Chronic kidney disease represents a true inflammatory state, and is related to multiple cardiovascular risk factors. Coronary artery disease is the major complication, and has usually been associated with non-classical or uraemic related factors that include the disturbance of calcium and phosphorus metabolism, among others. Recent clinical evidence shows that specific body fat deposition like epicardial adipose tissue is an additional factor to consider when evaluating cardiovascular risk in the general population and kidney patients. Direct interaction of this tissue and coronary vessels with consequent mediation of pro-atherogenic substances have a local process ending in endothelial damage. Although the population of renal patients has been poorly evaluated, future studies should determine precisely whether an increase in epicardial fat is truly associated with cardiovascular morbidity and mortality in this risk group.
La enfermedad renal crónica representa un verdadero estado inflamatorio y está relacionada con múltiples factores de riesgo cardiovascular. La enfermedad arterial coronaria es una de sus principales complicaciones y usualmente ha sido asociada con factores de riesgo cardiovascular no clásicos o propios de pacientes urémicos como las alteraciones del metabolismo del calcio y el fósforo, entre otros. Evidencia clínica reciente muestra que el depósito de grasa órgano específico, como el tejido adiposo epicárdico, es un factor de riesgo adicional a tener en cuenta en el momento de la evaluación de riesgo cardiovascular en la población general y en los pacientes renales. La interacción directa de este tejido con los vasos coronarios y la consecuente mediación de sustancias proaterogénicas generan un proceso local que termina en la producción de daño endotelial. Aunque la población de enfermos renales ha sido evaluada escasamente, estudios futuros determinarán con precisión si un incremento en la adiposidad epicárdica está verdaderamente asociado a la morbimortalidad cardiovascular en este grupo de riesgo.
Chronic kidney disease (CKD) is a true inflammatory condition and it is associated with multiple cardiovascular (CV) risk factors.1 In fact, patients with CKD are at significantly higher risk of suffering a CV event than individuals in the general population with normal kidney function.2 The main causes of CV complication in these patients are left ventricular hypertrophy and coronary arterial disease.3,4
Clinical evidence has shown that visceral adipose tissue (VAT) is an important CV risk marker.5,6 For example, there seems to be a connection between VAT and CV disease in diseases which are as common as metabolic syndrome, diabetes and arterial hypertension.7 Recognition that adipocytes are a type of cell with complex functioning and endocrine, paracrine and autocrine capacity has aroused interest in researching them in disciplines such as molecular biology, endocrinology, nephrology, cardiology and immunology. This makes it a fertile ground for scientific debate, given their broad heterogeneity in terms of location, functions, proteomics and metabolomics. In fact, these characteristics are of particular importance in VAT, where changes in function and volume are associated with greater cardiometabolic risk.8
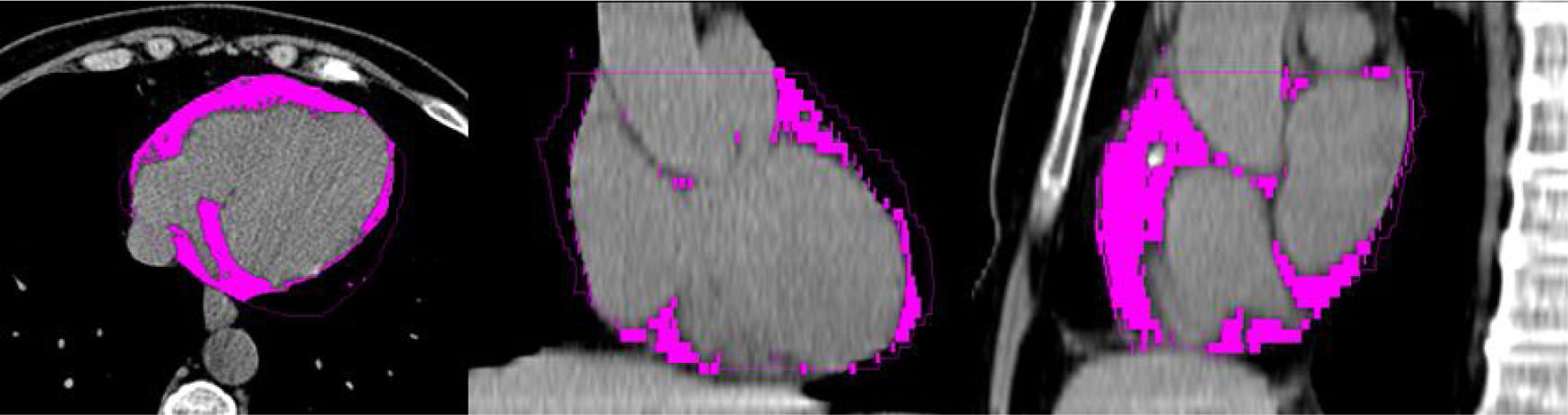
Epicardial adipose tissueAnatomy and functionDifferent regions of extra-abdominal adipose tissue have been studied in recent years. Thus epicardial adipose tissue (EAT) has been said to be a specific risk marker in coronary disease.9,10 More specifically, EAT represents the fat that is confined within the pericardial sac and located on the surface of the heart, covering the epicardial portion of the coronary arteries.11 It varies in volume within a range from 68cm3 to 124cm3, representing approximately from 15% to 20% of total cardiac volume and covering approximately 80% of the cardiac surface.10 In physiological terms it has mechanical, biochemical and heat-regulating functions.12 Nevertheless, under pathological conditions EAT affects the heart and coronary arteries locally by means of paracrine and vasocrine activity, with the secretion of proinflammatory cytokines.13–15
The EAT may be detected by means of different imaging techniques. Ultrasound scan was one of the first techniques used for this purpose, and it is both economical and rapid to use. Nevertheless, magnetic resonance imaging and computed axial tomography (CAT) are able to give a better and more suitable visualisation and measurement of the tissue, due to their high spatial resolution.16,17 Additionally, CAT makes it possible to simultaneously evaluate coronary arterial calcium (CAC).18,19Fig. 1 describes a cardiac CAT study, showing the EAT coloured.
