To ascertain the formalities and procedures required for the prescription of PCSK9 inhibitors in the cardiology departments of Spanish hospitals, making proposals for improvement to optimize the prescription process.
MethodsA first phase of collecting information about the variables and administrative procedures required for the prescription of PCK9 inhibitors and the elaboration of a specific questionnaire and a second phase of collecting data with an online self-administered questionnaire.
ResultsA total of 88 hospitals participated in the study (mean number of beds 625; mean number of cardiologists 18 ± 10; 78% university hospitals). There was underuse of PCSK9 inhibitors (real prescription of 30 treatments/year; potential prescription of 80), mainly because of not fulfilling the therapeutic positioning report (52%) and application refusal (31%). Beyond the requirements of the therapeutic positioning report, 1.2 ± 0.4 applications are required with 8.5 ± 4.2 variables. Only 21% of hospitals did not require a previous authorization process and in the remaining hospitals, approval from a committee was necessary. The accumulated time of the prescription process was 6 weeks. Discontinuation rates during follow-up were 9% ± 12%.
ConclusionsTreatment with PCSK9 inhibitors is clearly underused in Spain. This is mainly due to both inappropriate identification of patients, and complex administrative procedures that could inhibit/discourage prescription by cardiologists and consequently, limit their use. In addition, there is a substantial delay from drug approval tadministration.
Conocer las tramitaciones y gestiones requeridas en la prescripción del tratamiento con inhibidores PCSK9 en los servicios de cardiología de los hospitales españoles, haciendo propuestas de mejoras para optimizar el proceso de prescripción.
MétodosUna primera fase de recogida de información sobre variables y procedimientos administrativos requeridos en la prescripción de inhibidores PCSK9 y elaboración de un cuestionario específico. Una segunda fase de recogida de datos a través de un cuestionario electrónico autoadministrado.
ResultadosParticiparon 88 hospitales (número medio de camas 625; número medio de cardiólogos 18 ± 10; 78% hospitales universitarios). Hubo una infrautilización de inhibidores PCSK9 (prescripción real 30 tratamientos/año; prescripción potencial 80), principalmente por no cumplir con informe de posicionamiento terapéutico (52%), con la denegación de solicitud en un 31%. Se requirieron una media de 1,2 ± 0,4 formularios, con un promedio de 8,5 ± 4,2 variables, además de los requisitos del informe de posicionamiento terapéutico. Sólo en el 21% de los hospitales no es necesario un proceso de autorización previa, y en el resto es necesaria la aprobación por una comisión. El tiempo acumulado en el proceso de prescripción es de 6 semanas. La discontinuación del tratamiento durante el seguimiento es de 9 ± 12%.
ConclusionesLos inhibidores PCSK9 se encuentran claramente infrautilizados en España. Esto se debe a una incorrecta identificación de los pacientes, y a la existencia de complejos procedimientos de tipo administrativo que podrían inhibir/desmotivar su prescripción por parte de los cardiólogos, y consecuentemente, limitar su prescripción. Asimismo, existe un retraso notable desde la aprobación del fármaco hasta su administración.
Cardiovascular diseases are the leading cause of death in Spain, and atherosclerotic disease is the main cause.1 Low-density lipoprotein cholesterol (LDL-C) is not only one of the main risk factors for development of atherosclerosis, but also plays an essential role in its aetiopathogenesis.2 Multiple studies have shown that lowering LDL-C by lipid-lowering therapy is associated with a substantial decrease in cardiovascular events.3
Unfortunately, LDL-C control in patients with coronary heart disease is currently very poor. Therefore, both the REPAR study, conducted in Spain, and the EUROASPIRE V study, conducted in Europe, showed that LDL-C is controlled in barely 30% of patients with coronary heart disease.4,5 More recently, it has been found in Spain that, one year after an acute coronary syndrome, only 56% of patients achieve the former LDL-C target (<70 g/dL).6 Although patient adherence to lipid-lowering therapy clearly plays a role in the failure to meet these targets, and is associated with an increased risk of cardiovascular complications,7 several studies have also shown that in a large proportion of cases, this lack of control is mainly due to insufficient intensification of lipid-lowering therapy.4–6 Following the publication of the 2019 European guidelines for the management of dyslipidaemia, the LDL-C target in these patients at very high cardiovascular risk was lowered to <55 mg/dL.2 The Da Vinci study has shown that achievement of these targets is even lower: only 18% of patients achieve a LDL-C < 55 mg/dL.8
Proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) are the most potent lipid-lowering therapy currently available for lowering LDL-C and their use has been associated with a marked reduction in the risk of developing cardiovascular complications in secondary prevention.9,10 However, despite the evidence from clinical trials,9,10 the latest European guidelines with an IA recommendation for its use in subjects not adequately controlled with oral lipid-lowering therapy, in subjects with atherosclerotic cardiovascular disease,2 and the recommendations made by the therapeutic positioning report (TPR) in patients with cardiovascular disease and LDL-C levels > 100 mg/dL, despite optimal maximum tolerated lipid-lowering therapy,11,12 PCSK9i use in Spain is still very marginal.6
We need, therefore, to determine the reasons for the underuse of PCSK9i in Spain. The IKIGAI study (analysis of the PCSK9i prescription process in cardiology services and proposal for optimisation) was conducted with the aim of understanding and describing the procedures and steps required for PCSK9i prescription in Spain’s cardiology services, and the time required to complete the process, refusal/non-authorisation, and treatment discontinuation rates. The expert group will also make a series of recommendations to expedite appropriate prescribing.
