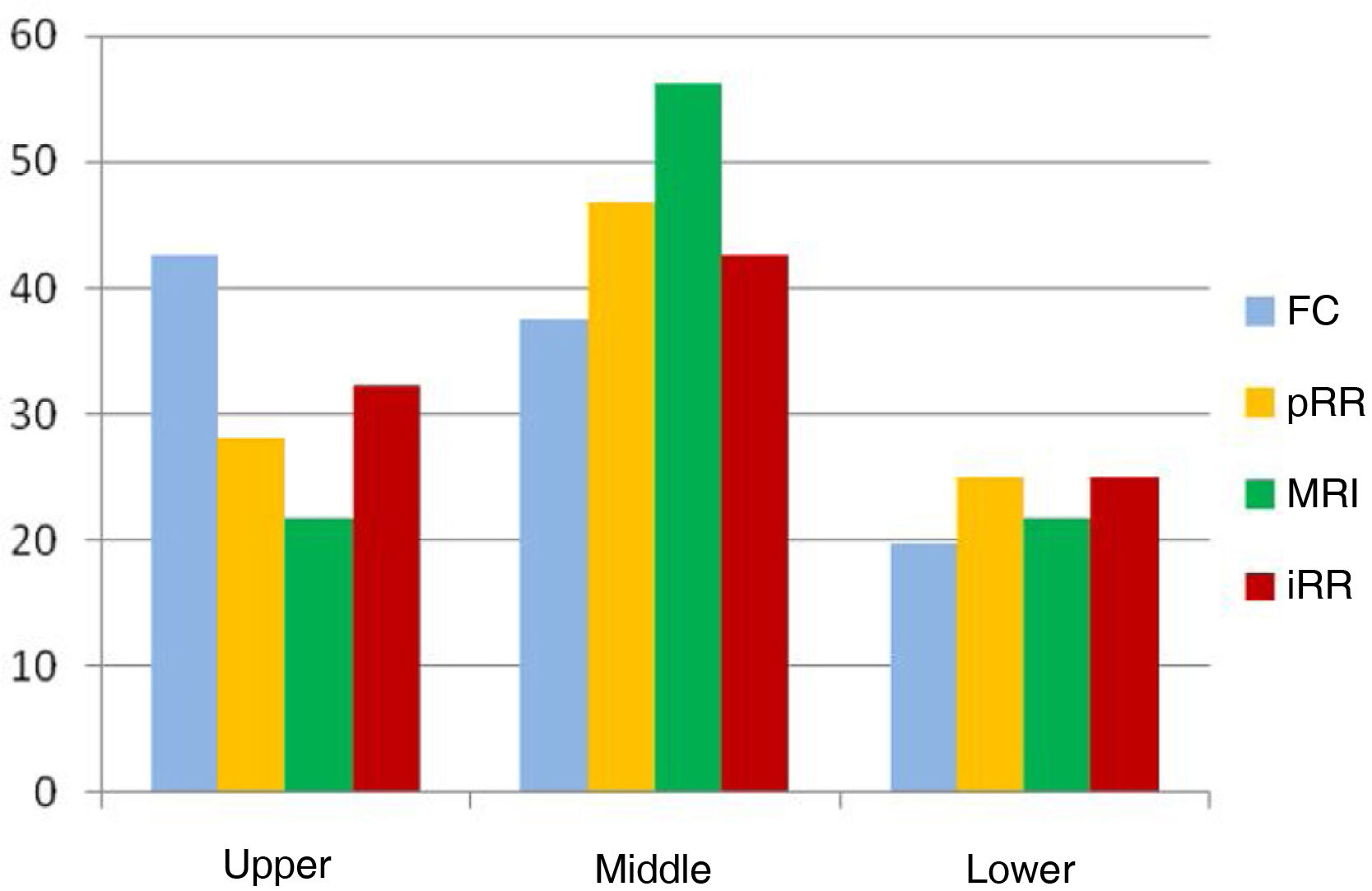
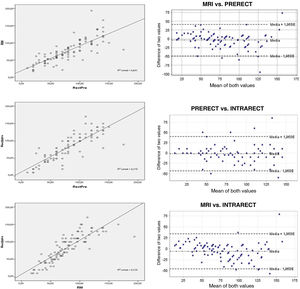
The mean distance from the anal verge to the lower edge of the tumour was significantly higher using FC than the other measurements (p < .001), placing 13.6% of tumours entirely outside the rectum at a height of more than 15 cm, which is why we excluded it from the subsequent detailed analysis (Table 2). The rest of the measurements obtained, both by rigid rectosigmoidoscopy and MRI, were comparable, the majority of cases were located between 5.1 cm and 10 cm from the anocutaneous verge (Fig. 2). This linear relationship, determined by Pearson's coefficient (r), remained in the "high positive" range when compared with the intraoperative measurement (iRR), even after neoadjuvant treatment or after both total and subtotal excision of the mesorectum.
