PCSK9 is a pivotal molecule in the regulation of lipid metabolism. Previous studies have suggested that PCSK9 expression and its function in LDL receptor regulation could be altered in the context of diabetes. The aim was to assess PCSK9 plasma levels in patients with type 2 diabetes (T2DM) and other related metabolic disorders as well as its relation to the metabolomic profile generated by nuclear magnetic resonance (NMR) and glucose homeostasis.
MethodsThere were recruited a total of 457 patients suffering from T2DM and other metabolic disorders (metabolic syndrome (MetS), obesity and atherogenic dyslipidaemia (AD) and other disorders). Anamnesis, anthropometry and physical examinations were conducted, and vascular and abdominal adiposity imaging were carried out. Biochemical studies were performed to determine PCSK9 plasma levels 6 weeks after lipid lowering drug wash-out in treated patients. A complete metabolomic lipid profile was also generated by NMR. The rs505151 and rs11591147 genetic variants of PCSK9 gene were identified in patients.
ResultsThe results showed that PCSK9 levels are increased in patients with T2DM and MetS (14% and 13%; p<0.005, respectively). Circulating PCSK9 levels were correlated with an atherogenic lipid profile and with insulin resistance parameters. PCSK9 levels were also positively associated with AD, as defined by lipoprotein particle number and size. The rs11591147 genetic variant resulted in lower levels of circulating PCSK9 and LDL cholesterol (LDL-C).
ConclusionsPCSK9 plasma levels are increased in T2DM and MetS patients and are associated with LDL-C and other parameters of AD and glucose metabolism.
PCSK9 es una molécula clave en la regulación del metabolismo lipídico. Estudios previos sugieren que la expresión y función de PCSK9 entorno a la regulación del receptor LDL puede alterarse en la diabetes. El objetivo del estudio fue determinar los niveles circulantes de PCSK9 en pacientes con diabetes tipo 2 (DM) y otras enfermedades metabólicas y su relación con las lipoproteínas estudiadas mediante resonancia magnética nuclear (RMN) y la homeostasis de la glucosa.
MétodosSe estudiaron un total de 457 pacientes, afectos de DM y otras alteraciones metabólicas (síndrome metabólico [SMet], obesidad y dislipidemia aterogénica [DA] y otros). Se realizó anamnesis, antropometría, exploración física y estudio vascular de carótidas y adiposidad abdominal. Se realizó bioquímica incluyendo PCSK9 circulante (tras 6 semanas de lavado en pacientes con hipolipemiantes). Se estudió mediante RMN el perfil de lipoproteínas. Se determinaron las variantes genéticas rs505151 y rs11591147 del gen PCSK9.
ResultadosLos niveles circulantes de PCSK9 están aumentados en pacientes con DM y SMet (14 y 13%; p<0.005, respectivamente). Los niveles circulantes de PCSK9 se correlacionaron de forma positiva con el perfil lipídico aterogénico y parámetros de resistencia insulínica. Los niveles circulantes de PCSK9 también se asociaron positivamente a DA, definida mediante el número y el tamaño de lipoproteínas analizado mediante RMN. Los portadores de la variante genética rs11591147 mostraron niveles inferiores de PCSK9 plasmática y cLDL.
ConclusionesLos niveles circulantes de PCSK9 están aumentados en pacientes con DM y SMet junto con parámetros de DA y metabolismo de la glucosa, más allá del cLDL.
Proprotein Convertase Subtilisin Kexin 9 (PCSK9) is a circulating protein that is synthesized and secreted predominantly by hepatocytes and plays a pivotal role in regulating LDL receptor (LDLR) expression, thereby acting as the main regulator of LDL cholesterol (LDL-C) concentration in plasma,1,2 a key risk factor in the development of cardiovascular disease. PCSK9 binds to the extracellular domain of the LDLR, thereby promoting the degradation of the LDLR in the lysosomal pathway and leading to an increase in LDL-C level in plasma.3,4 Gain-of-function mutations in the PCSK9 gene confer an increase in PCSK9 activity, reducing the number of LDLRs in the cell membrane and leading to high LDL cholesterol levels in plasma that result in familial hypercholesterolemia and vascular disease acceleration.5–7 Loss-of-function mutations in PCSK9 produce the opposite results and have been associated with a reduction in carotid intima-media thickness (cIMT), calcium coronary score and overall coronary disease risk.8,9 The most important low frequency genetic variant associated with low circulating levels of PCSK9 is R46L (rs11591147). The minor allele of this polymorphism has a frequency of 1–2% among the European population,10 explaining only a minor fraction of the variation in circulating PCSK9 level in healthy subjects.