MethodsThe IKIGAI study is a project of the Research Agency of the Spanish Society of Cardiology and was conducted in two phases.
During the first phase, a scientific committee comprising cardiologists with expertise in the management of patients with dyslipidaemia and in the use of PCSK9i, and experts in sociology and communication who helped focus the questions to derive the best from them, developed a self-administered electronic questionnaire specifically designed to meet the objectives of the study.
Hospitals were then selected as representative of all the regions of Spain, considering both the size and characteristics of their cardiology services, to increase the external validity of the study. The head of each cardiology department was contacted to select the representative with the most experience in prescribing PCSK9i (Annex I) to oversee completion of the questionnaire. Cardiologists had to have prescribed and processed at least one PCSK9i in the last month to be eligible.
The questionnaire asked about the type of hospital (number of beds, university/referral, number of cardiologists, existence of lipid unit, cardiac rehabilitation unit), aspects of the discharge report (therapeutic objectives, management, and follow-up) and about the continuity of care between the cardiology department and primary care. Regarding PCSK9i prescriptions, questions were asked on the criteria to be considered by the physician (guidelines/recommendations, patient characteristics) and actual and potential prescription figures. Specific questions were also asked about the authorisation strategy for prescribing PCSK9i, including questions about the number and type of forms needed, as well as the requirements of these forms, potential fees, the time to approval or refusal of authorisation, and the percentage and reason for any refusal. Finally, questions were also asked about the follow-up of patients treated with PCSK9i (percentage of discontinuations, requirements for continuation of treatment, number of vials administered, place of administration, and patient-specific training).
The results were analysed descriptively. Qualitative variables were presented as absolute (n) or relative (%) frequencies, and quantitative variables according to measures of central tendency (mean and median, as appropriate) and dispersion (standard deviation). The analysis was performed using the SPSS statistical package (v24.0 or higher, IBM Corp., Armonk, NY).
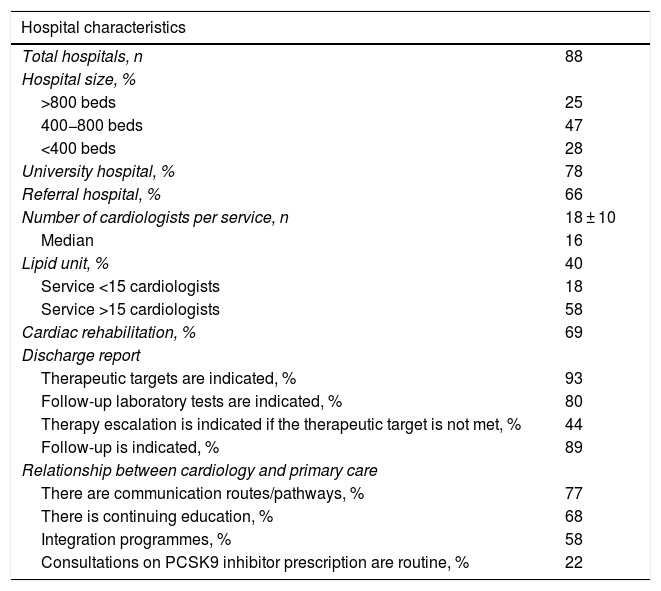
ResultsA total of 88 representative hospitals from all over Spain participated, with a mean of 625 beds, 78% were university hospitals, 66% were referral hospitals, and 40% of the hospitals had lipid units; this percentage was higher in the larger hospitals. The mean number of cardiologists in the cardiology department per hospital was 18 ± 10, and 69% had cardiac rehabilitation units. In most cases, the discharge report indicated the therapeutic objectives (93%), the follow-up laboratory tests (80%), and the actual follow-up to be performed (89%). However, only 44% of the reports referred to therapeutic escalation in the event of failure to achieve the control targets. Regarding continuity of care between cardiology and primary care, there were communication pathways/channels in 77% of the hospitals, continuous training in 68%, and regular interconsultations on PCSK9i prescription in only 22% (Table 1).
Hospital characteristics, discharge report, and relationship with primary care.
