Heart failure (HF) is a growing medical problem and it is of interest to study new biomarkers for better characterisation. In this sense, nitric oxide, reactive oxygen species (ROS), NADPH, and superoxide dismutase (SOD) were evaluated, along with their possible predictive value in patients with HF. An analysis was also performed on the potential differences between patients with and without secondary pulmonary hypertension (SPH), considered to have a worse prognosis. A significant decrease of nitric oxide and SOD was noted in HF, whereas ROS and NADPH were increased. These results agree with the pathophysiological changes characteristic of HF. It was also demonstrated that in patients with HF and SPH that nitric oxide and SOD were decreased when compared to HF without SPH, whereas ROS and NADPH were increased. Therefore, our results suggest that nitric oxide, ROS, NADPH, and SOD, could be considered as possible markers in HF, and could also characterise patients with SPH.
La insuficiencia cardíaca (IC) es un creciente problema médico por lo que resulta de interés el estudio de nuevos biomarcadores para su mejor caracterización, y en este sentido, evaluamos productos metabólicos (nitritos y nitratos) del óxido nítrico, especies reactivas del oxígeno (ROS), NADPH y superóxido dismutasa (SOD) y su posible valor predictivo en pacientes con IC. Además, estudiamos potenciales diferencias entre pacientes con y sin hipertensión pulmonar secundaria (HPS), considerados de peor pronóstico. En IC, demostramos disminución significativa de los niveles plasmáticos de nitritos y nitratos y SOD, mientras que ROS y NADPH se encontraban incrementados. Estos resultados concuerdan con los cambios fisiopatológicos propios de la IC. También demostramos en pacientes con IC e HPS que los niveles plasmáticos de nitritos y nitratos así como SOD estaban disminuidos respecto a IC sin HPS, mientras que ROS y NADPH estaban aumentados. Por lo tanto, nuestros resultados indican que óxido nítrico, ROS, NADPH y SOD podrían ser considerados como posibles marcadores en IC y además permitirían caracterizar a los pacientes con HPS.
This study approached heart failure (HF) from the perspective of determining possible new biomarkers such as nitric oxide (NO) and other related substances, such as reactive oxygen species (ROS), NADPH oxidase and superoxide dismutase (SOD), at the time of the disease's evolution. It is specifically noted that we determined plasma levels of nitrites and nitrates as an index of systemic NO production; whilst when we studied ROS, this indirectly referred to the sum of known products as superoxide and hydroxyl radicals.
Of particular interest is the fact that HF is a growing medical, health and socioeconomic problem, primarily in developing countries, and which was clearly defined over two decades ago by Braunwald (1992) as the “pathophysiological state in which an abnormality of cardiac function is responsible for the failure of the heart to pump blood at a rate commensurate with the requirements of the metabolising tissues”. Moreover, recent years have seen the introduction of elements that contribute to the diagnosis and/or prognosis of this disease. Thus, the aforementioned biomarkers are defined as biological variables that provide information on specific diseases. In this sense, and paying particular attention to HF, many molecules are studied, including neurohumoral and inflammatory markers, as well as markers of oxidative stress, interstitial matrix remodelling and myocyte damage.1 The most significant neurohumoral systems evaluated to date in HF, published by Richards in 20091 and Braunwald,2 include the renin–angiotensin–aldosterone system, adrenergic nervous system, arginine vasopressin and endothelin-derived peptides.
However, the use of these markers and the drugs indicated in clinical practice is costly and difficult-to-access for patients, particularly in the field of public health. Conversely, and of interest to this article, the NO system, which plays an important role in the development of HF syndrome, has not been sufficiently evaluated from the perspective of it constituting a potential biomarker, and it therefore remains a topic pending further study. In light of the above, a series of NO signalling pathway markers have been proposed, but their clinical utility has proven limited as the techniques have not been optimised.
In this context, our working group set out to assess NO in chronic HF (CHF) by means of determining its metabolic end products: nitrates (NO3−) and nitrites (NO2−); we also determined two of the most relevant ROS, these being hydroxyl (HO) and superoxide anion (O2). We also studied the activity of NADPH oxidase and SOD enzymes.