Other cardiac fat depositsEAT must not be confused with another type of localised cardiac fat deposit: pericardial adipose tissue (PAT) which, unlike EAT, is located outside the visceral pericardium over the external surface of the parietal pericardium.20 Both tissues have different origins: EAT originates in the splacno pleural layer of the mesoderm, as does VAT, while PAT originates in the primitive thoracic mesenchyme; this is why local circulation too differs in both tissues; thus epicardial fat is irrigated by the coronary arteries (sharing the same microcirculation as the myocardium), while pericardial fat is irrigated by the pericardiophrenic branches of the internal mammary artery.12 Due to this close relationship between EAT, the myocardium and the coronary arteries, the volume and function of EAT has been linked to the genesis and progression of coronary atherosclerosis.
Epicardial adipose tissue and metabolismCurrent evidence indicates that it is possible that adipose tissue next to coronary vessel walls permits the diffusion or transport through the vasa vasorum of proinflammatory cytokines or adipocytokines produced by the adipocytes, such as tumour necrosis factor alpha, interleukin 6 (IL-6), leptin, resistin and visfatin, among others.14 As a whole these elements cause local endothelial dysfunction, with an increase in the lipotoxicity-mediated expression of adhesion and oxidative stress molecules towards the endoplasmatic reticule via overload of free fatty acids in the surrounding tissues, where hypertrigliceridemia and insulin resistance associated with obesity and metabolic syndrome degrade the conditions of this microenvironment.14,21 Finally, this process favours the binding and increased dwell time in the subendothelium of low-density lipoproteins and other proatherogenic ApoB lipoproteins, such as intermediate density lipoprotein and (a) lipoprotein. The oxidative and non-oxidative modifications of these proteins make the subendothelium a niche for immunocompetent cells such as macrophages, T cells, monocytes and modified smooth muscle cells. When these cells interact within this medium they give rise to the functional and morphological alterations that typify the atherosclerotic plaque.22,23
The possibility that EAT may activate myocardiocyte pathways that alter its proteomic pattern and in turn its endocrine secretor capacity is far more interesting. The discovery of natriuretic peptides therefore altered the view of the heart as a simple mechanical pump, showing it to be an organ that is able to modify its performance by interacting with the rest of the organism by producing these hormonal messengers. Three natriuretic peptides have been studied to date, including atrial natriuretic peptide (ANP, produced in the atria), type B natriuretic peptide B (BNP, produced in the ventricles) and type C natriuretic peptide (CNP, produced in the endothelial cells). In general, ANP and BNP are secreted in very small amounts, although some conditions (such as CKD), which involve an increase in the mechanical loading and stress of the wall due to ventricular hypertrophy, hypertension, inflammation, fibrosis and hypoxia, may stimulate the production and secretion of these mediators. High levels of both markers are therefore well-known cardiovascular risk markers.24–26
Classically, research in this area has concentrated on exploring the haemodynamic effects of these hormones on the heart-kidney axis. Nevertheless, in the last decade the discovery of receptors in the adipose tissue for natriuretic peptides has opened a productive area of study of hormonal signalling between these tissues and its metabolic effects. These effects go beyond the initial concept that adipose tissue solely intervenes in the removal of natriuretic peptides from the circulation. However, it was then found that adipose tissue expresses natriuretic peptide receptor-1 and 2 (NPR-1 and NPR-2) protein, which is able to interact with ANP as well as with BNP with a high degree of affinity, activating c-protein kinase G signalling via guanilate cyclase-GMP translocation, activating the hormone-sensitive lipase within the adipocyte and then lipolysis. On the other hand, the activation of the MAP kinase/p38 cascade causes an increase in the oxidisation of fatty acids and the activation of thermogenesis by induction of UCP1 synthesis.27 Thus the overload of fatty acids within adipose tissue may affect many tissues, such as hepatic tissue (stimulating the production of hepatic glucose), beta cells (apoptosis and alterations in the insulin secretion pattern), nerve tissue (altering the production and clearance of Tau proteins), striated muscle tissue (insulin resistance) and cardiomyocyte tissue (changing the energy consumption pattern, with higher O2 consumption, cardiotoxicity and increased arrythmogenesis).28
On the contrary, signalling by receptor NPR-3—the expression of which increases in type 2 diabetes and obesity—leads to internalisation and degradation of the natriuretic peptides, cancelling out any systemic beneficial effect of these hormones. In fact, a low concentration of ANP, BNP and CNP is associated with the presence of insulin resistance and diabetes.29
