To present the first registry used to analyse the clinical profile of patients treated with evolocumab in Spain, including the effectiveness on the lipid profile and safety in the “real world” setting.
MethodsMulticentre, retrospective, and observational study of patients starting treatment with evolocumab from February 2016 to May 2017 in clinical practice in Spanish cardiology units.
ResultsA total of 186 patients (mean age 60.3±9.8 years were included, 35.5% with familial hypercholesterolaemia, and 94.1% with a previous cardiovascular event) from 31 cardiology units. Baseline lipid profile: Total cholesterol 219.4±52.2mg/dL, LDL-cholesterol 144.0±49.0mg/dL, HDL-cholesterol 47.7±13.0mg/dL, and triglycerides 151.0±76.2mg/dL. At the time of initiating evolocumab, 53.8% of patients were taking statins (50% had partial or total intolerance to statins), and 51.1% ezetimibe. In all cases, the dose of evolocumab used was 140 mg, mainly every 2 weeks (97.3%). Evolocumab compliance was high (92.3%).
Treatment with evolocumab was interrupted in 6 patients (3.2%), with only 1 (0.5%) due to a probable side effect. Evolocumab significantly reduced total cholesterol (30.9% at week 2, and 39.3% at week 12; P<.001), LDL cholesterol (44.4% and 57.6%, respectively; P<.001), and triglycerides (14.8% and 5.2%, respectively; P<001), with no significant changes in HDL-cholesterol (6.7% and 2.0%; P=.14).
ConclusionsIn clinical practice, evolocumab is associated with reductions in LDL cholesterol, with nearly 60% after 12 weeks of treatment, and with low rates of interruptions due to side effects and high medication compliance. These results are consistent with those reported in randomised clinical trials.
Presentar el primer registro que analiza el perfil clínico de los pacientes tratados en España con evolocumab y la efectividad sobre el perfil lipídico y su seguridad en el «mundo real».
MétodosEstudio multicéntrico, retrospectivo, observacional, de los pacientes que empezaron tratamiento con evolocumab entre febrero de 2016 y mayo de 2017 en la práctica clínica en unidades de cardiología en España.
ResultadosSe incluyó a 186 pacientes de 31 unidades de cardiología (edad media 60,3 ± 9,8 años; 35,5% con hipercolesterolemia familiar y 94,1% con evento cardiovascular previo). Perfil lipídico basal: colesterol total 219,4 ± 52,2 mg/dL, colesterol-LDL 144,0 ± 49,0 mg/dL, colesterol-HDL 47,7 ± 13,0 mg/dL y triglicéridos 151,0 ± 76,2 mg/dL. En el momento del inicio de evolocumab, el 53,8% estaba tomando estatinas (el 50% presentaba intolerancia a las estatinas, total o parcial) y el 51,1% ezetimiba. En todos los casos se empleó la dosis de evolocumab de 140 mg y, principalmente, cada 2 semanas (97,3%). El cumplimiento terapéutico fue elevado (92,3%). En 6 pacientes (3,2%) se interrumpió el tratamiento, pero solo en uno (0,5%), por posible efecto adverso. Evolocumab redujo significativamente el colesterol total (30,9% a las 2 semanas y 39,3% a las 12 semanas; p < 0,001), colesterol-LDL (44,4 y 57,6%, respectivamente; p < 0,001) y triglicéridos (14,8 y 5,2%; p < 0,001), sin modificar significativamente el colesterol-HDL (6,7 y 2,0%; p = 0,14).
ConclusionesEn la práctica clínica, evolocumab se asocia con reducciones del colesterol-LDL cercanas al 60% tras 12 semanas de tratamiento, con una tasa de retiradas por efectos adversos muy baja y con un elevado cumplimiento terapéutico. Estos resultados son consistentes con los obtenidos en los ensayos clínicos aleatorizados.
Cardiovascular diseases are the major cause of morbidity and mortality in developed countries, of which atherosclerosis is the key underlying factor. Hypercholesterolaemia is not only a risk factor for the development of atherosclerosis, but a major aetiological factor.1–3 Dyslipidaemia is very common in our setting, in the population and, in particular, in patients who have already had a prior cardiovascular event.4 Reduction of LDL cholesterol (LDL-C) with hypolipidaemic treatment has been shown to reduce the risk of cardiovascular complications in both secondary and primary prevention, and that the risk of presenting a cardiovascular event is lower the more intense and the earlier this reduction.1,3,5
Despite treatment with statins or ezetimibe, in reality, less than 30% of patients with ischaemic heart disease achieve LDL-C control objectives in clinical practice in Spain. Although there are several reasons, therapeutic inertia (non-intensification of hypolipidaemic therapy despite insufficient control), intolerance to statins or ezetimibe or failure to control LDL-C despite hypolipidaemic therapy are the most frequent.6–8
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors effectively reduce LDL-C levels and the risk of cardiovascular events.9,10 In the FOURIER study, in patients with established atherosclerotic cardiovascular disease, treatment with evolocumab resulted in a significant reduction in cardiovascular events.10 However, since there may be important differences between patients included in clinical trials and clinical practice, it is essential to determine whether efficacy and safety in patients is maintained in real life.11–13 Unfortunately, the information available at this time on the role of evolocumab in conditions of daily clinical practice is scarce and on few patients.14–19
The RETOSS-CARDIO study (RETrospective Observational Study of Evolocumab Use in Spanish Cardiology Units), supported by the Spanish Research Agency of the Spanish Society of Cardiology, aimed to analyse the clinical profile of the first patients treated in Spain with evolocumab, the effect on lipid profile and the safety of the drug in the "real world" of patients treated in hospital cardiology units.20
MethodsTo this end, a multicentre retrospective observational study was carried out to review the clinical histories of patients who began treatment with evolocumab in hospital cardiology units in Spain under real-life conditions. The study was developed over the initial years following publication of the therapeutic positioning report on evolocumab (March 2016).21 There were 2 conditions for selecting the hospitals, geographical distribution and type of hospital (small: <200 beds; medium: 200-500 beds; large: >500 beds), seeking to ensure a representative population of the Spanish population served by different models of cardiology units.