Recently, the effects of PCSK9 on metabolism beyond LDL have been actively studied. In particular, the association of PCSK9 level with insulin and glucose homeostasis remains controversial. Although some studies have shown a positive correlation between circulating PCSK9 and insulin and glucose levels,11,12 more recent work suggests that there is no association.13 In contrast, patients with type 2 diabetes exhibit a relationship between PCSK9, non-HDL cholesterol, and apolipoprotein B levels,13 suggesting a role for PCSK9 in the pathogenesis of atherogenic dyslipidaemia (AD). Additional results have suggested that diabetes impairs the effect of PCSK9 on LDLR activity.14
The regulatory mechanisms affecting PCSK9 expression are under investigation. It has been demonstrated that insulin induces hepatic PCSK9 expression in vivo in mice and rats and in vitro in human hepatocytes.15–17 It has been shown that SREBP2 regulates PCSK9 expression, but under certain conditions, SREBP1c can also bind to the SRE in the PCSK9 promoter.15,18 SREBP1c activation is regulated by fatty acids and insulin, thereby linking glucose and insulin metabolism to PCSK9 and cholesterol regulation. Moreover, the transcription factor HNF1A has been reported to activate PCSK9 transcription, providing new evidence for its association with glucose metabolism. No cases of PCSK9 nullizygosity have been reported in humans, and PCSK9-deficient mice have yielded contradictory results.19,20
Guardiola et al. has reported an association between circulating PCSK9 levels and AD in a subgroup of patients with high cardiovascular risk.21 In this study, we have examined the effect of two main Single Nucleotide Polymorphism (SNP) (rs505151, rs11591147) on circulating PCSK9 levels and clinical, vascular, biochemical parameters, as well as nuclear magnetic resonance (NMR)-assessed lipid profile, in patients with type 2 diabetes and related metabolic alterations.
Material and methodsStudy design and participantsFor this cross-sectional study, were recruited 457 consecutive individuals who were treated between 2010 and 2012 in the vascular medicine and metabolism unit of our University Hospital due to type 2 diabetes or related disorders, such as metabolic syndrome (MetS), obesity, and AD. Subjects with chronic lung, renal or liver disease, cancer, or any other serious disease were excluded. Patients on lipid-lowering drugs underwent a 6-week wash-out period (8 weeks if they were on fibrates). Anamnesis, anthropometry and physical examination data were recorded. The Hospital Ethical Committee approved the study, and all patients provided written consent to participate in the study.
Carotid intima-media thickness (cIMT) and arterial stiffnessThree hundred and twenty-seven subjects underwent a vascular study with MyLab 50 X-Vision ultrasound (Esaote, Italy). A 7.5MHz linear array and semiautomated software were used to measure cIMT in the far wall of both common carotid arteries. The cIMT mean was the average of 2 territories. Bifurcations and internal carotids were measured manually. Plaques were defined as focal structures that either encroached into the arterial lumen by at least 0.5mm or 50% of the surrounding cIMT value or that demonstrated a thickness >1.5mm, as measured from the media-adventitia interface to the intima-lumen interface, according to the Mannheim Carotid Intima-Media Thickness Consensus.22
Arterial wall functional properties with respect to the elasticity state were measured by ultrasonography using the MyLab X-60 (Esaote, Genova, Italy) with the QAS software system and an LA533 linear transducer (Quality Arterial Stiffness software) (n=121). The examination was performed according to standardized measurements.23 The augmentation index (AIx) and pulse wave velocity (PWV) were measured directly in the right and left common carotid arteries and were adjusted for arterial pressure, age, and gender to determine the pulse wave velocity as well as for heart rate and gender to determine the augmentation index. An average for Aix and PWV was calculated for both sides.
Body fat distributionBody fat distribution was assessed via MyLab 50 X-Vision ultrasonography (Esaote, Italy). The subcutaneous (SAT) and preperitoneal fat (VAT) thicknesses were measured by placing a 7.5-MHz linear array perpendicular to the skin on the epigastrium according to the ultrasound image review consensus.24 The thickness of the SAT is defined as the distance between the anterior surface of the linea Alba and the fat-skin barrier. The VAT extends from the anterior surface of the liver (left lobe) to the posterior surface of the linea Alba. Preaortic intraabdominal fat (PIF), as measured with a 3.5-MHz convex array, was measured as the distance between the anterior aortic wall and the posterior surface of the rectus abdominis muscle at 1–5cm above the umbilicus along the xipho-umbilical line.
Blood sample collection and storageA blood sample was obtained after overnight fasting. Plasma and serum aliquots were prepared and stored at −80°C in the BioBanc of our centre until further use. The cellular buffy coat was obtained, and the cells were stored at −80°C until DNA analyses were performed.
Standard biochemical analysesBiochemical parameters, lipids and apolipoproteins were measured using colorimetric, enzymatic and immunoturbidimetric assays (Spinreact, SA, Spain; Wako Chemicals GmbH, Germany; Polymedco, NY, US) adapted to a Cobas Mira Plus autoanalyzer (Roche Diagnostics, Spain). Circulating PCSK9 and insulin was measured using commercial ELISA kits (R&D Systems, MN, US and Mercodia AB, Uppsala, Sweden). Insulin resistance was estimated using the homeostasis model assessment index (HOMA-IR).25
2D NMR lipid profile evaluationThe Liposcale test was used in all samples. This advanced lipoprotein test is based on 2D diffusion-ordered 1H NMR spectroscopy. This method adds diffusion coefficients to classical NMR determinations to provide a direct measure of mean particle size and number.26
SNP selection and genotypingIn the present study were genotyped 2 SNPs; rs505151, an E670G polymorphism, and rs11591147, an R46L polymorphism, of the PCSK9 gene, that were selected from the International HapMap database (http://hapmap.ncbi.nlm.nih.gov/). These SNPs were selected due to previous reports supporting their association with PCSK9 level and subclinical atherosclerosis. rs11591147 is associated with lower levels of plasmatic PCSK9 and reduced subclinical atherosclerosis.8 rs505151 is associated with higher cIMT and its progression.6 Genomic DNA was extracted from peripheral leukocytes that had been isolated from anticoagulated venous blood using the QIAamp DNA Blood Kit (Qiagen Iberia SL, Madrid, Spain) according to the manufacturer's instructions. The two SNPs were genotyped on the Sequenom MassARRAY platform using the iPLEX Gold protocol as specified by the manufacturer (Sequenom Inc., San Diego, CA).27 The genotypes of 5 of the samples were confirmed using duplicate SEQUENOMH runs and showed 100% consistency. Genotyping was performed at the Spanish National Genotyping Centre.