Epicardial adipose tissue and chronic kidney diseaseAlthough the literature which evaluates EAT in CKD is always developing, some studies have detected a connection between EAT and the presence of CAC in small groups of patients with kidney involvement. One of the first studies was undertaken in 80 patients with CKD under dialysis and 27 controls.30 They were tested to detect atherosclerosis-inflammation-malnutrition syndrome (AIM) by measuring serum levels of albumin and reactive C protein, while CAT and EAT were quantified by CAT. EAT was significantly associated with the components of AIM syndrome, and there was a proportional relationship between the increase in the volume of EAT and the presence of CAC. Kerr et al. studied 94 patients with grade 3–5 CKD who were not in dialysis, reporting a lineal correlation with increased EAT and CAC and including other cytokines, such as IL-6 and fibroblastic growth factor 23, among others.31 Another study in a cohort of 411 patients with grade 4–5 CKD in dialysis who were being evaluated for kidney transplant found that EAT is a risk factor for alterations in myocardial perfusion, as is the presence of CAC.19 EAT was found to be independently associated with CAC when predicting myocardial perfusion defects. Karatas et al. recently proved that the thickness of the EAT measured by echocardiograph is significantly greater in patients undergoing haemodialysis in comparison with those in pre-dialysis stages or healthy subjects.32 Likewise, using a sub-analysis of the RIND study (Renagel in new dialysis trial), which firstly studied the presence of CAC and calcium and phosphorus alterations in patients who were commencing treatment with dialysis, it was found that each 10cc increase in the volume of EAT increased mortality by 6%.33 Factors such as CAC and age were also associated with increased mortality. Other publications have reported similar findings.34–36 However, other researchers found that EAT is not an isolated risk predictor, but rather that it is a cofactor associated with the body mass index (BMI) and other obesity scales.37,38
In populations at risk and in patients with CKD, EAT is not only associated with CAC. Recent studies have shown that there is an association between EAT and extracoronary vascular calcifications in the aorta, heart valves and carotid arteries.35 A lineal correlation has also been reported between an increase in EAT volume, carotid intima-media thickness and left ventricular hypertrophy.39,40
Obesity, nutrition and the kidneysNutritional alterations are often found in patients with kidney disease. The impact of kidney disease on body composition is in itself a morbimortality factor in this population.41 The most outstanding factors which lead to morbimortality include the presence of diabetes, hypertension, dyslipidemia, chronic inflammatory states, a lack of protein and calories in the diet, immune dysfunction, the depletion of lean mass, micronutrient deficiency and a negative nitrogen balance, among others.42,43
Although obesity is known to be a CV risk factor and obese patients are at higher risk of suffering CKD and even progressing more swiftly to advanced CKD once they are under dialysis, paradoxically survival is longer the higher their BMI (inverse epidemiology).43–45 Although this phenomenon is contrary to what is observed in the general population, it has been observed in some risk groups (the elderly and patients with congestive heart failure).46,47 Possible explanations for these findings include protein-calorie deficiency, a reduction in nutritional reserves, metabolic acidosis and inflammation.48
More specifically, this paradox observed in patients with CKD is associated with PEW (protein-energy wasting) and inflammation.49 Thus patients who have a lower BMI or bodyweight may have a higher degree of PEW, and this would be the cause of increased morbimortality. On the contrary, overweight patients would have less deficiency in their ingestion of protein or energy and lower probability of developing PEW.50,51 Moreover, it is known that undernourished patients are more susceptible to the harmful effects of the inflammatory process.49
ConclusionsFinally, EAT is associated with cardiovascular risk in the general population as well as in kidney patients. It has yet to be determined whether, once CKD progresses to advanced stages or treatment with dialysis begins, changes are caused at EAT level which influence the prognosis of patients. Future studies will have to be designed to cover this interesting question.
- •
The risk of suffering a cardiovascular event is 10 times higher in chronic kidney patients than it is in the general population.
- •
Vascular calcifications consist of calcium deposits in the vascular intima and media; calcifications of the heart valves are also included within this denomination. Patients with CKD exhibit all of their forms.
- •
Epicardial adipose tissue is fat that is confined within the pericardial sac located on the surface of the heart; it covers the epicardial portion of the coronary arteries.
- •
Nutritional alterations are common in kidney patients. They run from severe protein-calorie deficiencies to chronic inflammatory conditions.
The authors have no conflict of interests to declare.
Please cite this article as: D’Marco L, Cortez M, Salazar M, Lima-Martínez M, Bermúdez V. Tejido adiposo epicárdico: un marcador de riesgo cardiovascular a evaluar en la enfermedad renal crónica. Clin Investig Arterioscler. 2020;32:129–134.