A consecutive review of the clinical records of patients attended in 31 centres throughout the country was conducted between February 1, 2016 and May 15, 2017. All the patients who started treatment with evolocumab during that period and who met the inclusion/exclusion criteria were included in the study. Each centre was able to include up to a maximum of 10% of the total sample, to ensure adequate representativeness. Patients were included who were at least 18 years of age and prescribed evolocumab in the abovementioned period, according to the clinical criteria of the physician in charge, had received at least one dose of evolocumab, had at least one LDL-C measurement in the 12 weeks prior to starting evolocumab, and agreed to participate in the study by signing their informed consent. Patients who had received a PCSK9 inhibitor in the previous 12 weeks or who had been included in a clinical study during the observation period (from 12 weeks before the start of treatment with evolocumab to 12 weeks after) were excluded. The study was approved by the Ethics Committee of the Hospital Universitario La Paz.
Data were collected retrospectively from 12 weeks prior to treatment to 12 weeks after treatment and recorded in an electronic data collection notebook specifically designed for the study. Information was collected on biodemographic data (age, sex, employment status), physical examination (body mass index, heart rate, blood pressure), cardiovascular risk factors (high blood pressure, diabetes, smoking, family history of premature cardiovascular disease, familial hypercholesterolaemia [FH) and cardiovascular disease (angina, myocardial infarction, heart failure, peripheral arterial disease, chronic renal disease, carotid disease and cerebrovascular disease).
Information was also collected on hypolipidaemic treatment prior to starting evolocumab: statins, ezetimibe, fibrates, omega-3 fatty acids and resins. Statins were characterised according to their ability to reduce LDL-C,22 as well as total or partial intolerance to statin therapy (to which and at what dose). The reason for initiation, form of administration (self-administration, dose, frequency), temporary or permanent discontinuation and therapeutic compliance (good compliance if the number of administrations was ≥80% of the total theoretical administrations)23 were analysed. During the treatment period with evolocumab, changes in concomitant lipid-lowering medication were examined. Routine visits to the doctor in the year following the start of the drug and the specialist who made the initial diagnosis of hypercholesterolaemia were also recorded.
Baseline lipid profile test parameters (total cholesterol, LDL-C, HDL-C, non-HDL cholesterol and triglycerides) were collected (12 weeks previously), as well as the results of the analyses that would have been carried out on the patient over the 12 subsequent weeks according to the usual clinical practice of each centre, grouped at 4 points: 2 weeks (data obtained between day 1 and 21 from start of treatment with evolocumab); 4 weeks (from day 22 to 35); 8 weeks (from day 36 to 63), and 12 weeks (from day 64 to 112). Changes in the different lipid profile parameters were analysed, comparing levels at the different follow-up points (2, 4, 8 and 12 weeks) with the baseline values, both absolute and relative. Likewise, the percentage of patients who achieved LDL-C levels < 100mg/dL, LDL-C < 70mg/dL and LDL-C < 50mg/dL was calculated. Results were analysed in the general population and according to whether the patients had FH or were primary or secondary preventable.3 In cases of heterozygous and homozygous FH the diagnosis was genetic. FH was considered indeterminate in cases of negative mutation and clinical diagnosis was based on DLCN criteria.
Statistical analysisQuantitative variables were expressed as means and standard deviation and qualitative variables as absolute (n) and relative (%) values. Changes in lipid profile variables over the course of the study were expressed in both absolute and relative terms, with a 95% confidence interval. The quantitative variables (12 weeks vs. baseline) were compared by means of parametric (Student’s t-) or non-parametric (U for Mann-Whitney) tests, according to the characteristics of the variables under study (normality of the variables) and the qualitative variables by means of the χ2 or the exact Fisher test, depending on the sample size.
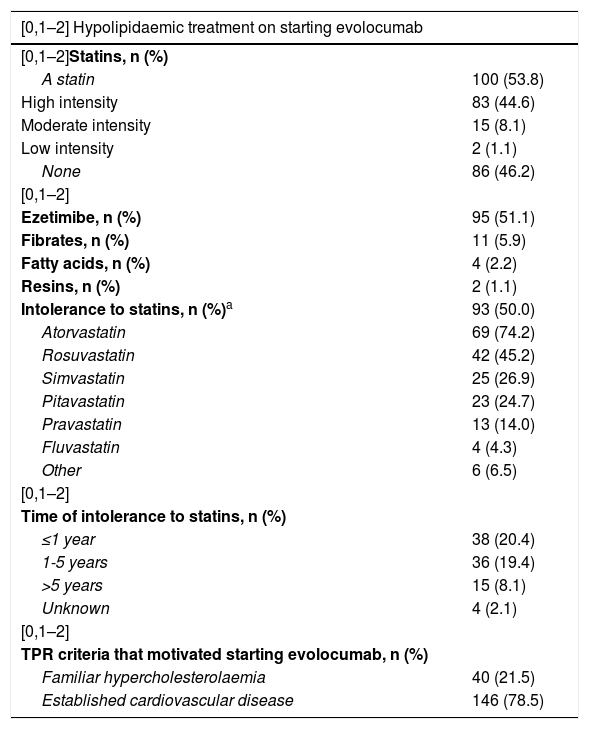
ResultsA total of 186 patients were included. The mean age was 60.3±9.8 years, 72.0% were male and the average body mass index was 28.5±4.3kg/m2. A total of 35.5% of the patients had FH, 58.6% had high blood pressure, 26.4% diabetes and 94.1% a previous cardiovascular event. The mean LDL-C before treatment was 144.0±49.0mg/dL (Table 1). Hypercholesterolaemia was chiefly diagnosed by cardiology (59.7%), followed by primary care (28.0%). Compared to patients without FH, the patients with FH were younger (61.9±8.8 vs. 57.6±10.9 years; p=.004), had less high blood pressure (65.0 vs. 47.0%; p=.01) and less diabetes mellitus (33.3 vs. 13.6%; p=.004). Compared to patients in primary prevention, patients in secondary prevention were more frequently male (45.5 vs. 73.7%; p=.04) and had less FH (81.8 vs. 25.7%; p<.01) (Table 2).