In CHF, an endothelial dysfunction develops that particularly compromises the peripheral arteries, thus rendering the vessel unable to dilate in response to the increased blood flow. In this sense, the complete mechanism behind this phenomenon is not fully understood. However, three hypotheses3 have been put forward in an attempt to explain it, including the reduced availability of the substrate for nitric oxide synthase, reduced NO synthesis and the extracellular inactivation of NO by superoxide anion. As such, NO plays a pivotal role.
In patients with chronic heart failure, an increase in oxygen free radical production has been demonstrated, particularly superoxide anion, thus leading to significant oxidative stress characterised by a relative ROS excess in relation to endogenous defence mechanisms. In this respect, there is abundant evidence that has demonstrated the role of ROS and reactive nitrogen species on the physiopathology of cardiovascular disease, including HF.3 In recent years, various markers of oxidative stress have been assessed as predictors of functional impairment. Of particular interest, the deterioration of NO signalling pathways has also been characterised recently, associated in some cases with a worse prognosis and mortality. Nevertheless, its clinical utility has proven limited.4
Of relevance, greater O2 production would lead to NO inactivation, giving rise to peroxynitrites, which although less toxic than O2, would also cause several forms of endothelial dysfunction, including a decrease in NO bioavailability and thus vasodilation.5 Moreover, increased activity of the NADPH oxidase enzyme is one of the most efficient ROS-producing systems in HF. NADPH oxidase activity is also greatly involved in inducing apoptosis as a result of high O2 production. Thus, cell damage occurs due to oxidative stress, among other variables, when ROS accumulate in excess and overwhelm the cellular defence mechanisms. The SOD enzyme, on the other hand, catalyses O2 disproportionation in oxygen and hydrogen peroxide.6 This mechanism justifies its important participation in antioxidant defence in the majority of oxygen-exposed cells. This metabolic pathway has not yet been sufficiently analysed as a provider of biomarkers in this disease.
In this respect, various neurohumoral systems have been investigated to identify biomarkers as candidates for being good predictors, but few meet the clinical utility criteria and also prove costly. Thus the need arises to find new markers to make a breakthrough contribution to the set that have already been studied.
As such, the central hypothesis of this paper arises from the assumption that plasma nitrite and nitrate values (NO) are lower in patients with CHF. Moreover, another supposition is that patients with secondary pulmonary hypertension with pulmonary pressure (PP)>40mmHg present lower levels of nitrites and nitrates than those with PP<40mmHg. We also propose that these patients would present with higher hydroxyl and superoxide (ROS) concentrations, as well as greater NADPH system activity, thus potentially inactivating the available NO, together with a decrease in SOD activity, aggravating symptoms further, with a clear implication on lower superoxide anion catalysis. Finally, the precision of these hypotheses would allow us to contribute, among other elements of interest, to the key objective of finding new biomarkers in HF as predictors of CHF progression, with particular regard to the development (or not) of secondary pulmonary hypertension.
Material/Patients and methodsThe design of this study took into account the basic principles of bioethics. The protocol was approved by the Independent Ethics Committee of the Hospital Central de Mendoza. Prior to implementing the protocol, each patient was asked to sign the informed consent form. 30 patients were included (average age 53±7.43; 7 women [23%] and 23 men [93%]), who were separated into groups according to their systolic pulmonary pressure (SPP), measured by Doppler echocardiography. Group A (SPP≥40mmHg) had 13 patients (43%) and group B (SPP<40mmHg) had 17 (57%). The SPP cut-off point was set at 40mmHg rather than 30mmHg (considered the normal maximum level). This way, we left a margin of error of 10mmHg, taking into account the variability of the method.
It was a cross-sectional, observational study, with patients assigned to the protocol according to inclusion and exclusion criteria in a number not yet sufficient to render it a preliminary study.