| Hospital characteristics | |
|---|---|
| Total hospitals, n | 88 |
| Hospital size, % | |
| >800 beds | 25 |
| 400−800 beds | 47 |
| <400 beds | 28 |
| University hospital, % | 78 |
| Referral hospital, % | 66 |
| Number of cardiologists per service, n | 18 ± 10 |
| Median | 16 |
| Lipid unit, % | 40 |
| Service <15 cardiologists | 18 |
| Service >15 cardiologists | 58 |
| Cardiac rehabilitation, % | 69 |
| Discharge report | |
| Therapeutic targets are indicated, % | 93 |
| Follow-up laboratory tests are indicated, % | 80 |
| Therapy escalation is indicated if the therapeutic target is not met, % | 44 |
| Follow-up is indicated, % | 89 |
| Relationship between cardiology and primary care | |
| There are communication routes/pathways, % | 77 |
| There is continuing education, % | 68 |
| Integration programmes, % | 58 |
| Consultations on PCSK9 inhibitor prescription are routine, % | 22 |
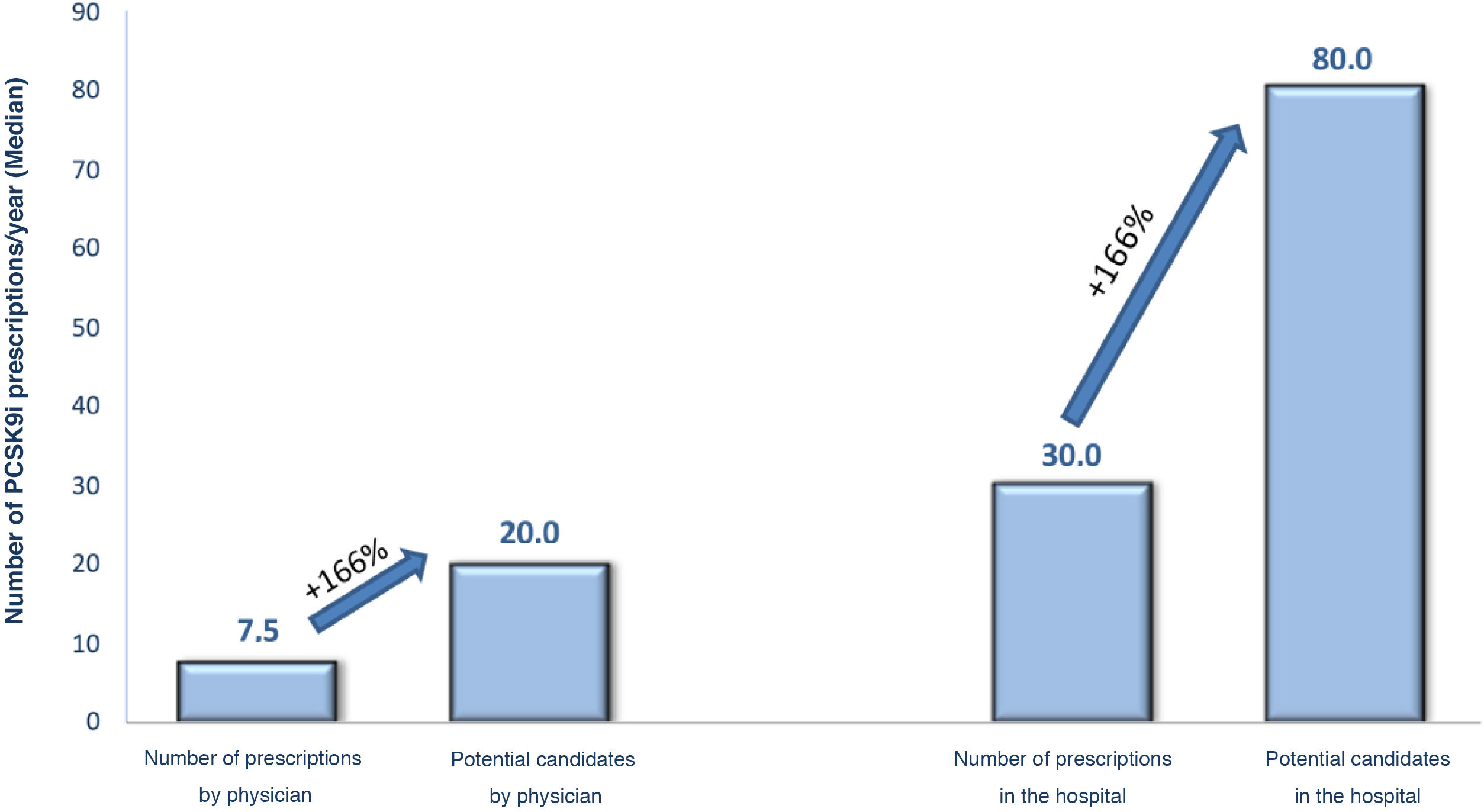
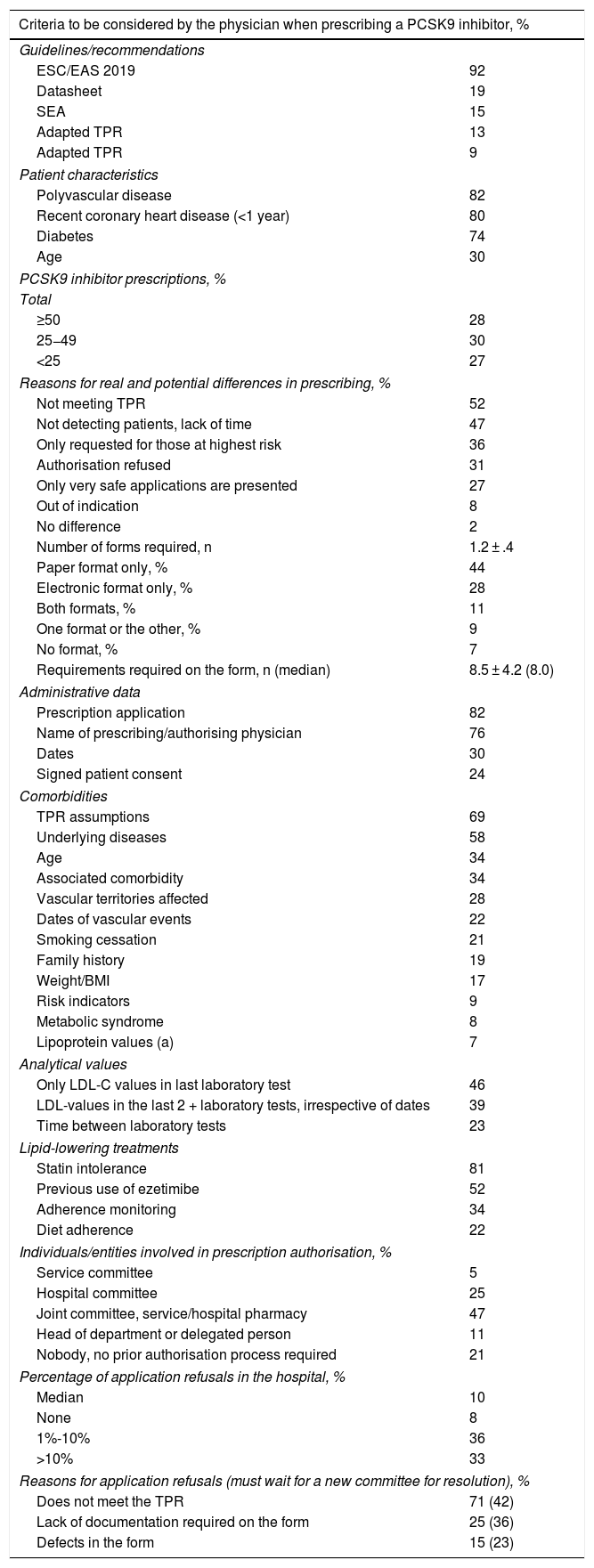
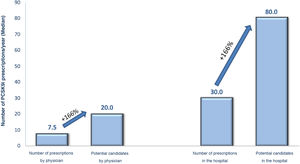
Regarding the criteria for selecting candidates for PCSK9i treatment, the cardiologists replied that the criteria for identifying PCSK9i candidates were those of the 2019 European guidelines for the management of dyslipidaemia, and the most important of the clinical criteria were having polyvascular disease (82%), recent coronary heart disease (80%), or diabetes (74%) (Table 2). Participating cardiologists prescribed an average of 7.5 PCSK9i treatments per year, although according to their criteria, this figure should be as high as 20 prescriptions per year. They also estimated the annual prescription volume in the hospital as a whole at 30 PCSK9i per year, this figure increasing to 80 potential candidates (Fig. 1). The prescribing volume was higher in hospitals with more than 15 cardiologists, where over one third prescribed more than 50 treatments per year. These differences between actual and potential prescribing were, in the opinion of the participants, mainly due to indications that did not comply with the TPR (52%), lack of patient detection/lack of time (47%), or situations where refusal was expected “only those at higher risk are presented” (36%), or those where “you are very sure” (27%) (Table 2).
Prescription of PCSK9 inhibitors and strategy for authorising the prescription of PCSK9 inhibitors.
| Criteria to be considered by the physician when prescribing a PCSK9 inhibitor, % | |
|---|---|
| Guidelines/recommendations | |
| ESC/EAS 2019 | 92 |