Baseline patient characteristics.
| Variable | Total (n=186) |
|---|---|
| Biodemographic characteristics | |
| Age (years) n (%) | 60.3±9.8 |
| ≤65 years | 122 (65.6) |
| 65-75 years | 54 (29.0) |
| >75 years | 10 (5.4) |
| Sex (male), n (%) | 134 (72.0) |
| Employment status, n (%) | |
| Active | 71 (38.2) |
| Unemployed | 9 (4.8) |
| Pensioner | 75 (40.3) |
| Other | 31 (16.7) |
| Non-familiar hypercholesterolaemia, n (%) | 120 (64.5) |
| Familiar hypercholesterolaemiaa, n (%) | 66 (35.5) |
| Heterozygous | 36 (19.4) |
| Homozygous | 4 (2.2) |
| Indeterminate | 26 (14.0) |
| Time since diagnosis of familiar hypercholesterolaemia (years) | 10.7±10.9 |
| Primary prevention (familiar hypercholesterolaemia), n (%) | 11 (5.9) |
| Secondary prevention, n (%) | 175 (94.1) |
| Baseline LDL cholesterol (mg/dL) n (%) | 144.0±49.0 |
| <100 | 15 (8.1) |
| ≥100-<130 | 72 (38.7) |
| ≥130-<160 | 52 (28.0) |
| ≥160-<190 | 23 (12.4) |
| ≥190 | 24 (12.9) |
| [0,1–2] | |
| Cardiovascular risk factors | |
| High blood pressure, n (%) | 109 (58.6) |
| Time since diagnosis of high blood pressure (years) | 8.5±6.3 |
| Diabetes mellitus, n (%) | 49 (26.4) |
| Type 2 | 47 (25.3) |
| Type 1 | 2 (1.1) |
| Time since diagnosis of diabetes (years) | 8.6±10.4 |
| Smoker, n (%) | |
| Never smoked | 59 (31.7) |
| Ex-smoker | 111 (59.7) |
| Active | 16 (8.6) |
| Family history of cardiovascular disease, n (%) | 91 (48.9) |
| [0,1–2] | |
| [0,1-2]Vascular disease | |
| Ischaemic heart disease, n (%) | |
| Angina | 82 (44.1) |
| Myocardial infarction | 122 (65.6) |
| Time since angina n (%) | 7.2±7.3 |
| >2 years | 54 (65.9) |
| Time since infarction n (%) | 7.3±6.7 |
| >2 years | 90 (73.8) |
| Heart failure, n (%) | 19 (10.2) |
| Peripheral artery disease, n (%) | 16 (8.6) |
| Chronic kidney disease, n (%) | 16 (8.6) |
| Carotid disease, n (%) | 10 (5.4) |
| Stroke, n (%) | 9 (4.8) |
| [0,1–2] | |
| [0,1–2]Physical examination | |
| Body mass index (kg/m2) n (%) | 28.5±4.3 |
| Underweight (<18,5) | 1 (.5) |
| Normal weight (18,5-24,9) | 19 (10.2) |
| Overweight (grade I or II) (25,0-29,9) | 47 (25.3) |
| Grade I obesity (30,0-34,9) | 26 (14.0) |
| Grade II obesity (35,0-39,9) | 6 (3.2) |
| Morbid obesity (grade III or IV) (≥40) | 1 (.5) |
| No data | 86 (46.2) |
| Heart rate (lpm) | 66.1±10.3 |
| Systolic blood pressure (mmHg) | 131.6±17.5 |
| Diastolic blood pressure (mmHg) | 76.6±11.3 |
Baseline characteristics of patients according to the presence of familial hypercholesterolaemia or whether primary or secondary prevention.
| Variable | Total (n=186) | Non-familial hypercholesterolaemia (n=120) | Familial hypercholesterolaemia (n=66) | p | Primary prevention (n=11) | Secondary prevention (n=175) | p |
|---|---|---|---|---|---|---|---|
| [0,1–8]Biodemographic characteristics | |||||||
| Age (years) | 60.3±9.8 | 61.9±8.8 | 57.6±10.9 | .004 | 58.0±9.5 | 60.5±9.8 | .41 |
| Sex (male), n (%) | 134 (72.0) | 89 (74.2) | 45 (68.2) | .38 | 5 (45.5) | 129 (73.7) | .04 |
| Familial hypercholesterolaemia, n (%) | 66 (35.5) | 0 | 54 (81.8) | <.01 | 9 (81.8) | 45 (25.7) | <.01 |
| [0,1–8]Physical examination | |||||||
| Body mass index (kg/m2) | 28.5±4.31 | 28.3±4.1 | 28.7±4.6 | .54 | 28.4±3.3 | 28.5±4.4 | .94 |
| Heart rate (lpm) | 66.1±10.3 | 66.0±10.0 | 66.2±10.9 | .90 | 76.4±12.6 | 65.7±10.1 | .001 |
| Systolic blood pressure (mmHg) | 131.6±17.5 | 129.2±16.5 | 135.3±18.4 | .02 | 130.2±12.1 | 131.6±17.7 | .78 |
| Diastolic blood pressure (mmHg) | 76.6±11.3 | 76.5±10.0 | 76.7±13.2 | .91 | 80.4±7.1 | 76.4±11.4 | .25 |
| [0,1–8]Cardiovascular risk factors | |||||||
| High blood pressure, n (%) | 109 (58.6) | 78 (65.0) | 31 (47.0) | .01 | 4 (36.4) | 105 (60.0) | .12 |
| Diabetes mellitus, n (%) | 49 (26.4) | 40 (33.3) | 9 (13.6) | .004 | 1 (9.1) | 48 (27.4) | .18 |
| Smoker, n (%) | |||||||
| Never smoked | 59 (31.7) | 35 (29.2) | 24 (36.4) | 6 (54.5) | 53 (30.3) | ||
| Ex-smoker | 111 (59.7) | 76 (63.3) | 35 (53.0) | .38 | 5 (45.5) | 106 (60.6) | .35 |
| Active | 16 (8.6) | 9 (7.5) | 7 (10.6) | 0 | 16 (9.1) | ||
| Chronic kidney disease, n (%) | 16 (8.6) | 11 (9.2) | 5 (7.6) | .92 | 0 | 16 (9.1) | .67 |
*Includes patients diagnosed with familial hypercholesterolaemia (N=54) and patients diagnosed with combined familial hyperlipidaemia (N=12).