The patients analysed attended the Heart Failure Unit of the Hospital Central de Mendoza. The majority underwent general biochemistry tests, an ECG, a chest X-ray, echocardiogram and a six-minute walk test. Furthermore, having consumed a diet low in NO precursors (nitrites and nitrates) 24h beforehand, the following markers were analysed in samples of the patients’ venous blood:
Nitrites and nitratesBefore proceeding with the NO determination, each blood sample was deproteinised for the purposes of optimising the assay. For this method, 5% trichloroacetic acid was used. The samples were then centrifuged and the supernatant was taken to then centrifuge the samples again at 6400rpm for 20min; the supernatant was separated to determine nitrite production using the Griess method (stable metabolic compound of NO). The quantity of nitrates was corrected based on the protein in each sample. The protein was determined by the Bradford assay, using albumin as a reference. The quantitation of the nitrites was performed in a spectrophotometer at 540nm and the results were expressed as pmol of nitrites formed per microgram of protein per minute of incubation.
Reactive oxygen species (HO and superoxide anion free radicals)In the presence of HO− and O2− radicals, purple 5,5′-nitrilodibarbituric acid monoammonium salt becomes a colourless alloxan derivative and this decrease in colour is proportional to the radicals present and produced. These were quantitated spectrophotometrically at 515nm.6
NADPH oxidase activityThe NADPH oxidase activity in the samples was measured using the Luminol technique (5-amino-2,3-dihydro-1,4-phthalazinedione, Sigma–Aldrich). The samples were homogenised and centrifuged at 6000rpm for 30min. The supernatant was separated and centrifuged again, this time at 19,500rpm, and the concentration of proteins in the lysed membrane fraction was quantitated using the Lowry assay, with bovine serum albumin as the reference. A sample (40μl) of fraction was read quickly on the spectrofluorometer (FluoroCount™; AF10001, Cambers Company, USA) in order to establish the baseline value of each sample. Next, 2μl of β-NADH (Sigma) 0.1mmol/l and 2μl of luminol 5μmol/l in dimethyl sulphoxide were incorporated and read for 10min (360nm excitation and 460nm emission). The values were expressed in relative fluorescent units per microgram of protein and per minute of incubation.
Superoxide dismutase activityThe alkaline autoxidation of 5,6,6a,11b-tetrahydro-3,9,10-trihydroxybenzo(c)fluorene (R1) is accelerated by SOD, yielding a chromogen with maximal absorbance at 525nm. 