Measurement of the distance to the anal margin by different methods (N = 96).
| Range | Mean (mm) | SD | |
|---|---|---|---|
| FC | 15−250 | 103.6 | 52 |
| pRR | 10−175 | 81.1 | 37.6 |
| MRI | 17−194 | 77.4 | 33.8 |
| iRR | 10−170 | 82.9 | 38.8 |
FC: colonoscopy; iRR: intraoperative rectoscopy; MRI: magnetic resonance imaging; pRR: preop rectoscopy; SD: standard deviation.
pRR vs. MRI: p = .115; pRR vs. iRR: p = .420; FC vs. pRR, MRI, and iRR: p < .001.
MRI vs. pRR. The linear relationship between the two was "high positive" (r = .801; p < .001), as was the intraclass correlation coefficient ICC = .890; 95% CI (.830–.924), expressing excellent reproducibility between the two7. The concordance index (kappa) of .597 (p < .001) was acceptable, as was the coefficient of determination, R2 (Fig. 3).
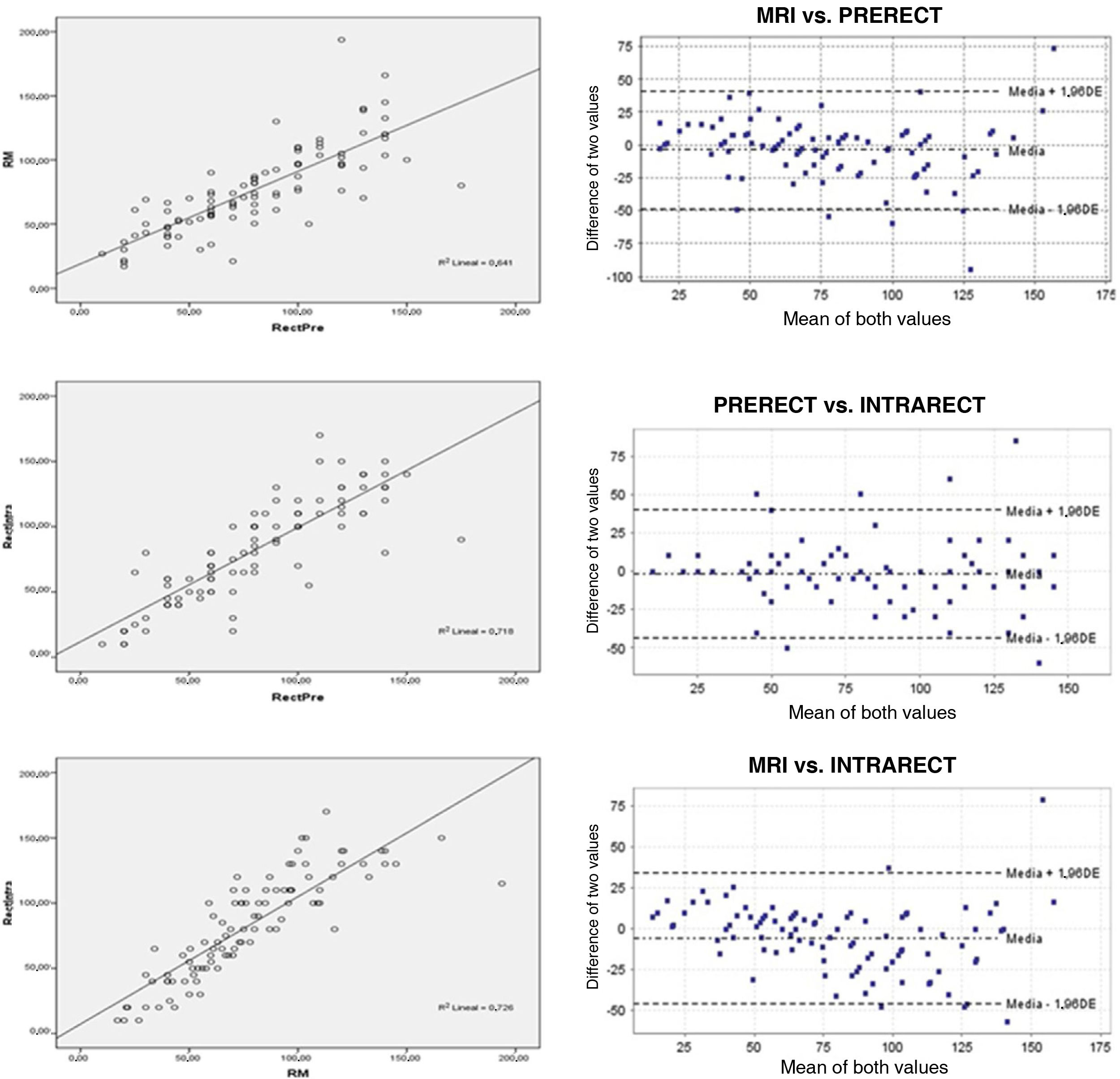
Lineal relationship (r) and Bland-Altman plot between: magnetic resonance (MRI) and preoperative rectoscopy (PRERECT); preoperative rigid rectoscopy (PRERECT) and intraoperative rectoscopy (INTRARECT) and MRI compared to rigid