Regarding baseline hypolipidaemic treatment, 53.8% of patients were taking statins (44.6% high intensity, 8.1% moderate intensity statins and 1.1% low intensity) and 51.1% ezetimibe. Fifty percent had intolerance to statins (total or partial), atorvastatin most frequently (74.2%), followed by rosuvastatin (45.2%) and simvastatin (26.9%). The median duration of treatment with high-intensity statins was 778 days (range 288-1,520) and with ezetimibe 763 days (range 273-1,469) (Table 3).
Hypolipidaemic treatment on starting evolocumab and thereafter.
| [0,1–2] Hypolipidaemic treatment on starting evolocumab | |
|---|---|
| [0,1–2]Statins, n (%) | |
| A statin | 100 (53.8) |
| High intensity | 83 (44.6) |
| Moderate intensity | 15 (8.1) |
| Low intensity | 2 (1.1) |
| None | 86 (46.2) |
| [0,1–2] | |
| Ezetimibe, n (%) | 95 (51.1) |
| Fibrates, n (%) | 11 (5.9) |
| Fatty acids, n (%) | 4 (2.2) |
| Resins, n (%) | 2 (1.1) |
| Intolerance to statins, n (%)a | 93 (50.0) |
| Atorvastatin | 69 (74.2) |
| Rosuvastatin | 42 (45.2) |
| Simvastatin | 25 (26.9) |
| Pitavastatin | 23 (24.7) |
| Pravastatin | 13 (14.0) |
| Fluvastatin | 4 (4.3) |
| Other | 6 (6.5) |
| [0,1–2] | |
| Time of intolerance to statins, n (%) | |
| ≤1 year | 38 (20.4) |
| 1-5 years | 36 (19.4) |
| >5 years | 15 (8.1) |
| Unknown | 4 (2.1) |
| [0,1–2] | |
| TPR criteria that motivated starting evolocumab, n (%) | |
| Familiar hypercholesterolaemia | 40 (21.5) |
