Beyond glucemic control there are other important goals when it comes to providing integral care to patients with Diabetes Mellitus. A bibliographic review was made in order to identify the role played by new antidiabetic drugs in cardiovascular prevention and heart failure. The use of SLGT2i and GLP1a leads to a significant decrease in cardiovascular events, with no difference between the two, except when it comes to hospitalizations for heart failure, where the superiority of the last ones (especially dapaglifozin and empaglifozin) is evident. The current evidence regarding the effect of dpp-4i is diverse, although an increased risk of hospitalizations for heart failure is observed with the use of some drugs of this class (saxagliptin).
Más allá del control de la glucemia existen otros objetivos importantes a la hora de brindar atención integral a pacientes con Diabetes Mellitus. Se realizó una revisión bibliográfica con el objetivo de identificar el papel que juegan los nuevos fármacos antidiabéticos en la prevención cardiovascular y la insuficiencia cardiaca. El uso de SGLT2i y GLP-1a acarrea una disminución significativa de eventos cardiovasculares, sin diferencias entre ambos, exceptuando las hospitalizaciones por insuficiencia cardiaca, en donde es evidente la superioridad de estos últimos (en especial dapaglifozina y empaglifozina). La evidencia actual respecto al efecto de los DPP-4i es diversa aunque se observa un aumento del riesgo de hospitalizaciones por insuficiencia cardiaca con el consumo de algunas drogas de esta clase (saxagliptina).
Diabetes mellitus (DM) is a complex chronic disease which requires continuous medical care with multifactorial strategies to reduce risk through glycaemic control.1
Beyond glycaemic control there are other major objectives in providing comprehensive care to patients with this condition. Due to the significant macrovascular and microvascular impact of this disorder, much emphasis is currently placed on the prevention and intervention in chronic kidney disease (CKD), ischaemic cardiopathy (IC) and heart failure (HF), all three being associated with this baseline pathology.1
There are 3 groups of drugs which have most recently been introduced into daily practice and which have been studied with the latter proposal: sodium-glucose co-transporter type 2 inhibitors (SGLT2i), glucagon-like peptide-1 receptor agonists (GLP-1a) and dipeptidyl peptidase-4 inhibitors (DPP-4i).1,2
Irrespective of the presence of coronary lesions there is a direct relationship between DM and HF. A term that has been coined to refer to this is diabetic cardiomyopathy. Its pathophysiological basis is the presence of microvascular disease, fibrosis, inflammation and myocardial metabolic disturbances.3
A recent study published in Circulation demonstrated that the presence of DM in patients with additional cardiovascular risk factors represented a significant increase in the risk of having HF of between 21% and 111%, depending on the number of risk-associated factors. In the same way, the increased risk of HF hospitalizations was between 31% and 182%.4
Due to the aforementioned situation the authors of this paper decided to carry out a bibliographic review aimed at identifying the role of new antidiabetic drugs on cardiovascular prevention and HF.
Material and methodsA bibliographic search was performed on the databases of Pubmed/Medline, ScienceDirect and AHA/ASA Journals complemented by the Google Scholar search engine (date of last consultation 1st December 2020).
To collect information a search strategy was applied using the key words Sodium-glucose co-transporter 2 inhibitor (SGLT2i), Glucagon-like peptide-1 receptor agonists (GLP-1 RA), Dipeptidylpeptidase-4 inhibitor (DPP-4i), Heart failure, Myocardial Ischemia and Type 2 Diabetes Mellitus. These were filtered by relevance and the original articles, reviews and meta-analysis were prioritised.
Eighty three articles were pre-selected which were reviewed in depth by the authors and a total of 53 bibliographic references were used for designing the final report.