Statistical analysesThe results are expressed as the mean±SD for normally distributed data, the median (interquartile range, IQR) for data that was not normally distributed, and the frequency for categorical data. The differences between groups were assessed using the t-test or ANOVA (for data that were normally distributed), the Mann–Whitney U-test or Kruskal–Wallis test (for data that were not normally distributed) or χ2 tests (for data that were collected as categorical variables). Correlations were performed using Spearman's test. Statistical analyses were performed using SPSS software (IBM SPSS Statistics, version 20.0). A p value of <0.05 was considered statistically significant in all analyses.
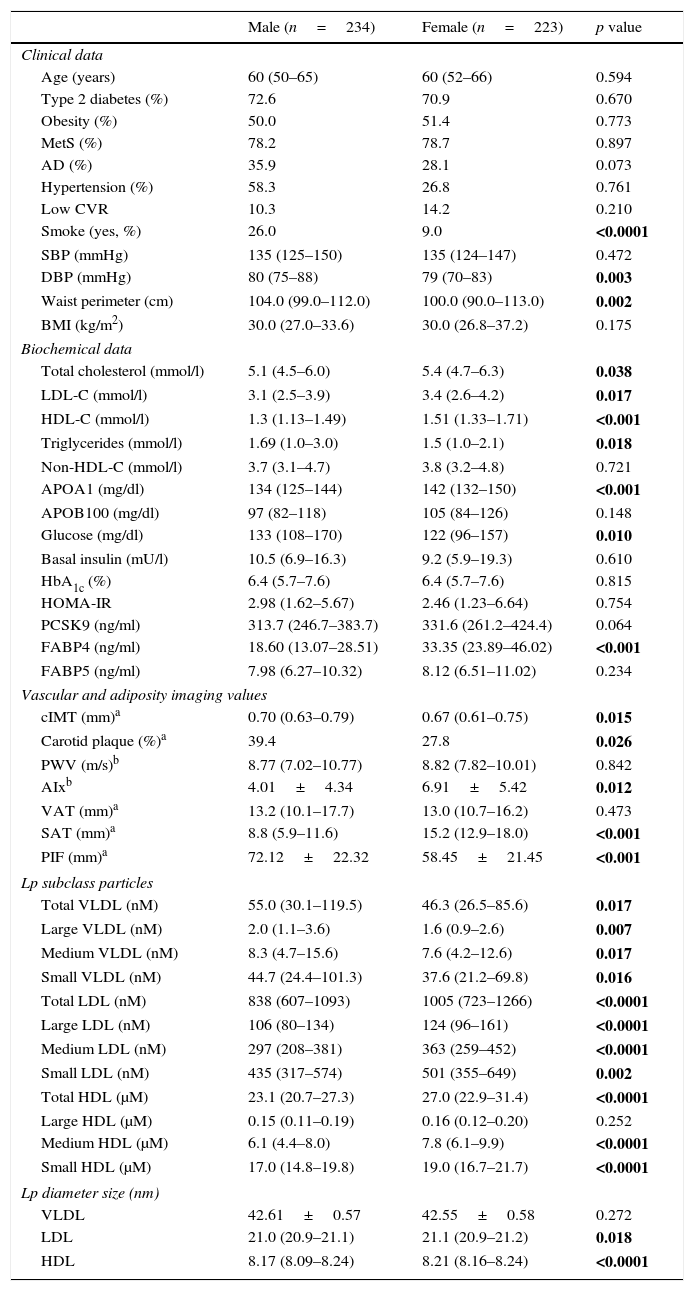
ResultsTable 1 summarizes the clinical, demographic, biochemical, vascular and adiposity imaging and lipoprotein NMR characteristics of patients by gender. We found a statistically significant increase in the incidence of smokers, waist circumference and diastolic blood pressure (DBP) in males (p<0.05). Regarding lipid and glucose parameters, females presented higher levels of total cholesterol, HDL cholesterol (HDL-C), LDL-C, triglycerides, Apo A1 and glucose with respect to males (p<0.05). Imaging revealed that, as expected, males presented higher cIMT, carotid plaque levels and arterial stiffness (p<0.05). The observed body fat distributions were in accord with the literature; males had a higher average PIF, while females had a higher average SAT (p<0.05). LDL and HDL size, as assessed by 2D-NMR, was lower in males, females had less total VLDL Lp particle number (p=0.017) and more total LDL and HDL Lp particle number (p<0.0001; p<0.0001, respectively).