| Established cardiovascular disease | 146 (78.5) |
| [0,1–2]Hypolipidemic treatment after starting evolocumab | |
|---|---|
| [0,1–2]Statins, n (%) | |
| [0,1–2]Initiation | |
| Statins | 5 (2.7) |
| Moderate intensity statins | 4 (2.2) |
| Low intensity statins | 1 (.5) |
| [0,1–2]Dose reduction | |
| High density statins | 1/74 (1.4) |
| Moderate intensity statins | 0 |
| Low intensity statins | 0 |
| [0,1–2]Discontinuation | |
| High intensity statins | 10/74 (13.5) |
| Moderate intensity statins | 5/12 (41.7) |
| Low intensity statins | 0 |
| [0,1–2] | |
| [0,1–2]Ezetimib, n (%) | |
| Initiation | 3 (1.6) |
| Discontinuation | 22 (22.4) |
| [0,1–2]Evolocumab, n (%) | |
| Self-administered | 181 (97.3) |
| Dose 140mg | 186 (100) |
| Every 2 weeks | 181 (97.3) |
| Every 4 weeks | 5 (2.7) |
| Increase/discontinuation or temporary interruption of dose | 0 |
| Permanent discontinuation | 6 (3.2) |
| Voluntarily by the patient | 5 (2.7) |
| Myalgia | 1 (.5) |
| Adequate compliance | 153 (92.3) |
| Visits in the year following starting evolocumab | 1.8±1.5 |
a Intolerance ≥1 statin; TPR: therapeutic positioning report.
In all cases the dose used for evolocumab was 140mg, and in almost all it was administered every 2 weeks (97.3%). Throughout the follow-up, neither the drug dose nor the regimen was modified in any of the patients, and only in 6 patients (3.2%) was treatment stopped, in 5 cases at the