1,4,6-trimethyl-2-vinylpyridinium (R2) was also used, which eliminates most of the interferences in the samples normally caused by mercaptans, such as reduced glutathione, trapping the mercaptans by means of an alkylation reaction.7
Data collection and statisticsThe data collection instrument was the “Patient inclusion form”. The results were expressed as percentages for categorical variables and as a mean with their standard deviation for continuous variables. The paired t-test was used to compare all of the HF patients to eight healthy volunteers (a comparison that passed the p-value normality test). To compare both groups (A and B), the GraphPad InStat 3® program was used to perform the corresponding statistical operations; a p-value<0.05 was deemed a statistically significant difference.
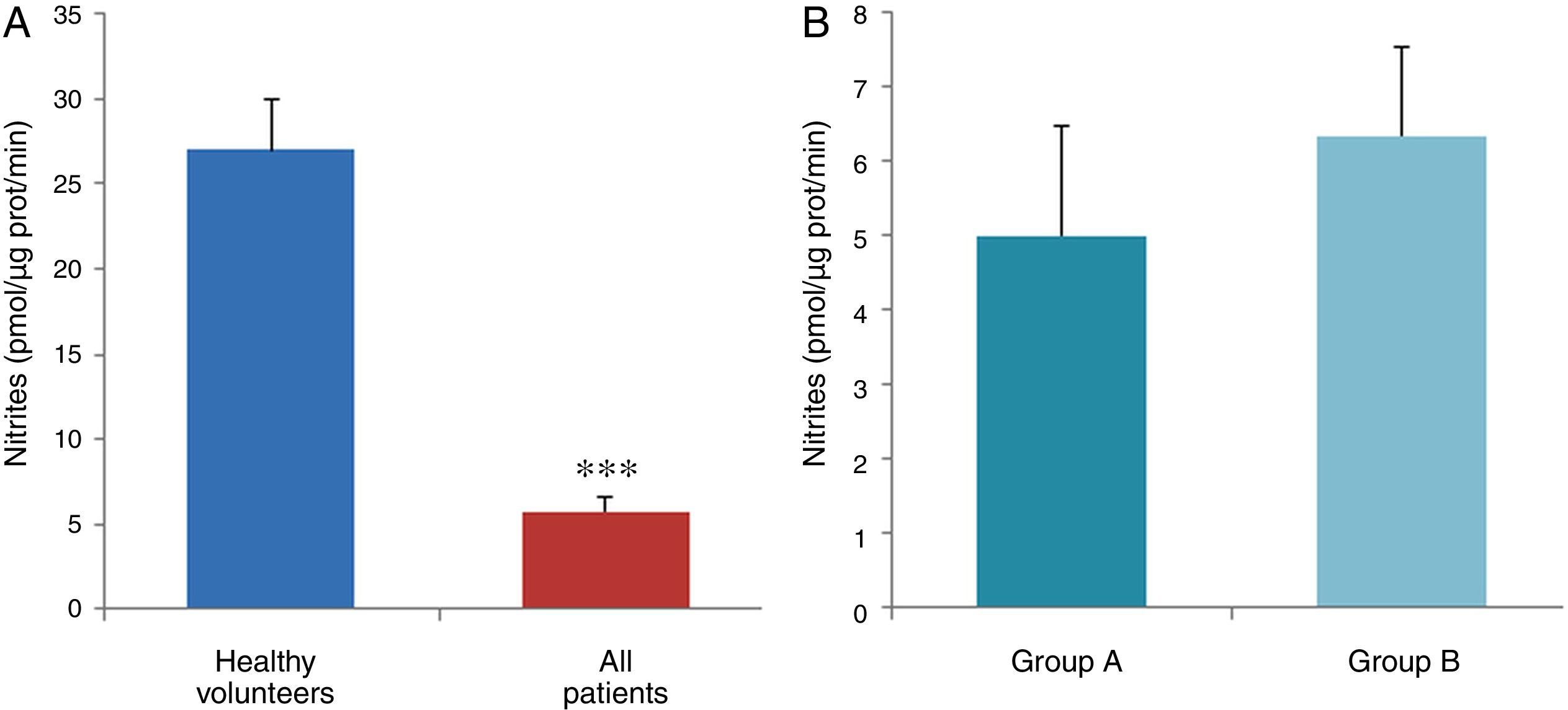
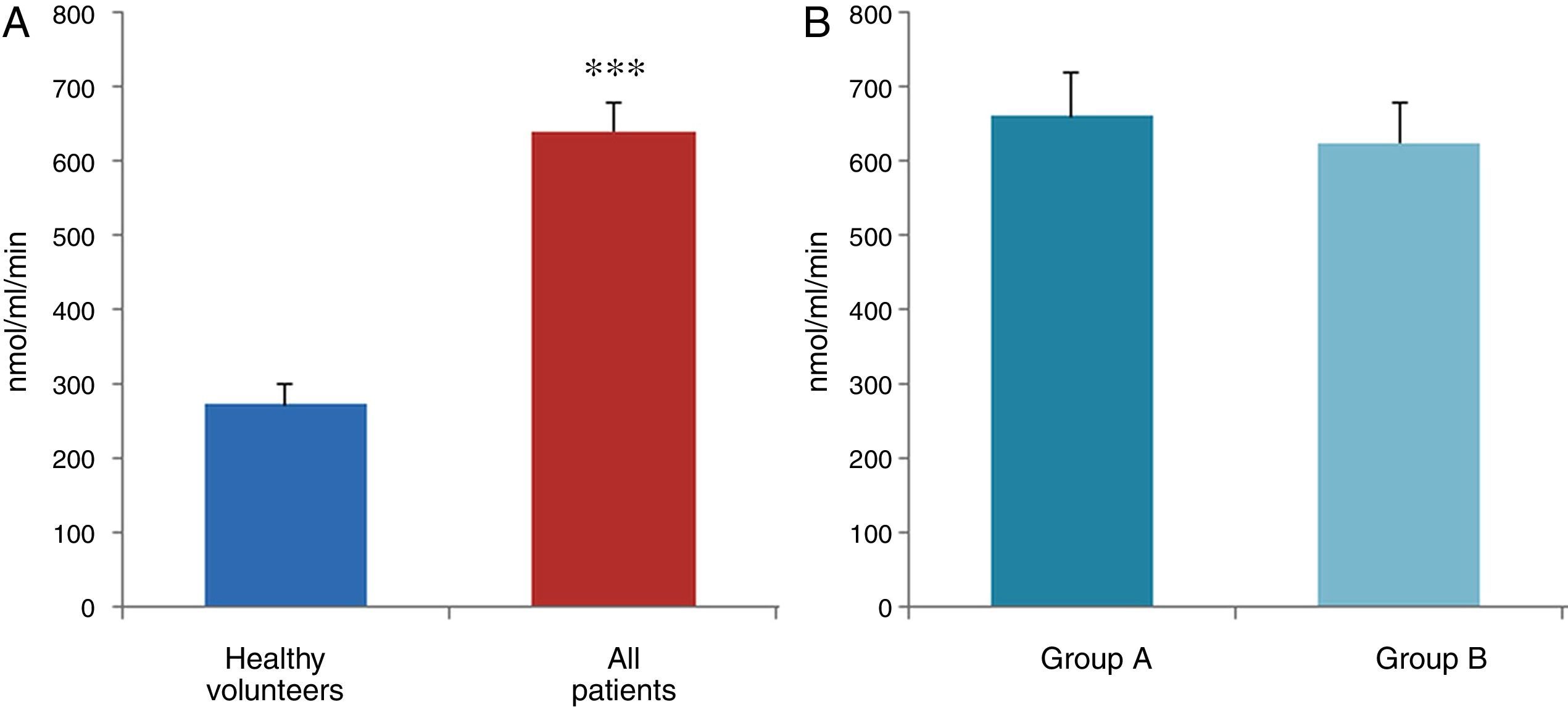
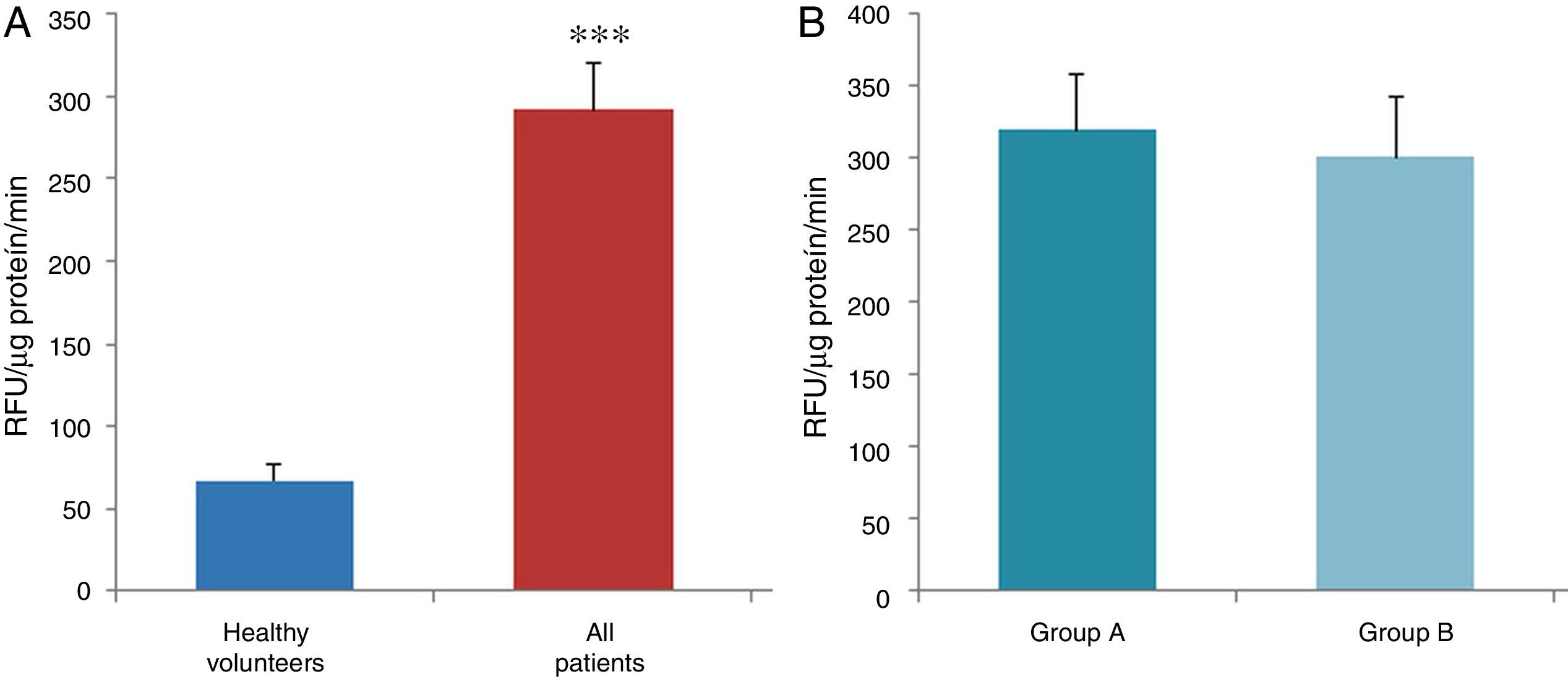
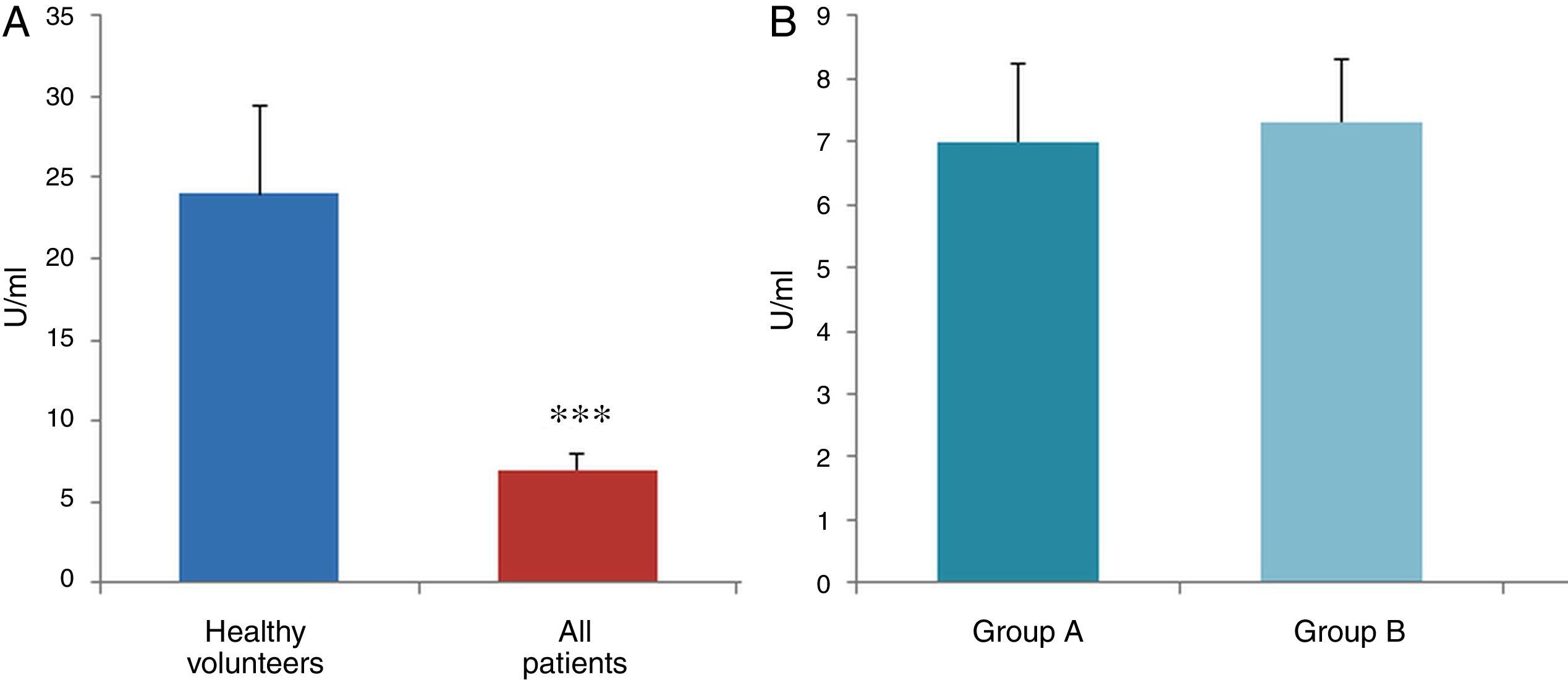
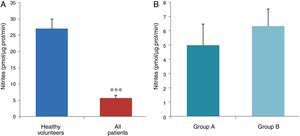
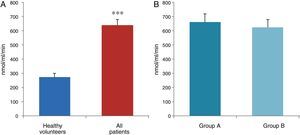
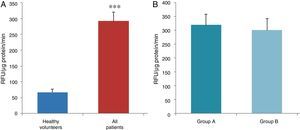
ResultsThe data used for the statistical analysis were SPP (mmHg), plasma levels of nitrites and nitrates (NO), superoxide anion and hydroxyl (ROS), NADPH oxidase and SOD, expressed as pmol of nitrites/μg of protein/min, nmol/ml/min