Characteristics of the study group sorted by gender.
| Male (n=234) | Female (n=223) | p value | |
|---|---|---|---|
| Clinical data | |||
| Age (years) | 60 (50–65) | 60 (52–66) | 0.594 |
| Type 2 diabetes (%) | 72.6 | 70.9 | 0.670 |
| Obesity (%) | 50.0 | 51.4 | 0.773 |
| MetS (%) | 78.2 | 78.7 | 0.897 |
| AD (%) | 35.9 | 28.1 | 0.073 |
| Hypertension (%) | 58.3 | 26.8 | 0.761 |
| Low CVR | 10.3 | 14.2 | 0.210 |
| Smoke (yes, %) | 26.0 | 9.0 | <0.0001 |
| SBP (mmHg) | 135 (125–150) | 135 (124–147) | 0.472 |
| DBP (mmHg) | 80 (75–88) | 79 (70–83) | 0.003 |
| Waist perimeter (cm) | 104.0 (99.0–112.0) | 100.0 (90.0–113.0) | 0.002 |
| BMI (kg/m2) | 30.0 (27.0–33.6) | 30.0 (26.8–37.2) | 0.175 |
| Biochemical data | |||
| Total cholesterol (mmol/l) | 5.1 (4.5–6.0) | 5.4 (4.7–6.3) | 0.038 |
| LDL-C (mmol/l) | 3.1 (2.5–3.9) | 3.4 (2.6–4.2) | 0.017 |
| HDL-C (mmol/l) | 1.3 (1.13–1.49) | 1.51 (1.33–1.71) | <0.001 |
| Triglycerides (mmol/l) | 1.69 (1.0–3.0) | 1.5 (1.0–2.1) | 0.018 |
| Non-HDL-C (mmol/l) | 3.7 (3.1–4.7) | 3.8 (3.2–4.8) | 0.721 |
| APOA1 (mg/dl) | 134 (125–144) | 142 (132–150) | <0.001 |
| APOB100 (mg/dl) | 97 (82–118) | 105 (84–126) | 0.148 |
| Glucose (mg/dl) | 133 (108–170) | 122 (96–157) | 0.010 |
| Basal insulin (mU/l) | 10.5 (6.9–16.3) | 9.2 (5.9–19.3) | 0.610 |
| HbA1c (%) | 6.4 (5.7–7.6) | 6.4 (5.7–7.6) | 0.815 |
| HOMA-IR | 2.98 (1.62–5.67) | 2.46 (1.23–6.64) | 0.754 |
| PCSK9 (ng/ml) | 313.7 (246.7–383.7) | 331.6 (261.2–424.4) | 0.064 |
| FABP4 (ng/ml) | 18.60 (13.07–28.51) | 33.35 (23.89–46.02) | <0.001 |
| FABP5 (ng/ml) | 7.98 (6.27–10.32) | 8.12 (6.51–11.02) | 0.234 |
| Vascular and adiposity imaging values | |||
| cIMT (mm)a | 0.70 (0.63–0.79) | 0.67 (0.61–0.75) | 0.015 |
| Carotid plaque (%)a | 39.4 | 27.8 | 0.026 |
| PWV (m/s)b | 8.77 (7.02–10.77) | 8.82 (7.82–10.01) | 0.842 |
| AIxb | 4.01±4.34 | 6.91±5.42 | 0.012 |
| VAT (mm)a | 13.2 (10.1–17.7) | 13.0 (10.7–16.2) | 0.473 |
| SAT (mm)a | 8.8 (5.9–11.6) | 15.2 (12.9–18.0) | <0.001 |
| PIF (mm)a | 72.12±22.32 | 58.45±21.45 | <0.001 |
| Lp subclass particles | |||
| Total VLDL (nM) | 55.0 (30.1–119.5) | 46.3 (26.5–85.6) | 0.017 |
| Large VLDL (nM) | 2.0 (1.1–3.6) | 1.6 (0.9–2.6) | 0.007 |
| Medium VLDL (nM) | 8.3 (4.7–15.6) | 7.6 (4.2–12.6) | 0.017 |
| Small VLDL (nM) | 44.7 (24.4–101.3) | 37.6 (21.2–69.8) | 0.016 |
| Total LDL (nM) | 838 (607–1093) | 1005 (723–1266) | <0.0001 |
| Large LDL (nM) | 106 (80–134) | 124 (96–161) | <0.0001 |
| Medium LDL (nM) | 297 (208–381) | 363 (259–452) | <0.0001 |
| Small LDL (nM) | 435 (317–574) | 501 (355–649) | 0.002 |
| Total HDL (μM) | 23.1 (20.7–27.3) | 27.0 (22.9–31.4) | <0.0001 |
| Large HDL (μM) | 0.15 (0.11–0.19) | 0.16 (0.12–0.20) | 0.252 |
| Medium HDL (μM) | 6.1 (4.4–8.0) | 7.8 (6.1–9.9) | <0.0001 |
| Small HDL (μM) | 17.0 (14.8–19.8) | 19.0 (16.7–21.7) | <0.0001 |
| Lp diameter size (nm) | |||
| VLDL | 42.61±0.57 | 42.55±0.58 | 0.272 |
| LDL | 21.0 (20.9–21.1) | 21.1 (20.9–21.2) | 0.018 |
| HDL | 8.17 (8.09–8.24) | 8.21 (8.16–8.24) | <0.0001 |
Data are expressed as the mean±SD for normally distributed data, as the median (IQR) for non-normally distributed data, or as a percentage for categorical variables. The statistical tests used were ANOVA (for data that was normally distributed), Kruskal–Wallis (for data that were not normally distributed) or χ2 (for data gathered as categorical variables). MetS, metabolic syndrome; AD, atherogenic dyslipidaemia; Low CVR, low cardiovascular risk; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; HbA1c, glycosylated haemoglobin; LDL-C, low density lipoprotein; HDL-C, high density lipoprotein; cIMT, carotid intima-media thickness; PWV, pulse wave velocity; AIx, augmentation index; VAT, visceral fat; SAT, subcutaneous fat; PIF, preaortic intraabdominal fat; Lp, lipoprotein particle.