request of the patient (without reporting side effects) and in one case due to myalgia (.5%), without demonstrating direct causality. Therapeutic compliance was adequate in most patients (92.3%). Regarding concomitant hypolipidaemic medication after starting treatment with evolocumab, after 12 weeks of treatment ezetimibe was discontinued in 22.4% of patients, high intensity statins in 13.5% and moderate intensity statins in 41.7%. The average number of visits in the following year after starting evolocumab was 1.8±1.5 (Table 3).
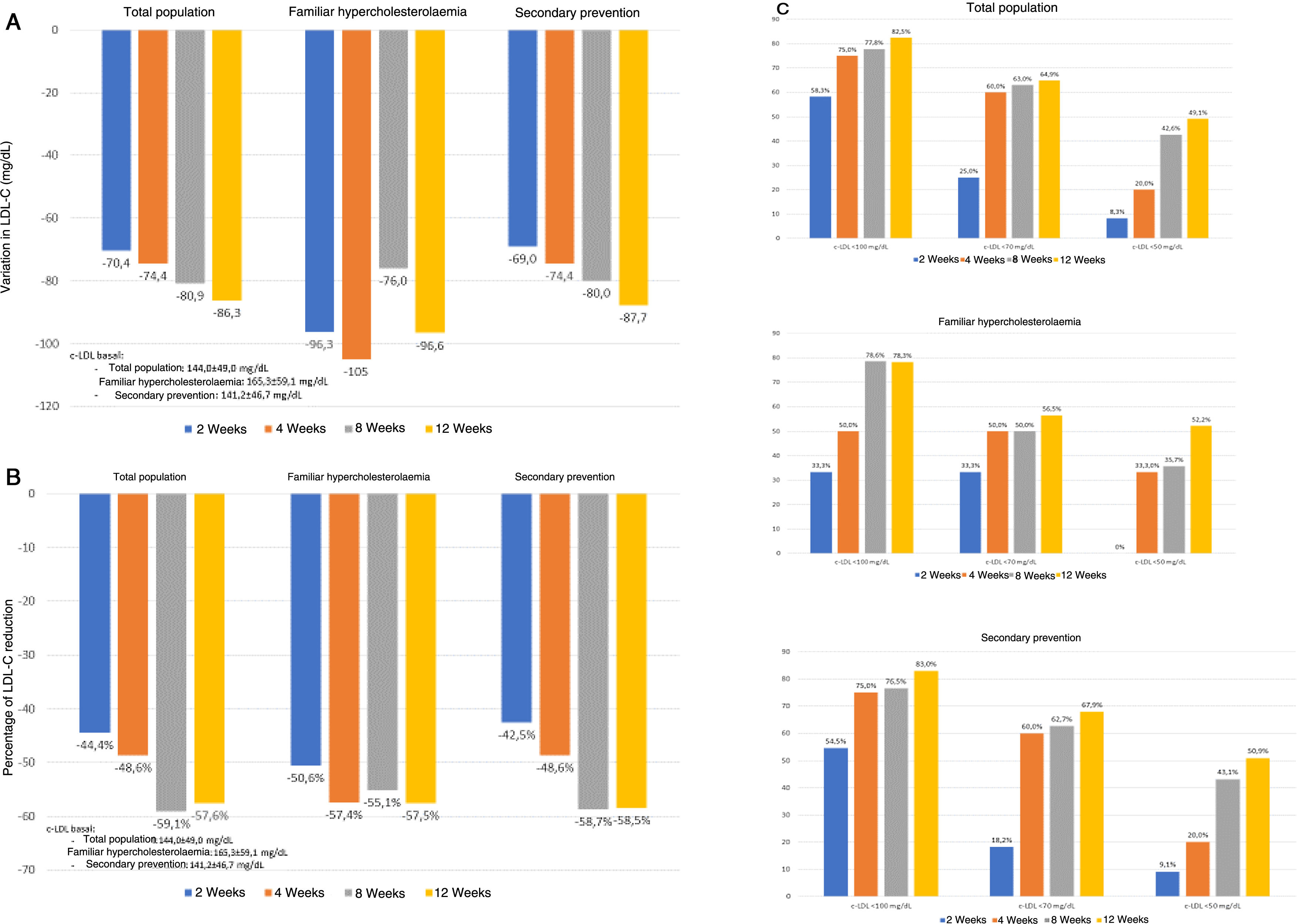
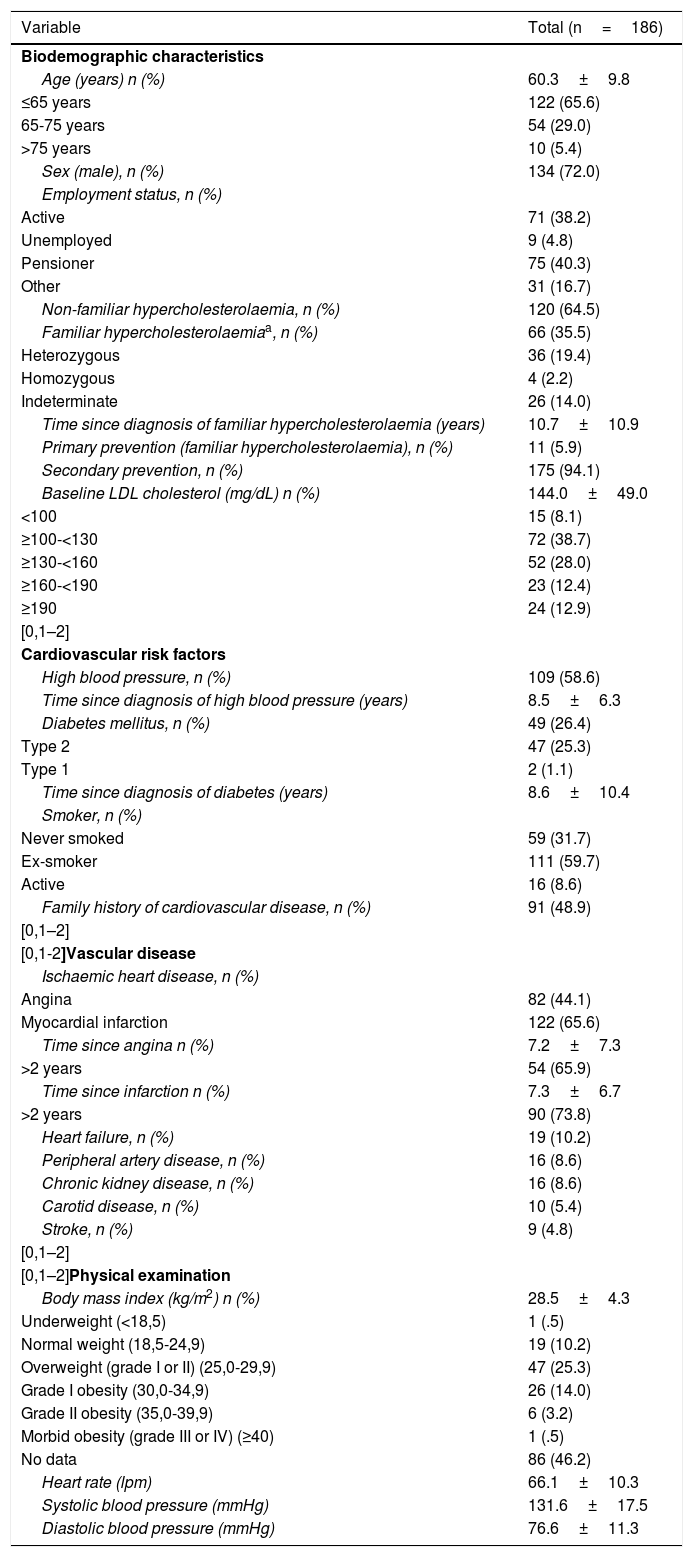
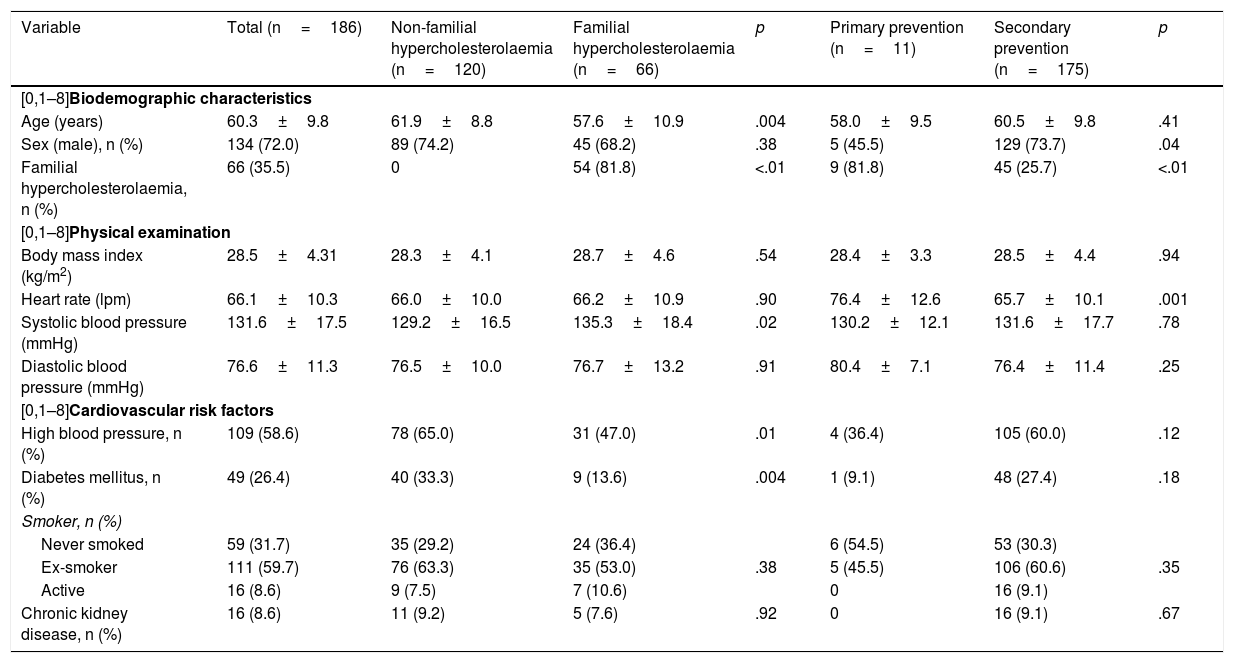
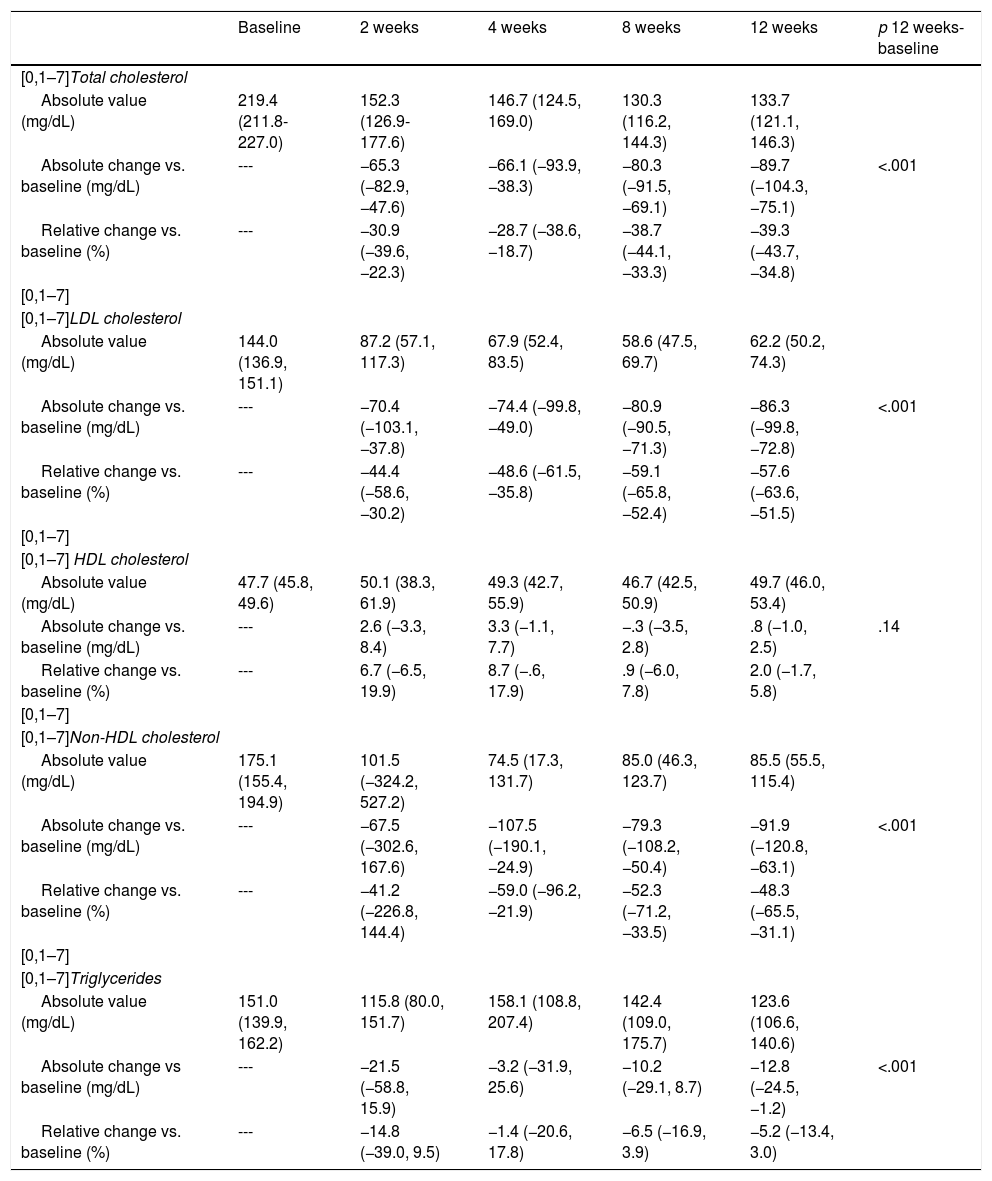
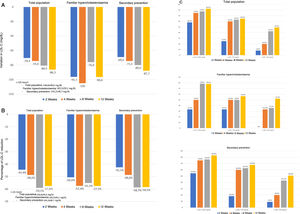
The changes in lipid profile during the treatment period are shown in Table 4 and Fig. 1. Treatment with evolocumab was associated with significant reductions in total cholesterol (30.9% at 2 weeks and 39.3% at 12 weeks; p<.001), LDL-C (44.4% and 57.6%, respectively; p<.001), non-HDL cholesterol (41.2% and 48.3%; p<.001) and triglycerides (14.8% and 5.2%; p<.001). HDL-C was not significantly modified during follow-up. At the end of follow-up, LDL-C < 100mg/dL, <70mg/dL, and <50mg/dL were 82.5%, 64.9%, and 49.1%, respectively. In the case of patients with FH, these percentages were 78.3%; 56.5% and 52.2%, respectively, and in secondary prevention, 83.0%; 67.9% and 50.9%, respectively.