incubation, relative fluorescence units/μg protein/min incubation and U/ml, respectively. The reference values, taken from eight healthy volunteers, were: 27±2.38; 274±28; 67±10 and 24±5.39, respectively. In group A, the mean SPP was 46±4mmHg, while group B's was 31±5mmHg. The nitrite, ROS, NADPH oxidase and SOD values detected for all patients (group A+group B) were: 5.73±0.93; 641±39; 292.66±29.5 and 7±0.77, respectively. When these values were compared to those of the healthy volunteers, the differences were highly significant for nitrites (p<0.0001) (Fig. 1A); as well as for ROS (p<0.0069) (Fig. 2A); NADPH oxidase (p<0.0092) (Fig. 3A); and SOD (p<0.0018) (Fig. 4A). Furthermore, the means with their corresponding standard error for each group were, in group A: 5±1.47; 661±59.34; 320±38 and 7±1.25, respectively; and in group B: 6.35±1.21; 625.58±53.37; 301±41 and 7.32±1, respectively. When these values were paired between both groups (A versus B), the results were as expected, demonstrating a clear trend, albeit without significant differences for nitrites (Fig. 1B), ROS (Fig. 2B), NADPH oxidase (Fig. 3B) and SOD (Fig. 4B).
The results of this study confirm previous evidence and also demonstrate that a series of interconnected humoral and enzymatic changes occur in HF. We specifically demonstrated an increase in NADPH oxidase activity – the main productive force behind ROS as a result of greater superoxide anion synthesis – and that this would be directly related to the stimulation of the renin–angiotensin system. Moreover, we determined a clear reduction in NO bioavailability (lower nitrite and nitrate levels) due, among other elements, to the probable inactivation mediated by superoxide anion. For its part, if the superoxide anion were to react with NO, this would yield the well-known peroxynitrite, which is also toxic and significantly contributes to several forms of endothelial dysfunction, including decreased NO accessibility. These events would stimulate further deterioration of the vasodilation mechanism, with the resulting tissue damage. In addition to the vessel's inability to dilate in response to increased blood flow, we would also see a rapid plasmatic elimination of NO as a result of its possible inactivation due to the combination with haemoglobin.8 Of particular interest is the fact that we were able to establish a reduction in SOD activity, a relevant finding in patients with a significant oxidative state, where higher superoxide anion production, as a result of increased NADPH oxidase activity, would play a key role in inducing apoptosis and fibrosis.