The p value in bold indicates that there is statistical significance (p<0.05).
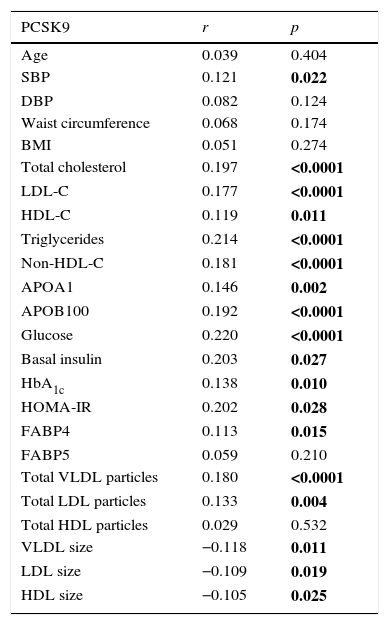
Table 2 shows the binary correlations between circulating PCSK9 and anthropometric, clinical, biochemical data, vascular and adiposity imaging, and 2D-NMR lipoprotein profile. The results show a positive and significant correlation between circulating PCSK9 and lipid and glucose homeostasis parameters, including total cholesterol, LDL-C, HDL-C, triglycerides, non-HDL-C, ApoA1, ApoB100, glucose, basal insulin, HbA1c and HOMA-IR (p<0.05). The PCSK9 levels were positively correlated with FABP4 (r=0.113, p=0.015) but not with FABP5. Significant positive correlations were observed between circulating PCSK9 and total VLDL and LDL Lp particle number (r=0.180, p<0.0001; r=0.133, p=0.004, respectively). In contrast, circulating PCSK9 levels were negatively correlated with VLDL, LDL and HDL Lp size (r=−0.118, p=0.011; r=−0.109, p=0.019; r=−0.105, p=0.025, respectively). In the studied population, there is no significant correlation between circulating PCSK9 concentration and vascular or adiposity imaging values.
Spearman's correlation of metabolic, lipid and metabolomic variables with PCSK9 plasma levels in all populations.
| PCSK9 | r | p |
|---|---|---|
| Age | 0.039 | 0.404 |
| SBP | 0.121 | 0.022 |
| DBP | 0.082 | 0.124 |
| Waist circumference | 0.068 | 0.174 |
| BMI | 0.051 | 0.274 |
| Total cholesterol | 0.197 | <0.0001 |
| LDL-C | 0.177 | <0.0001 |
| HDL-C | 0.119 | 0.011 |
| Triglycerides | 0.214 | <0.0001 |
| Non-HDL-C | 0.181 | <0.0001 |
| APOA1 | 0.146 | 0.002 |
| APOB100 | 0.192 | <0.0001 |
| Glucose | 0.220 | <0.0001 |
| Basal insulin | 0.203 | 0.027 |
| HbA1c | 0.138 | 0.010 |
| HOMA-IR | 0.202 | 0.028 |
| FABP4 | 0.113 | 0.015 |
| FABP5 | 0.059 | 0.210 |
| Total VLDL particles | 0.180 | <0.0001 |
| Total LDL particles | 0.133 | 0.004 |
| Total HDL particles | 0.029 | 0.532 |
| VLDL size | −0.118 | 0.011 |
| LDL size | −0.109 | 0.019 |
| HDL size | −0.105 | 0.025 |
The p value in bold indicates that there is statistical significance (p<0.05).