| Datasheet | 19 |
| SEA | 15 |
| Adapted TPR | 13 |
| Adapted TPR | 9 |
| Patient characteristics | |
| Polyvascular disease | 82 |
| Recent coronary heart disease (<1 year) | 80 |
| Diabetes | 74 |
| Age | 30 |
| PCSK9 inhibitor prescriptions, % | |
| Total | |
| ≥50 | 28 |
| 25−49 | 30 |
| <25 | 27 |
| Reasons for real and potential differences in prescribing, % | |
| Not meeting TPR | 52 |
| Not detecting patients, lack of time | 47 |
| Only requested for those at highest risk | 36 |
| Authorisation refused | 31 |
| Only very safe applications are presented | 27 |
| Out of indication | 8 |
| No difference | 2 |
| Number of forms required, n | 1.2 ± .4 |
| Paper format only, % | 44 |
| Electronic format only, % | 28 |
| Both formats, % | 11 |
| One format or the other, % | 9 |
| No format, % | 7 |
| Requirements required on the form, n (median) | 8.5 ± 4.2 (8.0) |
| Administrative data | |
| Prescription application | 82 |
| Name of prescribing/authorising physician | 76 |
| Dates | 30 |
| Signed patient consent | 24 |
| Comorbidities | |
| TPR assumptions | 69 |
| Underlying diseases | 58 |
| Age | 34 |
| Associated comorbidity | 34 |
| Vascular territories affected | 28 |
| Dates of vascular events | 22 |
| Smoking cessation | 21 |
| Family history | 19 |
| Weight/BMI | 17 |
| Risk indicators | 9 |
| Metabolic syndrome | 8 |
| Lipoprotein values (a) | 7 |
| Analytical values | |
| Only LDL-C values in last laboratory test | 46 |
| LDL-values in the last 2 + laboratory tests, irrespective of dates | 39 |
| Time between laboratory tests | 23 |
| Lipid-lowering treatments | |
| Statin intolerance | 81 |
| Previous use of ezetimibe | 52 |
| Adherence monitoring | 34 |
| Diet adherence | 22 |
| Individuals/entities involved in prescription authorisation, % | |
| Service committee | 5 |
| Hospital committee | 25 |
| Joint committee, service/hospital pharmacy | 47 |
| Head of department or delegated person | 11 |
| Nobody, no prior authorisation process required | 21 |
| Percentage of application refusals in the hospital, % | |
| Median | 10 |
| None | 8 |
| 1%-10% | 36 |
| >10% | 33 |
| Reasons for application refusals (must wait for a new committee for resolution), % | |
| Does not meet the TPR | 71 (42) |
| Lack of documentation required on the form | 25 (36) |
| Defects in the form | 15 (23) |
BMI: body mass index; EAS: European Atherosclerosis Society; ESC: European Society of Cardiology; SEA: Spanish Atherosclerosis Society; TPR: therapeutic positioning report.