intraoperative rectoscopy (INTRARECT). The linear correlations are adequate in all of them, the highest being in the last comparative group. The Bland-Altman plot shows high concordance and generally symmetrical and clustered data with few scattered values.
pRR vs. iRR. The linear relationship between the two was "high positive" (r = .848, p < .001), with good distribution in the scatter plot and an acceptable degree of agreement (k = .599, p < .001). The ICC value = .847, 95% CI (.780–.895) also expressed excellent reproducibility7.
MR vs. iRR. The correlation coefficient (r = .852, p < .001) reached the highest value of the comparison, with an adequate coefficient of determination (R2) (Fig. 3) and an again "acceptable" kappa index (.542, p < .001) in the contingency tables. The ICC = .916, 95% CI (.873–.944) was also the highest of those compared, and finally the Bland-Altman plot visually demonstrated the high degree of concordance between the two (Fig. 3).
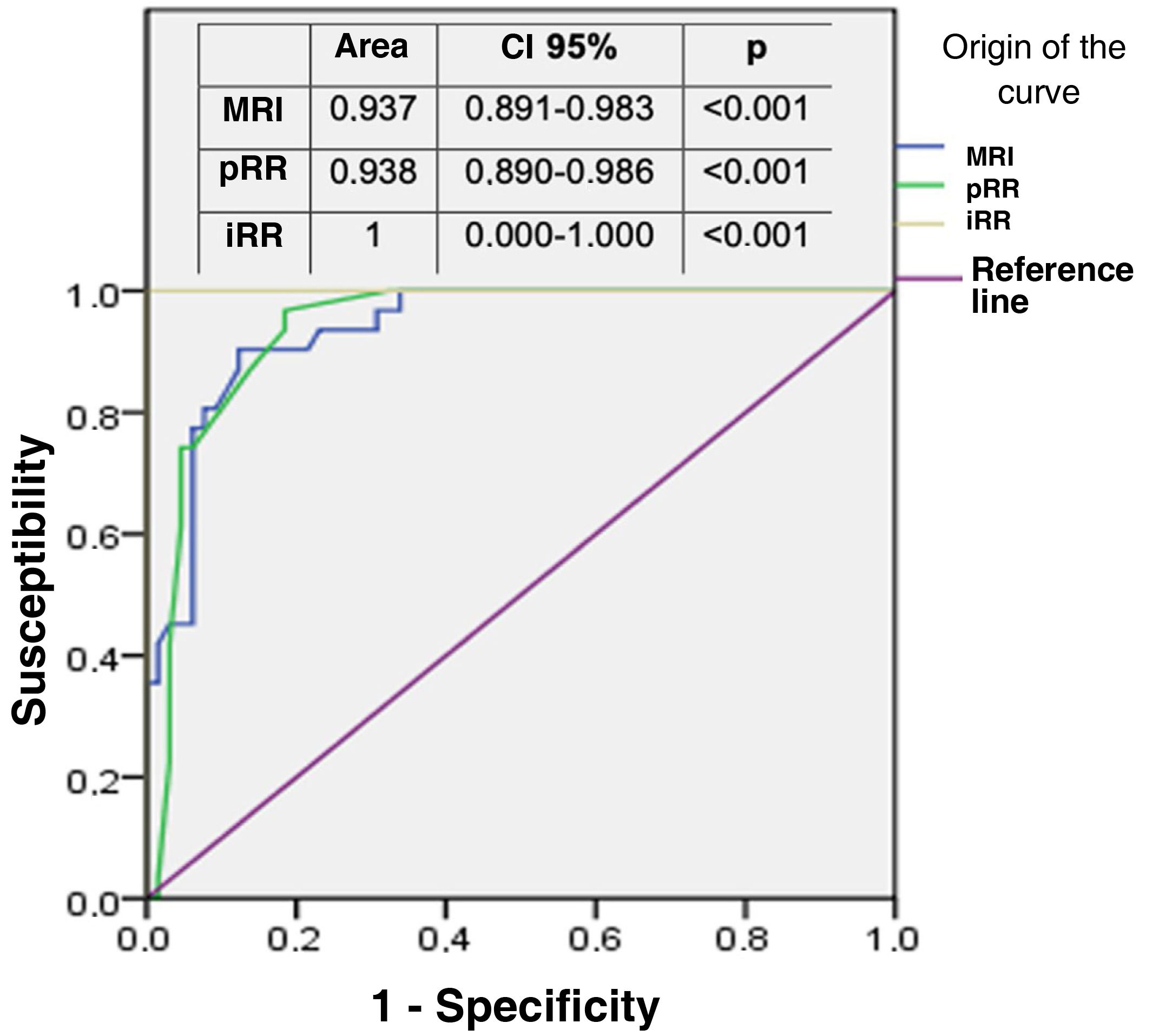
The area under the ROC curve of both MRI and pRR was then compared, taking as the "exact" reference that determined by iRR at 10 cm from the anal verge. Both areas were close to the reference value (value 1) and similar to each other (Fig. 4), thus corroborating the reproducibility between the two. MRI determined in all cases the relationship of the tumour with the sphincteric apparatus, affected by infiltration in 15 patients (15.6%), and with the anterior peritoneal reflection (APR), considering 75 of the 96 tumours in the sample (78.1%) to be extraperitoneal because they were at or below its height.
DiscussionThe benefit of neoadjuvant chemoradiotherapy in advanced tumours of the middle and lower third of the rectum requires accurate measurement of the distance to the anal verge and precise pre-operative locoregional staging to maximise the chances of cure8.
Measurements of the rectum are not exact, and may vary between patients depending on size, gender, and other factors9–11. Therefore, using a globally accepted definition of its anatomical landmarks is key to planning the therapeutic strategy and comparing studies.