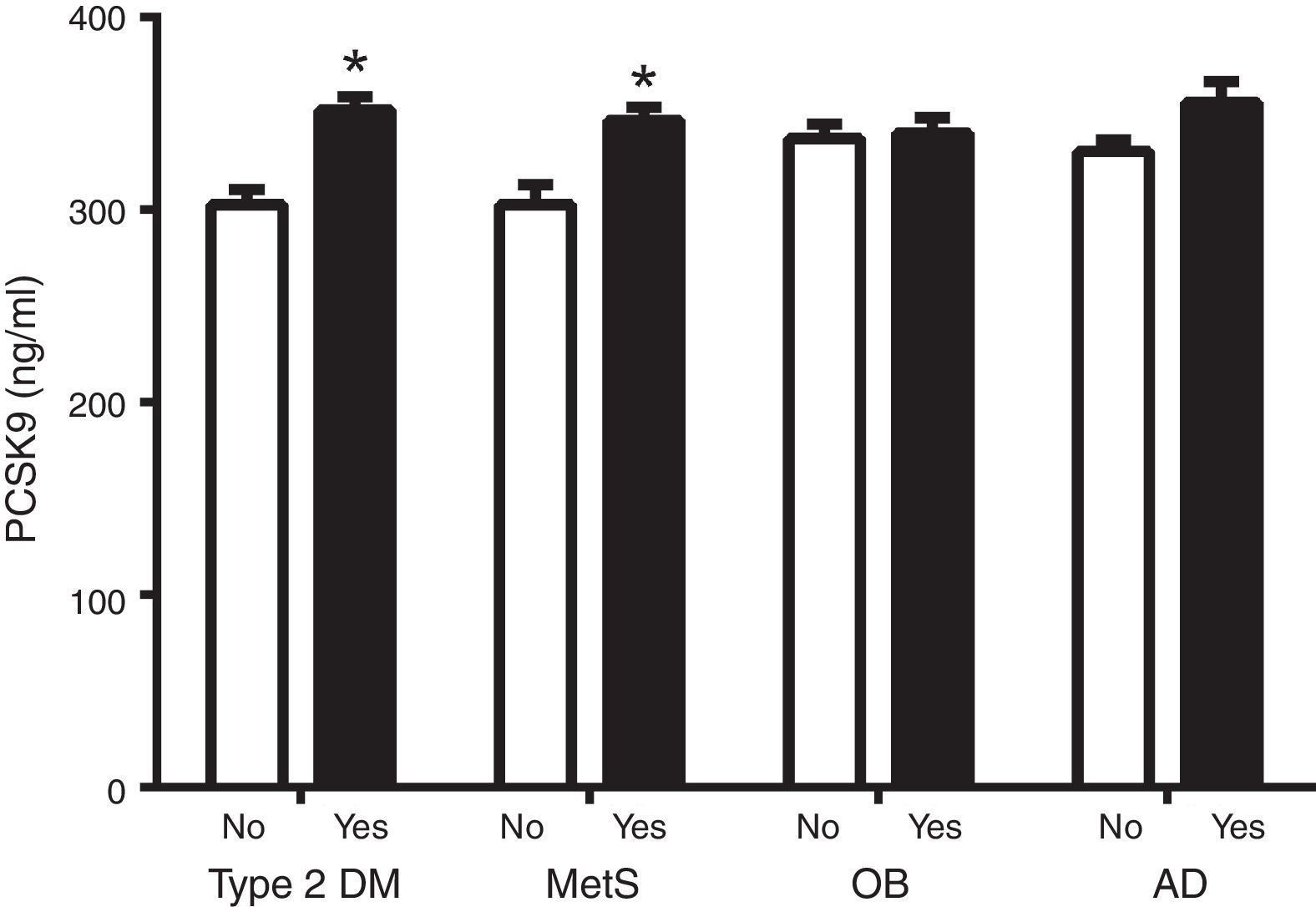
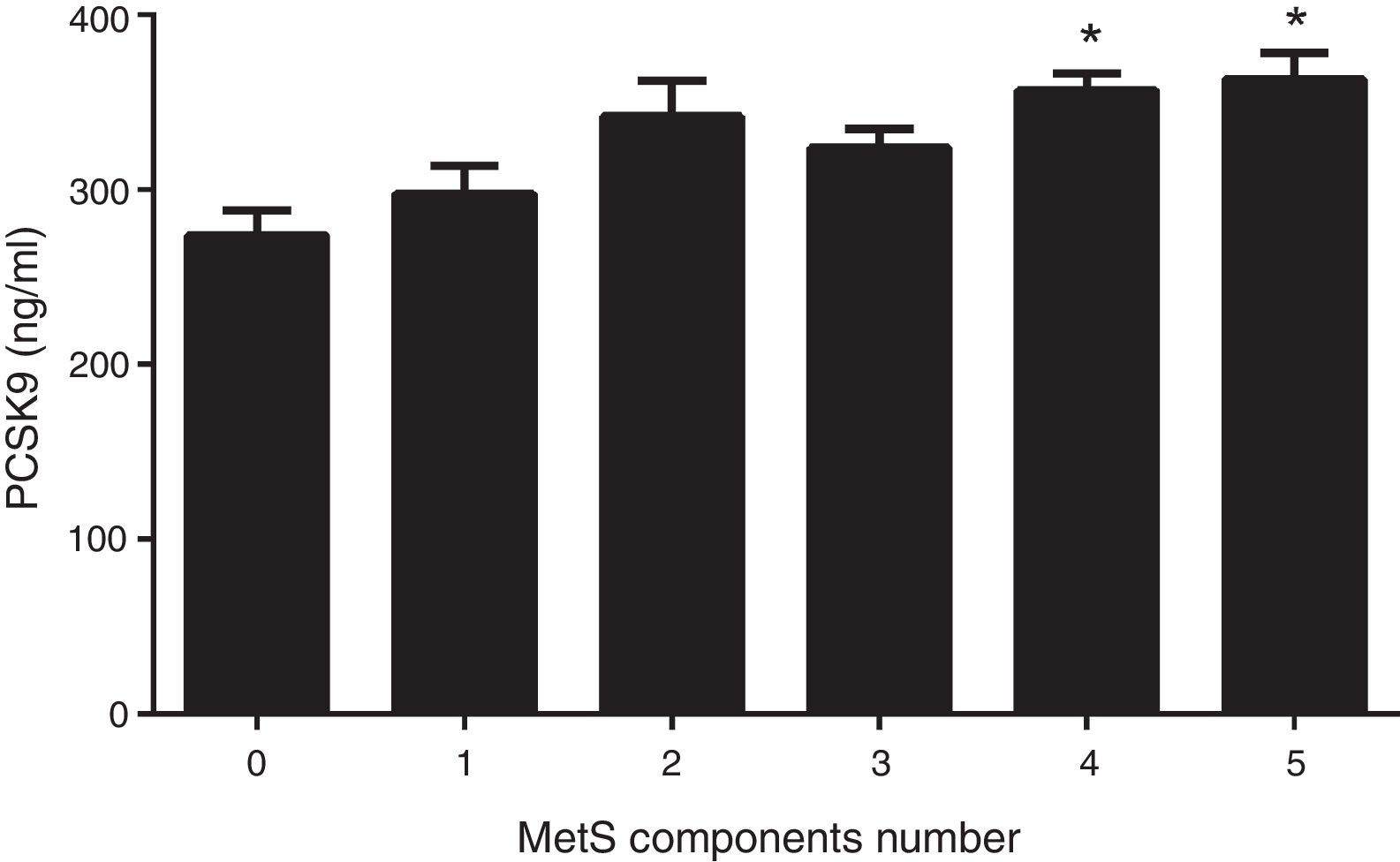
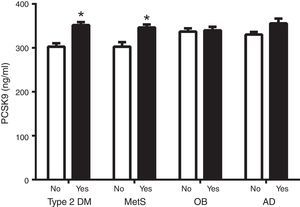
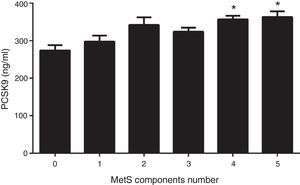
Fig. 1 shows circulating PCSK9 concentrations in the presence or absence of type 2 diabetes, MetS, obesity and AD. Circulating PSCK9 was higher in the presence of metabolic alterations, but only type 2 diabetes and MetS patients reached