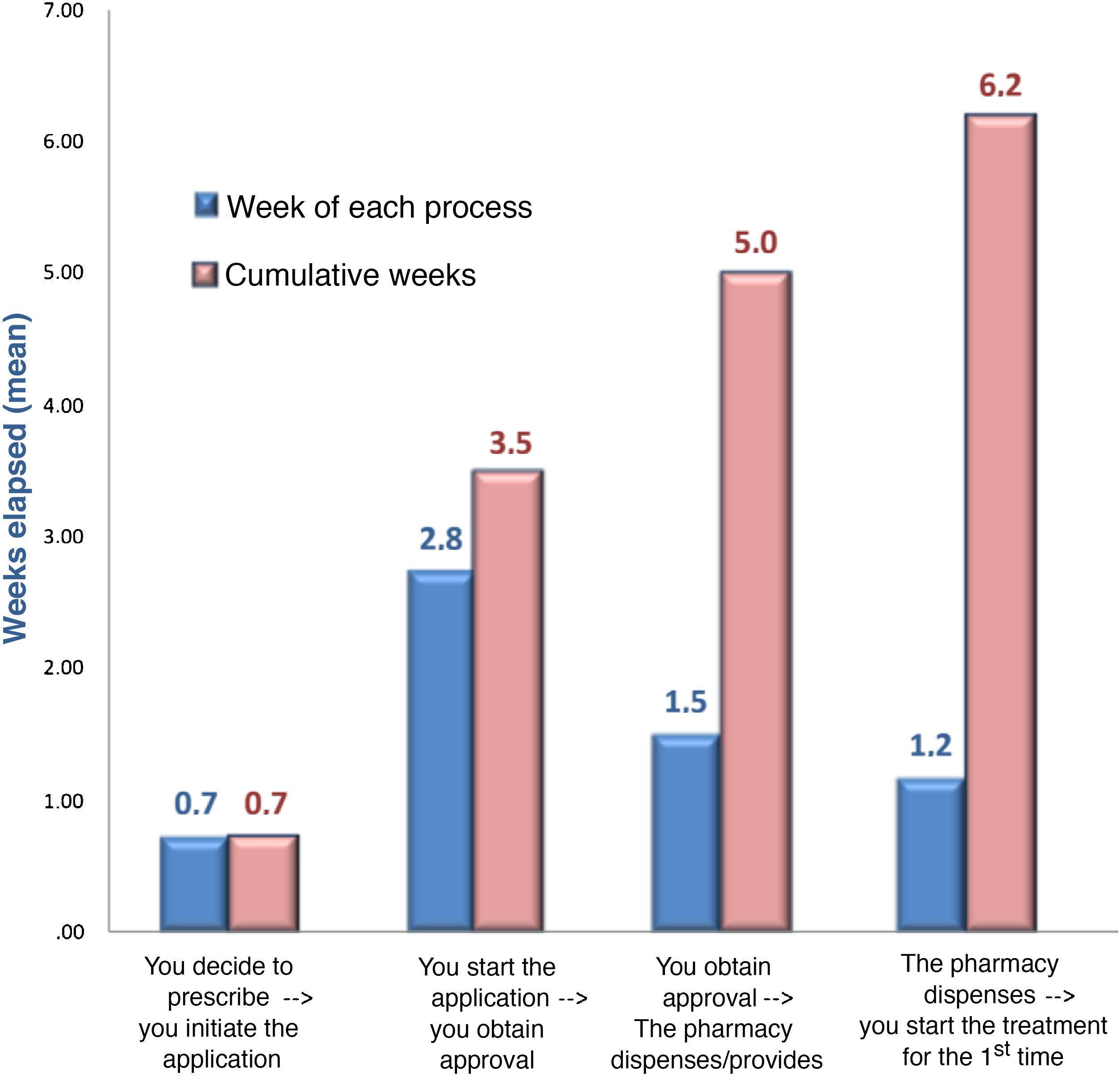
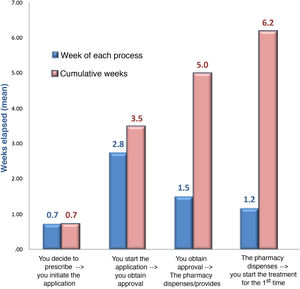
In terms of the authorisation strategy for PCSK9i prescription, an average of 1.2 ± .4 forms were required, most in paper format (44%), with a mean of 8.5 ± 4.2 variables, in addition to the TPR requirements. The most important requirements of the forms included information on intolerance to statins (81%), the assumptions described in the TPR (69%), underlying diseases (58%), previous use of ezetimibe (52%), or the last LDL-C test values (46%). Adherence to lipid-lowering therapy was required in 34% (41% for statins and 25% for ezetimibe), which had to reach 89% picked up in the pharmacy. The entity most involved in authorising prescription was the joint departmental-hospital pharmacy committee (47%), although in larger hospitals it was the hospital committee (48%) (Table 2). The departmental committee met every 2.5 weeks, the hospital committee every 5.9 weeks, and the joint committee every 6.4 weeks. The cumulative delay in the prescription process was 6.2 weeks; approval by the committee was responsible for the longest delay (2.8 weeks) (Fig. 2). The median number of refusals per year in the hospital was 10%. The main reason for refusal was non-compliance with the TPR (71%), in which case 42% of cases had to wait for a new committee to resolve the situation (Table 2).