Pelvic MRI is currently the method of choice for local and regional staging of rectal cancer, with almost unanimous measurement and interpretation criteria and a high histopathological correlation12,13. It is also considered the most commonly available method (98%) and chosen by the largest number of centres (67%) to specifically define the beginning of the rectum as the junction between the mesocolon and the mesorectum (sigmoid take-off)14. Although there are classifications proposed exclusively using MRI to precisely locate a tumour in the rectum15, in most Western countries the subdivision of the rectum into 3 equivalent segments of 5 cm in length (lower, middle and upper) measured from the anal verge by rigid rectosigmoidoscopy is still used. Only the classification of the Japanese Colon and Rectum Society (JCCCR), based on more anatomical criteria, divides it into 4 sections: rectosigmoid (Rs), superior rectum (Ra), inferior rectum (Rb) and anal canal (P) respectively, considering the line that marks the anterior peritoneal reflection (APR) the dividing line between Ra-Rb at the level of the medial valve of Houston16,17. Hence, as important as the distance to the anal verge is the relationship of the tumour to the APR, which determines the difference between intra- and extraperitoneal tumours, which tends to be 10 cm from the anal verge, although anatomically it would be closer to 6−8 cm according to the studies18,19. During preoperative assessment, the anterior and lateral APR can be visualised by endorectal ultrasound with some limitations, but not by rigid rectosigmoidoscopy20,21. Using MRI it is a visible structure in most studies22, and in our case it was found in all patients, with 75 (78%) of the tumours evaluated at the level of or below the APR, and we were able to accurately determine its infiltration and prognostic implication (T4a)23,24. The involvement of the sphincteric apparatus is further preoperative data that can be recognised preoperatively by MRI, and is key to the design of the therapeutic strategy. We were also able to assess infiltration in all cases, showing 15% infiltration.
MR imaging offers advantages at all levels: in the upper third it allows us to identify the APR, and establish its infiltration and relationship with the tumour18,25,26; in the middle third it determines the patients who may be candidates for neoadjuvant RCT and will require TME to achieve radicality, the surgical dissection plane being the mesorectal fascia; and in the lower third it will determine the relationship with the sphincter apparatus to plan surgery27.
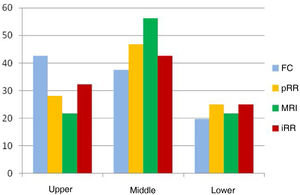
Our study comparing the measurement of the distance to the anal verge in rectal cancer confirms the variability between the different tests, and that these are accentuated the further away from the anus the tumour is located; the differentiation between the middle and upper third has the greatest implication in terms of prognosis and survival. Although there is a high positive linear relationship between all the tests evaluated, we observed that colonoscopy — because it is a long, flexible instrument designed to evaluate the entire colon — offers measurements up to 20−25 mm higher than the other tests, which is why we do not consider it to be reliable in this measurement. These data coincide with those of Schoellhammer et al.26, and contrast to some extent with those published by Tanaka et al.28, who found no significant differences between FC and pRR, only very slightly higher in lesions of the upper third of the rectum. However, when comparing pRR with MRI, their mean distances are observed to be very close (less than 4 mm), and both tests place 47% and 56% respectively in the middle third of the rectum (between 5.1 and 10 cm from the anal verge), where diagnostic accuracy plays a fundamental role (Fig. 1). When comparing these measurements with intraoperative rectoscopy (iRR) we found almost exact values, even among the subgroups created to avoid measurement bias after TME (65 patients) vs. the subtotal (31 patients), or after clinical response to neoadjuvant therapy (58 patients).
The original contribution of our study is that we performed rigid rectoscopy during the surgical procedure (iRR), once the rectum had been freed, to validate the distance to the anal verge obtained by both pRR and MRI performed preoperatively. After the statistical analysis, we found a high degree of concordance of both with the intraoperative measurement, which was considered confirmatory or reference.
Therefore, to conclude, we consider that during the preoperative pelvic MRI can be as accurate as pRR in determining the distance to the anal verge of rectal tumours, both being superior to colonoscopy, and that its three-dimensional image also provides complete and individualized topographic information of the rectum and the tumour in its anatomical position, defining its exact relationship with the peritoneal reflection and the anorectal sphincter complex, the craniocaudal length and the orientation of the tumour, among others. pRR, as a gold standard test, correctly determines the distance of the tumour to the anal verge, but does not consider individual anatomical variations or the relationships of the tumour to other structures, and thus must be considered to be of limited usefulness in decision making. Therefore, in our opinion, MRI measurement can replace endoscopic methods.
Conflict of interestsThe authors have no conflict of interests to declare.