statistical significance (14% and 13%, respectively; p<0.005). Moreover, the PCSK9 level rose with an increase in MetS components, reaching statistical significance beyond 4 components. (p<0.05) (Fig. 2).
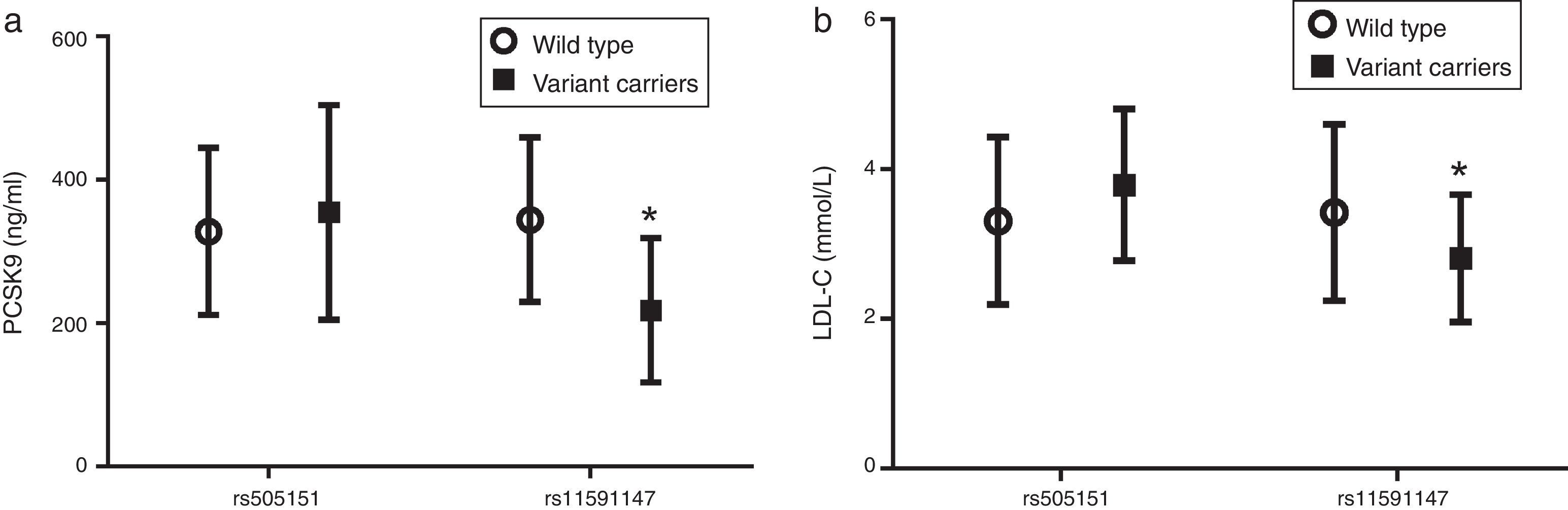
Fig. 3 shows that carriers of the rare variant rs11591147 have significantly lower levels of both circulating PCSK9 (Fig. 3a) and LDL-C (Fig. 3b) (p<0.05).