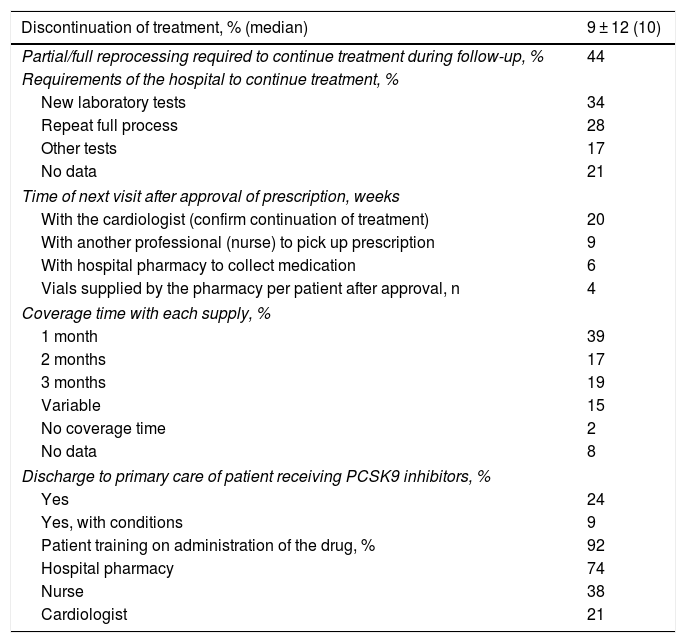
The median treatment discontinuations during follow-up were 10%. In 44%, partial/full re-processing was required to continue treatment during follow-up. In-hospital follow-up of patients on PCSK9i treatment was mainly in the context of comprehensive cardiac monitoring (82%), the remainder exclusively for monitoring of PCSK9i treatment. The survey results show that the patient must visit the hospital an average of three times in approximately five months for pharmacological and clinical monitoring. The hospital pharmacy supplied an average of 3.9 vials. The first time the drug was administered was in the hospital (92%), although this percentage decreased in following visits. In 92% of cases, the patient was trained in drug administration, usually by the hospital pharmacist (74%). In hospitals with cardiac rehabilitation units, these units played an important role both in prescribing (89%) and in patient training (75%), or in the design of the care route or pathway to be followed (75%) (Table 3).
Follow-up of treatment with PCSK9 inhibitors.
| Discontinuation of treatment, % (median) | 9 ± 12 (10) |
|---|---|
| Partial/full reprocessing required to continue treatment during follow-up, % | 44 |
| Requirements of the hospital to continue treatment, % | |
| New laboratory tests | 34 |
| Repeat full process | 28 |
| Other tests | 17 |
| No data | 21 |
| Time of next visit after approval of prescription, weeks | |
| With the cardiologist (confirm continuation of treatment) | 20 |
| With another professional (nurse) to pick up prescription | 9 |
| With hospital pharmacy to collect medication | 6 |
| Vials supplied by the pharmacy per patient after approval, n | 4 |
| Coverage time with each supply, % | |
| 1 month | 39 |
| 2 months | 17 |
| 3 months | 19 |
| Variable | 15 |
| No coverage time | 2 |
| No data | 8 |
| Discharge to primary care of patient receiving PCSK9 inhibitors, % | |
| Yes | 24 |
| Yes, with conditions | 9 |
| Patient training on administration of the drug, % | 92 |
| Hospital pharmacy | 74 |
| Nurse | 38 |
| Cardiologist | 21 |
The main results of the IKIGAI study show how PCSK9i are not prescribed to the extent to which some studies estimate they could be, according to the approved indications. Consequently, the IKIGAI study from the Research Agency of the Spanish Society of Cardiology, conducted in a representative sample of cardiology departments in hospitals throughout Spain, is a key instrument for cardiologists, health managers, and politicians to analyse the need to improve the conditions of the authorisation and use of iPCSK9i in Spain.