DevelopmentSodium-glucose co-transporter type 2 inhibitorsSGTL2i are drugs which act by inhibiting the sodium-glucose co-transporter in the proximal convoluted tubule of the nephrons. In this way they inhibit glucose reabsorption in the kidney, causing glycosuria, with a consequent decrease in glycaemia.1,5
Irrespective of this mechanism there are others which intervene in the cardiovascular benefit produced by these drugs. Myocardial effects, renal haemodynamic and non-haemodynamic effects, non-glycaemic metabolic effects, reduction of renal hypoxia, increased haematocrit, hypotensive effect and uricosuria have been reported.5,6
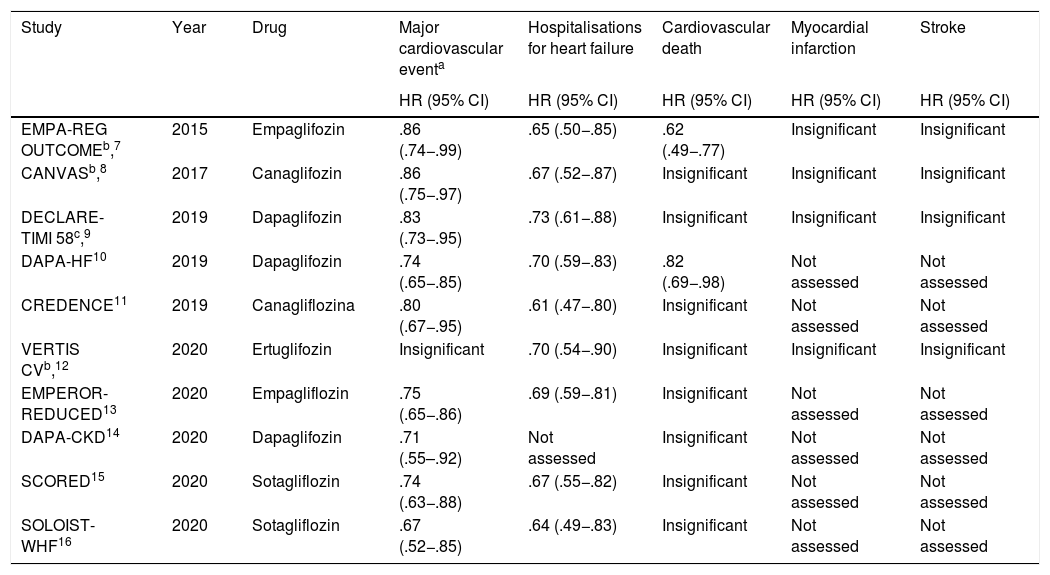
Until now a total of 10 relevant randomised multicentre clinical trials have been published, which have demonstrated the real benefit of these drugs in reducing major cardiovascular events, hospitalisations for HF and death from cardiovascular causes (Table 1).
Clinical trials which assess the impact of SGLT2i in cardiovascular outcomes.
| Study | Year | Drug | Major cardiovascular eventa | Hospitalisations for heart failure | Cardiovascular death | Myocardial infarction | Stroke |
|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| EMPA-REG OUTCOMEb,7 | 2015 | Empaglifozin | .86 (.74−.99) | .65 (.50−.85) | .62 (.49−.77) | Insignificant | Insignificant |
| CANVASb,8 | 2017 | Canaglifozin | .86 (.75−.97) | .67 (.52−.87) | Insignificant | Insignificant | Insignificant |
| DECLARE-TIMI 58c,9 | 2019 | Dapaglifozin | .83 (.73−.95) | .73 (.61−.88) | Insignificant | Insignificant | Insignificant |
| DAPA-HF10 | 2019 | Dapaglifozin | .74 (.65−.85) | .70 (.59−.83) | .82 (.69−.98) | Not assessed | Not assessed |
| CREDENCE11 | 2019 | Canagliflozina | .80 (.67−.95) | .61 (.47−.80) | Insignificant | Not assessed | Not assessed |
| VERTIS CVb,12 | 2020 | Ertuglifozin | Insignificant | .70 (.54−.90) | Insignificant | Insignificant | Insignificant |
| EMPEROR-REDUCED13 | 2020 | Empagliflozin | .75 (.65−.86) | .69 (.59−.81) | Insignificant | Not assessed | Not assessed |
| DAPA-CKD14 | 2020 | Dapaglifozin | .71 (.55–.92) | Not assessed | Insignificant | Not assessed | Not assessed |
| SCORED15 | 2020 | Sotagliflozin | .74 (.63−.88) | .67 (.55−.82) | Insignificant | Not assessed | Not assessed |
| SOLOIST-WHF16 | 2020 | Sotagliflozin | .67 (.52−.85) | .64 (.49−.83) | Insignificant | Not assessed | Not assessed |
The CANVAS 2017 study assessed canaglifozin in patients with type 2 DM. Compared with placebo, this drug reduced the rate of the primary composite of cardiovascular death, myocardial infarction (MI) and stroke. It also reduced HF hospitalisations.8
In concordance with these results, CREDENCE, EMPEROR REDUCED, SCORED and SOLOIST-WHF trials showed a reduction in HF hospitalisations between 31% (EMPEROR REDUCED) and 39% (CREDENCE). However, these studies reported a reduction in cardiovascular death in primary composites but not when assessing this variable alone.11,13,15,16
In a pre-clinical study, Iborra-Egea et al.17 demonstrated that empaglifozin causes direct blockage in the sodium-hydrogen type 1 exchanger, which reduces apoptosis of cardiomyocytes in rats with HF, with no differences between diabetics and non-diabetics.