DiscussionIn this study, we have described the association between total PCSK9 plasma level and clinical parameters in patients with type 2 diabetes or associated metabolic alterations. The main observation is that PCSK9 concentration is associated not only with LDL-C values, but with MetS and type 2 diabetes. Despite a lack of correlation with BMI, PCSK9 concentrations are higher in patients with type 2 diabetes mellitus, AD and MetS. Moreover, a higher number of MetS components were correlated with higher PCSK9 concentrations. The association of PCSK9 with triglycerides, non-HDL-cholesterol and apoB was reinforced by its correlation with the AD profile, as assessed by 2D-NMR. PCSK9 was positively associated with VLDL and LDL particle number and VLDL size, while it was negatively associated with VLDL, LDL and HDL size. These data suggest a pivotal role of PCSK9 on the lipid profile beyond LDL metabolism. This association is concordant with the effects observed on triglyceride levels in patients treated with PCSK9 inhibitors when a reduction on the above mentioned lipid has been communicated.28 The positive correlation between HDL-C is paradoxical, and we cannot explain this result. The results strongly support an effect of PCSK9 on triglyceride rich lipoprotein (TRL). The mechanisms associated with this effect are not yet fully understood. It has been demonstrated that PCSK9 can interfere directly with apoB synthesis to modulate all apoB-containing particles.29 Moreover, the interaction of PCSK9 with VLDL receptors has also been suggested.30 However, in this work there are a correlation between PCSK9 level and insulin metabolism: higher PCSK9 levels are linked to higher insulin, glucose, HbA1c and HOMA levels. Taking into account the impact of insulin on TRL metabolism, it cannot exclude an insulin-mediated effect of PCSK9 on these particles. The correlation between glucose and insulin metabolism and PCSK9 is the subject of intense debate.11,12 Here, the results show that diabetic patients exhibit higher PCSK9 levels. The mechanism underlying this association is not clear, but it is known that the PCSK9 gene is regulated by the SREBP1c and HNF1a transcription factors, both of which are involved in insulin metabolism.15,18 Interestingly, PCSK9 levels were correlated to FABP4 concentrations which is considered an emerging biomarker of adiposity related alterations including type 2 diabetes, MetS and hypertriglyceridemia as AD component. In our hands, PCSK9 concentration did not serve as a subclinical arteriosclerosis marker in this population. Its concentration was not associated with cIMT values, nor did patients with increased cIMT exhibit different PCSK9 levels. This observation could be in conflict with genetic data showing that individuals wearing PCSK9 loss-of-function mutation have less subclinical atherosclerosis. Previous statin treatment could explain in part our data. Also interesting was the lack of association between PCSK9 and the adiposity indexes. PCSK9 did not differ significantly according to BMI or sonography-assessed fat distribution. These data are not surprising, given that PCSK9 is not expressed in adipose tissue.
Finally, the results of the present study corroborate the impact of loss-of-function mutations on PCSK9 and LDL concentrations, suggesting that these gene variants contribute to common lipid profiles.
The study has several limitations. The cross-sectional design precludes extrapolating any causality association. The sample size is relatively small particularly for genetic studies but the significant results are strengthened. We studied a metabolically altered population, so the extrapolation of these data to other groups must be done with caution. In the present study, PCSK9 levels are total and not free fraction. Moreover, although the patients were washout for lipid lowering treatment we cannot exclude a residual impact on PCSK9 after the washout period.
ConclusionPCSK9 concentrations are elevated in patients with MetS and type 2 diabetes. Its concentration is associated with insulin and glucose levels and AD profile as assessed by NMR, suggesting that PCSK9 is highly correlated with insulin metabolism, thereby modulating its impact on lipid profile.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
FundingsThis study was funded in part by CIBERDEM and FIS-PI11/02216 and FEDER founds.
Contribution statementThe authors’ responsibilities were as follows: DI, NP, RF and LM designed the study; DI, JG, NP, RF and LM conducted research; JG, AC, SG performed the biochemical analyses; NA and RM performed NMR analyses; DI, JG and LM performed the statistical tests and wrote the final manuscript. All authors have read and approved the final manuscript.
Conflict of interestThe authors declared no conflict of interest.
This work was supported by grants from the Spanish Atherosclerosis Society. PCSK9 como marcador del patrón metabolómico asociado a la dislipemia aterogénica. Relación con la arteriosclerosis subclínica. Beca FEA/SEA 2012 Investigación clínico-epidemiológica presented at the XXV SEA Congress, 2012. Spanish Atherosclerosis Society to Anna Cabré.