Progression of total cholesterol levels, LDL cholesterol, HDL cholesterol, Non-HDL cholesterol and triglycerides during treatment with evolocumaba.
| Baseline | 2 weeks | 4 weeks | 8 weeks | 12 weeks | p 12 weeks-baseline | |
|---|---|---|---|---|---|---|
| [0,1–7]Total cholesterol | ||||||
| Absolute value (mg/dL) | 219.4 (211.8-227.0) | 152.3 (126.9-177.6) | 146.7 (124.5, 169.0) | 130.3 (116.2, 144.3) | 133.7 (121.1, 146.3) | |
| Absolute change vs. baseline (mg/dL) | --- | −65.3 (−82.9, −47.6) | −66.1 (−93.9, −38.3) | −80.3 (−91.5, −69.1) | −89.7 (−104.3, −75.1) | <.001 |
| Relative change vs. baseline (%) | --- | −30.9 (−39.6, −22.3) | −28.7 (−38.6, −18.7) | −38.7 (−44.1, −33.3) | −39.3 (−43.7, −34.8) | |
| [0,1–7] | ||||||
| [0,1–7]LDL cholesterol | ||||||
| Absolute value (mg/dL) | 144.0 (136.9, 151.1) | 87.2 (57.1, 117.3) | 67.9 (52.4, 83.5) | 58.6 (47.5, 69.7) | 62.2 (50.2, 74.3) | |
| Absolute change vs. baseline (mg/dL) | --- | −70.4 (−103.1, −37.8) | −74.4 (−99.8, −49.0) | −80.9 (−90.5, −71.3) | −86.3 (−99.8, −72.8) | <.001 |
| Relative change vs. baseline (%) | --- | −44.4 (−58.6, −30.2) | −48.6 (−61.5, −35.8) | −59.1 (−65.8, −52.4) | −57.6 (−63.6, −51.5) | |
| [0,1–7] | ||||||
| [0,1–7] HDL cholesterol | ||||||
| Absolute value (mg/dL) | 47.7 (45.8, 49.6) | 50.1 (38.3, 61.9) | 49.3 (42.7, 55.9) | 46.7 (42.5, 50.9) | 49.7 (46.0, 53.4) | |
| Absolute change vs. baseline (mg/dL) | --- | 2.6 (−3.3, 8.4) | 3.3 (−1.1, 7.7) | −.3 (−3.5, 2.8) | .8 (−1.0, 2.5) | .14 |
| Relative change vs. baseline (%) | --- | 6.7 (−6.5, 19.9) | 8.7 (−.6, 17.9) | .9 (−6.0, 7.8) | 2.0 (−1.7, 5.8) | |
| [0,1–7] | ||||||
| [0,1–7]Non-HDL cholesterol | ||||||
| Absolute value (mg/dL) | 175.1 (155.4, 194.9) | 101.5 (−324.2, 527.2) | 74.5 (17.3, 131.7) | 85.0 (46.3, 123.7) | 85.5 (55.5, 115.4) | |
| Absolute change vs. baseline (mg/dL) | --- | −67.5 (−302.6, 167.6) | −107.5 (−190.1, −24.9) | −79.3 (−108.2, −50.4) | −91.9 (−120.8, −63.1) | <.001 |
| Relative change vs. baseline (%) | --- | −41.2 (−226.8, 144.4) | −59.0 (−96.2, −21.9) | −52.3 (−71.2, −33.5) | −48.3 (−65.5, −31.1) | |
| [0,1–7] | ||||||
| [0,1–7]Triglycerides | ||||||
| Absolute value (mg/dL) | 151.0 (139.9, 162.2) | 115.8 (80.0, 151.7) | 158.1 (108.8, 207.4) | 142.4 (109.0, 175.7) | 123.6 (106.6, 140.6) | |
| Absolute change vs baseline (mg/dL) | --- | −21.5 (−58.8, 15.9) | −3.2 (−31.9, 25.6) | −10.2 (−29.1, 8.7) | −12.8 (−24.5, −1.2) | <.001 |
| Relative change vs. baseline (%) | --- | −14.8 (−39.0, 9.5) | −1.4 (−20.6, 17.8) | −6.5 (−16.9, 3.9) | −5.2 (−13.4, 3.0) | |
The data are expressed as means (95% confidence interval).
This study analyses in a large sample the first patients who received evolocumab in cardiology units in Spain. In fact, the therapeutic positioning report was published in March 2016.21 The most relevant results show that evolocumab produced significant reductions in LDL-C, with few side effects, and that it achieved the control objectives for LDL-C in a high percentage of patients.
Although the results of randomised clinical trials with evolocumab are truly relevant,10 it is particularly important to know whether these results can be transferred to clinical practice. This is not a minor issue, as several studies have determined that, in both FH patients and secondary prevention, a significant percentage of patients with dyslipidaemia will require PCSK9 inhibitor therapy to achieve the LDL-C control objectives recommended in the guidelines.24,25 Our paper is interesting in that it is the first national 'real life' study with evolocumab conducted in our country.