A simulation study in the United States showed that to achieve the previous target of LDL-C control in patients with atherosclerotic cardiovascular disease (<70 mg/dL), treatment intensification included the use of statin combination therapy with ezetimibe in about 19% of patients, and treatment with PCSK9i in 14% of subjects.13 Importantly, this intensification of lipid-lowering therapy is associated with a substantial reduction in cardiovascular events.14 Moreover, not only clinical trials8,9 but also “real-life” studies in Spain have confirmed the efficacy and safety of PCSK9i.15–17 Along these lines, the EVACS and EVOPACS studies showed that starting evolocumab early in patients with coronary syndrome was effective and safe.18,19 Moreover, a recent study shows that, in Spain, as lipid-lowering treatment is intensified and, consequently, a greater reduction in LDL-C levels is obtained, not only cardiovascular events decrease but also the associated healthcare costs in patients who have had a myocardial infarction.20 In fact, several studies have shown that PCSK9i are a cost-effective alternative in higher risk patients with coronary heart disease.21,22 Current TPRs have estimated a number needed to treat (NNT) of 67 for evolocumab for the primary endpoint of the study and 62 for alirocumab.11,12 However, in patients with chronic coronary syndrome, data from the REPAR study show the percentages of patients with LDL-C > 100 mg/dL despite taking high-intensity statins were 27% if not taking ezetimibe, and 19% otherwise, 23 there are still many patients, therefore, who are candidates for PCSK9i within the TPR funding criteria. The Spanish Society of Arteriosclerosis has recently updated its recommendations on the use of PCSK9i, considering the patient’s LDL-C levels and clinical situation, additional risk factors, and the cost-effectiveness of its use.24 Furthermore, to help correctly identify patients who are candidates for PCSK9i, the Spanish Society of Cardiology has recently published three simple and practical algorithms to optimise the use of lipid-lowering treatment, with the two-fold objective of achieving the recommended LDL-C levels in the shortest possible time.25 In a study conducted in a Spanish hospital, applying these algorithms would mean that 3% of patients with acute coronary syndrome would be eligible for treatment with high-potency statins and initiation of PCSK9i from hospital discharge.26 Thus, our analysis only confirms the underuse of PCSK9i in routine clinical practice, which could increase the risk of cardiovascular complications in inadequately controlled patients.14 Furthermore, our analysis shows that there are marked differences between hospitals, even within the same autonomous community.
The results of the IKIGAI study help to better understand the causes of this marked underuse of PCSK9i in Spain. Firstly, potential cases are under-detected. Thus, although European dyslipidaemia management guidelines are the main criterion for optimising lipid-lowering treatment, the patient requiring this treatment is not correctly identified in half of cases, the TPR is not followed, or the administrative procedure is abandoned due to lack of time or lack of follow-up by the clinician. In slightly more than one third of patients, it is only requested for the highest risk patients, so that pre-selection among candidate patients is identified under the study. It should be noted that the TPR funding criteria for both PCKS9i are very simple (statins at maximum dose and LDL-C > 100 mg/dL), but an average of 8.5 additional criteria to TPR have been observed in the approval form. Correct optimisation of lipid-lowering therapy is necessary to better meet therapeutic targets. To improve this situation, our results show that it is necessary to optimise the discharge report, indicating not only the therapeutic targets, but also how the follow-up (clinical and analytical) and therapeutic escalation should be carried out, and to simplify the application forms and approval processes. It is also important to improve the continuity of care between cardiology and primary care, as integration and training percentages could be improved. In this regard, departments with a cardiac rehabilitation unit show higher percentages of coordination and training. Unfortunately, there are still insufficient cardiac rehabilitation units.27 In addition, a consensus of the Spanish Society of Cardiology has recently been published to enable better identification of the patients who will benefit most from the use of PCSKi9.28
According to our results, the prescription authorisation strategy for PCSK9i is the other area for improvement. Overall, an average of six weeks elapses from the time it is decided that a patient should be treated with these drugs until the first dose is finally administered to initiate treatment, a very significant delay that clearly means a delay in achieving LDL-C targets and, consequently, an increased risk of a cardiovascular event.2,14 The European dyslipidaemia management guidelines make a 1B recommendation for the incorporation of PCSK9i at four to six weeks after the event, when the recommended targets are not achieved in patients at very high cardiovascular risk,2 and therefore time is an important variable in the management of these patients. This administrative delay adds to the time it takes to identify and optimise a patient who is a candidate for PCSK9i, therefore it is necessary to reduce this time both in identifying patients and in approval. There are several areas for improvement in this regard. For example, 44% of the forms are in paper format, which could discourage their use. Moreover, the forms vary greatly between hospitals, as do the aspects requested for approval, including administrative data, comorbidities, analytical parameters, and lipid-lowering treatment. Simplifying and standardising the forms (better targeted forms), as well as the use of an electronic format, could facilitate further implementation. Once the application has been submitted, only in 20% of cases is a prior authorisation process not necessary. In the remaining cases, the form must be approved by a committee (departmental, hospital or joint), which is where the longest delay accumulates. Given that most refusals are due to failure to comply with the TPR criteria or lack of documentation, a similar procedure could be followed as for other cardiovascular drugs that require authorisation by medical inspection, which is a faster and simpler procedure, given that it is only necessary to check whether certain criteria have been met,29 and leave the committee to assess those situations that do not meet the TPR criteria, but where the cardiologist considers that the patients would benefit significantly from treatment with PCSK9i.28
Finally, regarding the follow-up of patients treated with iPCSK9, 44% of patients require partial or complete reprocessing, and the coverage time of each supply is rather limited. Given that both clinical trials9,10 and real-life studies15–17 have confirmed the safety of PCSK9i, once it has been confirmed that the drug is well tolerated, it would not be necessary to carry out visits so close together for the sole purpose of assessing for adverse effects, since no additional benefit would be obtained, and they could be made to coincide with routine visits to cardiology or primary care clinics, depending on the case. This would mean that the hospital pharmacy would provide the medication until the next scheduled visit. In addition, treatment with PCSK9i is chronic and it has been shown that the benefit of PCSK9i increases with treatment time,9,10 and therefore it is recommended that treatment with these drugs should not be discontinued due to administrative procedures.