Furthermore, and of particular relevance to this study, there is abundant evidence that supports the concept that NO exerts a selective action on pulmonary circulation, thus strengthening our central hypothesis. The fact that cyclic GMP–the second messenger for NO–is also degraded by phosphodiesterase type 5 in the pulmonary vasculature is noteworthy.9 Moreover, the inhalation of NO prompts a rapid improvement in oxygenation in most patients, inducing pulmonary artery vasodilation. In addition, there is also evidence that shows the efficacy of phosphodiesterase type 5 inhibitors (such as sildenafil) in the pulmonary circulation relative to systemic vasculature. The effects of inhaled NO on the pulmonary circulation were described over two decades ago. By means of an experiment, it was verified that inhaled NO led to selective pulmonary vasodilation by reducing pulmonary artery pressure, without modifying systemic arterial pressure or cardiac output10; this is consistent with data on the efficacy of drugs that inhibit phosphodiesterase type 5 in the pulmonary circulation relative to systemic vasculature, hence why they are successfully used in patients with idiopathic pulmonary hypertension (group 1, Nice classification). Likewise, other drugs such as nebivolol, sodium nitroprusside, nitroglycerin, isosorbide dinitrate and isosorbide 5-mononitrate, S-nitrosothiols, NSAIDs, dihydropyridine calcium channel blockers, antioxidants in LDLs (alpha-tocopherol) and extracellular fluid (vitamin C) and l-arginine, also increase NO synthesis in the pulmonary circulation.
In spite of current knowledge and more than 20 years since the functions of endogenous NO were identified, attempts to generate new therapeutic strategies have not been fruitful, thereby reflecting that our knowledge is expanding very slowly, with other potential related biomarkers having been investigated without obtaining any solid evidence.
The information gathered on the relationship between NO and pulmonary circulation provides a rich mechanistic substrate for proposing new research into biomarkers and their implications on diagnosis, prognosis and a potential new treatment that enables PP to be reduced in HF. In this sense, our working group considered the effect of NO on pulmonary circulation and that its synthesis would be reduced in general HF, and probably greater in HF with pulmonary hypertension (group 2, Nice classification). Thus, the results obtained in this study by means of the biochemical methods described show that, up to this point in our research, in all of the patients analysed, the NO end products (nitrates/nitrites) and SOD activity fell. In contrast, ROS (superoxide and hydroxyl free radicals) and NADPH oxidase enzyme activity increased, as expected.
The authors consider this work to have methodological and design-related limitations. One of them is in regards to how we measured PP. The Hospital Central de Mendoza has a Haemodynamics Department. However, for budgetary reasons, right heart catheterisation was prohibited. Moreover, the protocol design did not allow for long delays in patient management and we had to schedule as few appointments as possible (invasive procedures were also forbidden). As such, the measurement of the main haemodynamic parameter, PP, had to be performed with Doppler echocardiography. In this sense, pulmonary hypertension is defined as mean pulmonary pressure >25mmHg, a reading that should be obtained from a direct recording by means of right heart catheterisation. However, PP was determined indirectly by measuring SPP with Doppler echocardiography, defining pulmonary hypertension as a SPP value >40mmHg. Recording PP directly is undoubtedly more suitable than indirect measurements, but Doppler echocardiography is a sensitive method for measuring SPP and has the advantage of being non-invasive. Non-invasive measurements were compared with simultaneous right catheterisation recordings. SPP estimations were performed for 89% of the patients with a high degree of accuracy (r=0.97).11 Another limitation, due to operational reasons at the Hospital Central de Mendoza, was that the echocardiograms were not performed by the same operator.
Data from some patients (six-minute walk, echocardiogram, general laboratory tests) could not be obtained due to various setbacks. However, this did not affect the pursuit of the primary objective proposed or the results obtained, and in this sense we can assert that the data set provides us with information on chronic patients with severe structural deterioration and a good functional class due to treatment response.
Finally, with regard to the design, a prospective, longitudinal follow-up study allowing us to assess the behaviour of these markers over time would have been preferable, with this remaining a task for both our group and others in the future.
Ethical disclosuresProtection of people and animalsThe authors declare that no experiments were conducted on human beings or animals for this research.
Data confidentialityThe authors declare that they have followed the protocols of their work site regarding the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data is contained in this article.
The authors acknowledge and thank Dr Elcira Maneschi for her contributions and statistical studies.
Please cite this article as: Bonafede RJ, Calvo JP, Fausti JMV, Puebla S, Gambarte AJ, Manucha W. Óxido nítrico: ¿un posible nuevo biomarcador en insuficiencia cardíaca? Relación con hipertensión pulmonar secundaria a insuficiencia cardíaca izquierda. Clin Invest Arterioscler. 2017;29:120–126.