The FOURIER study included patients with atherosclerotic cardiovascular disease, with an average age of 62.5 years, who often had other comorbidities. Regarding hypolipidaemic treatment, although approximately 69% of patients were taking high intensity statins and 30% moderate intensity, only about 5% were taking ezetimibe, with a baseline LDL-C of 92mg/dL.10 However, since only a percentage of patients in clinical practice meet the inclusion criteria of the FOURIER study,12 "real-life" studies are necessary to complete the information from clinical trials. In our study, the patients’ average age was 60 years, the vast majority had had a previous cardiovascular event (94%) and 35.5% had FH. Regarding previous hypolipidaemic treatment, 54% were taking statins (50% had total or partial intolerance to statins) and 51% were taking ezetimibe. The high figure for statin intolerance is probably because this is a selected population, as they were among the first patients to take evolocumab. Although the patients had been taking statins or ezetimibe for more than 2 years on average, the mean baseline cholesterol was 144mg/dL, far from the targets recommended by the clinical practice guidelines.3 In a recent retrospective study conducted in Spain with fewer than 100 patients treated with PCSK9 inhibitors (56% with evolocumab), the mean age was slightly younger (57 years), 82% had ischaemic heart disease, 56% were taking moderate-high intensity statins, and 52% ezetimibe. The baseline LDL-C was 159mg/dL.14 In another retrospective analysis of patients treated with PCSK9 inhibitors, approximately 35% of patients were not taking statins concomitantly.16 Although, as in these studies, the population group in which evolocumab is most frequently prescribed are subjects with ischaemic heart disease, followed by those with FH,14,16 in our paper there was a higher proportion of patients with FH, although most had prior cardiovascular disease, and who were not taking statins, most likely because our patients were the first to be treated with evolocumab in cardiology units in Spain. In any case, all these data indicate that, in clinical practice, there is a significant percentage of patients who do not tolerate statins or, at least, only at low-intermediate doses, and that despite the high risk they have of presenting a new cardiovascular event, treatment with evolocumab is often started at much higher LDL-C figures than recommended by the guidelines, and after waiting for probably too long with standard hypolipidaemic treatment, exposing patients to high risk and delaying the potential benefit of this type of drug.
According to the technical data sheet, 140mg of evolocumab every 2 weeks or 420mg once a month is indicated in patients with primary hypercholesterolaemia, mixed dyslipidaemia or established atherosclerotic cardiovascular disease; and in patients with homozygous FH, 420mg once a month which, if there is no adequate control after 12 weeks of treatment, can be titrated to 420mg every 2 weeks.26 In our study, the vast majority of patients received 40mg of evolocumab every 2 weeks (<3% every 4 weeks) without modifying the dose throughout follow-up. Therapeutic compliance was high (>92%) and only 2 patients discontinued the drug (.5%) due to adverse effects, in line with the results of clinical trials and other clinical practice studies.10,13,14,16,19,27–30 In a recent study of statin-intolerant patients with muscular problems, the tolerability of evolocumab was very good, with treatment persistence in 95% after 2 years of follow-up,30 indicating that evolocumab can also safely be used in patients who do not tolerate other lipid-lowering drugs.31 Therefore, this "real life" study, like previous studies, confirms the safety of evolocumab described in clinical trials in daily clinical practice. However, some studies have shown that in the long term there could be greater discontinuance with evolocumab, which would not be related to the incidence of adverse effects or with lower efficacy,18 but would be for other reasons, such as administrative barriers or lack of awareness of the importance of cholesterol reduction in the reduction of cardiovascular events.32–34
It is interesting to highlight that in our study, treatment with evolocumab did not imply a very significant increase in visits to the doctor (1.8 on average in the following year), and therefore, did not result in a relevant increase in the healthcare burden. In short, it is really important to make an effort to identify the patients who will benefit most from treatment with PCSK9 inhibitors, to ensure adequate long-term adherence and to overcome administrative obstacles through correct healthcare organisation.32,33,35–37
With regard to the effects on lipid profile, evolocumab was associated with very significant reductions in LDL-C, already observed early on (week 2), which persisted and even increased in the following weeks of treatment and reached nearly 60% at 12 weeks, both in patients with FH and in those in secondary prevention, with high control percentages. These figures are consistent with those observed in the FOURIER study,10 and in other studies in clinical practice,13,14,17,27 which endorses the high effectiveness of evolocumab in reducing LDL-C figures, regardless of the clinical context. It is noteworthy that, after treatment with evolocumab, there were 15 patients whose treatment with statins was discontinued, as the reduction in LDL-C would be less than if they had continued with them and, therefore, also the potential benefit, especially considering that no side effects due to marked decreases in LDL-C have been described.10
This study has some limitations that we must highlight. Primarily, the limitations of the retrospective design itself, without a control group. However, it is precisely this type of design that makes it possible to objectively determine how a certain therapeutic alternative is used in clinical practice, without the doctor or patient modifying their guidelines of action because they are participating in a clinical study. On the other hand, there was no imputation for the missing data and the analyses were based only on the observed data. For patients with incomplete data, the variables were included up to the last observation collected. However, the high number of patients included in the study significantly reduces this possible limitation. Finally, in order to avoid possible selection biases, patients from participating centres who started evolocumab between 1 February 2016 and 15 May 2017 (recruitment period) were included, with a maximum recruitment per centre of 10%, to ensure adequate representativeness of the sample at national level.
In conclusion, the RETOSS-CARDIO study is the first national registry of patients treated with evolocumab in hospital cardiology units in Spain, with a large and representative sample of patients. The main indication for the use of evolocumab was secondary prevention, followed by FH. Treatment with evolocumab showed very marked reductions in LDL-C during follow-up, with particularly good tolerability and high compliance with therapy. The data from our study confirm the results of several clinical trials on lipid metabolism, primarily the FOURIER study, in the population with dyslipidaemia treated in Spain’s different cardiology units.
FundingThe study was sponsored by Amgen España, who did not influence its development, and was endorsed by the Spanish Research Agency of the Spanish Society of Cardiology.
Conflict of interestsCecilia Roldán works at Amgen Spain, although this has not influenced the results of the study. The rest of the authors have received fees for papers/consultancy from Amge.
Please cite this article as: Barrios V, Escobar C, Arrarte V, García E, Fernández MR, Rincón LM, et al. Primer registro nacional sobre la efectividad y seguridad de evolocumab en la práctica clínica en pacientes atendidos en cardiología en España. Estudio RETOSS-CARDIO. Clin Investig Arterioscler. 2020. https://doi.org/10.1016/j.arteri.2020.05.002