In short, the results of the IKIGAI study will make it possible to review each centres’ local protocols, to streamline the process and optimise the treatment of patients in whom the administration of PCSK9i is indicated, attempting to make them as homogeneous as possible in all Spanish hospitals, to avoid disparities, not only between Autonomous Communities, but also between the hospitals themselves in each region, for which a series of recommendations are made (Table 4).
Recommendations to improve prescription of PCSK9i.
| - Implement electronic authorisation documents to facilitate their treatment and management. |
| - Implement simple and rapid forms to expedite the PCSK9i authorisation process when the drug is indicated. |
| - Strictly include the variables that the Ministry of Health includes in the TPR*, avoiding introducing additional requirements of the centre itself to prevent differences between hospitals that could result in disparities. |
| - Expedite the assessment of the PCSK9i treatment authorisation form, so that if the prescription application meets all the necessary conditions, the patient can receive the medication within a reasonable period (less than 14 days). |
| - In any case, it is essential that the assessment committee studies the applications with the necessary frequency to meet the time recommended by the European guidelines of 4−6 weeks between each change of treatment to achieve control. |
| - Implement guidelines for reasonable follow-up, which does not imply an additional burden on the physician or discomfort for the patient, if there is no condition (e.g., side effects) that would cause the treatment to vary. |
| - Therefore, it is recommended that pharmacological follow-up should match the clinical follow-up of the patient, dispensing sufficient doses of PCSK9i to cover the time until the next visit with the clinical team. |
PCSK9i: PCSK9 inhibitors; TPR: therapeutic positioning report.
When the patient who is indicated for PCKS9i treatment has LDL-C > 70 mg/dL and <100 mg/dL and maximum tolerated statin dose plus ezetimibe, data from the last LDL-C test and current lipid-lowering treatment should be included for assessment by the approval committee.
Statin intolerance will be documented on the form and authorisation will take place through medical inspection.
Current PCSK9i TPR: when the patient with indication for PCKS9i treatment has LDL-C > 100 mg/dL and maximum tolerated statins, it is recommended that authorisation be automatic through a medical inspection. The application form should be completed with details of the medical indication, the last LDL-C test, and the statin dose.
The study has several limitations. Although hospitals were scrupulously selected to achieve adequate representativeness, it is possible that there is a small percentage that would not have been included in the study. Furthermore, although the questionnaire was designed to answer the objectives of the study adequately, it is possible that some questions and answers may require further clarification or specification. The results of our study are also a "snapshot" at a given moment in time, and therefore some procedure(s) may have changed in some of the hospitals over this time.
ConclusionsDespite the evidence to show the clinical benefits of treatment with PCSK9i, it is clearly underused in Spain. There are several reasons for this, including, among others, a failure to correctly identify the patients who will benefit most from this therapy, and complex administrative procedures that could be inhibiting or discouraging its prescription by cardiologists, and be delaying its use. Better identification of the patients who would benefit most from this treatment, as well as simplifying and improving the authorisation and monitoring strategy, could facilitate its use, and consequently LDL-C control targets would be achieved more quickly and effectively, thus reducing the risk of cardiovascular complications.
FundingThe study was funded by an unconditional grant from Amgen.
Conflict of interestVivencio Barrios, Carlos Escobar, Marisol Bravo, and Lluís Recasens have received consultancy/presentation fees from Amgen and Sanofi. Vicente Arrarte has received fees for speaking from MSD, Sanofi, Rovi, Almirall, Daiichi-Sankyo. Alfredo del Campo has received fees from a grant from the Spanish Society of Cardiology. Ángel Cequier has received conference/consultancy fees from AstraZeneca, Amgen, Biotronik, Boehringer Ingelheim, Daiichi-Sankyo, Ferrer International, Sanofi.
Please cite this article as: Barrios V, Escobar C, Arrarte V, Bravo M, del Campo A, Hidalgo R, et al. Análisis del proceso de prescripción de inhibidores PCSK9 en los servicios de cardiología de los hospitales españoles y propuesta de optimización. Estudio IKIGAI. Clin Investig Arterioscler. 2021;33:296–305.