This could explain the clear benefit obtained with the SGLT2i in individuals with HF and the reduced ejection fraction.
Another pre-clinical study showed that in mice with heart failure and preserved left ventricular ejection fraction empaglifozin reduced the left ventricular mass, improving diastolic function.18
This result would support the hypothesis that the benefit of SGLT2i may also be observed in patients with HF with diastolic dysfunction as the predominant pathophysiological mechanism. At present the EMPEROR-Preserved study (NCT03057951) is underway to confirm this hypothesis.
Januzzi et al.19 also confirmed that consumption of SGLT2i in individuals with DM aged between 55 and 80 years was significantly statistically associated with lower serum concentrations of natriuretic peptides and troponin I at 2 years of follow-up.
Only 2 clinical trials have demonstrated a significant reduction in cardiovascular death when assessing this variable alone.
The EMPA-REG OUTCOME compared empaglifozin vs. placebo in individuals with type 2 DM. Empaglifozin reduced the rate of a primary composite of cardiovascular death, MI and stroke. It also reduced the rate of HF hospitalizations and cardiovascular mortality.7
The DAPA HF trial published in 2019 in the New England Journal of Medicine assessed dapaglifozin vs. placebo in both diabetic individuals and other people without this baseline condition. This SGLT2i was associated with a reduction in the primary endpoint (cardiovascular death or hospitalization from HF). It also reduced hospitalizations from HF and cardiovascular mortality.10
Most striking about the outcomes of this study was that they showed a reduction in cardiovascular mortality and in HF hospitalisations in both diabetics and non-diabetics.
The EMPA-TROPISM study demonstrated that in non-diabetic patients empaglifozin with HF and with reduced ejection fraction had a significant improvement in left ventricular volumes, left ventricular mass, ejection fraction, functional capacity and quality of life compared with placebo, 6 months after radnomisation.20
These results justify the reduction of cardiovascular mortality and HF hospitalisations reported by the DAPA HF.
In a cohort of diabetic patients with high cardiovascular risk conducted by Udell et al.21 the start of treatment with SGLT2i was associated with a lower risk of 2 primary objectives comprising all-cause death and HF hospitalisations, and all-cause death, MI and stroke.
In a recent study Couselo-Seijas et al.22 demonstrated that dapaglifozin improves glucose oxidation metabolism, with no changes to the genes involved in the lipid storage, with a significant reduction in lactate release and acidosis in pericardial fat.
This metabolic benefit may account for the effect of the SGLT2i in reducing the rate of MI and cardiovascular death.
In contrast with these results, the DECLARE TIMI 58 and VERTIS CV studies did not report a significant reduction in cardiovascular mortality alone or assessed in a primary composite objective with MI or stroke. The DECLARE TIMI 58 revealed a 27% reduction in HR hospitalisations comparing dapaglifozin to placebo in diabetics, whilst in the VERTIS CV this reduction was 30% with ertuglifozin. Furthermore, in the DECLARE TIMI 58 there was a reduction in a secondary composite objective for cardiovascular death and HF hospitalizations.9,12
The DAPA-CKD study assessed dapaglifozin vs. placebo, reporting in the SGLT2i arm a reduction in the rate of terminal CKD, a drop above 50% in basal glomerular filtration rate and/or cardiovascular or renal death, and a reduction of the composite between cardiovascular death and HF hospitalizations.14
The above shows a clear benefit in the use of SGLT2i for HF hospitalisation reduction. Both dapagliflozin and empagliflozin have been associated with cardiovascular mortality reduction. There is evidence that these drugs are beneficial regarding major cardiovascular and cerebrovascular events, although no significant reduction has been observed in the rates of MR or stroke when assessing these variables individually, despite the fact that this advantage was observed within composite variables.
Glucagon-like peptide type 1 receptor agonistsGlucagon-like peptide type 1 (GLP 1) is an incretin hormone, synthesised by L cells of the distal ileum and colon, which stimulates postprandial insulin secretion in a blood glucose-dependent manner, suppressing postprandial glucagon secretion, decreasing gastric emptying and reducing food intake.2,23
The favourable effect of GLP1 receptor agonists on endothelial function, increased oxygen delivery to skeletal muscle, decreased blood pressure, C-reactive protein values, plasminogen activator type 1, brain natriuretic peptide, as well as possible direct effects on myocardial and ventricular systolic diastolic function prompted studies to evaluate their protective effect in patients with type 2 DM and cardiovascular disease.2,24
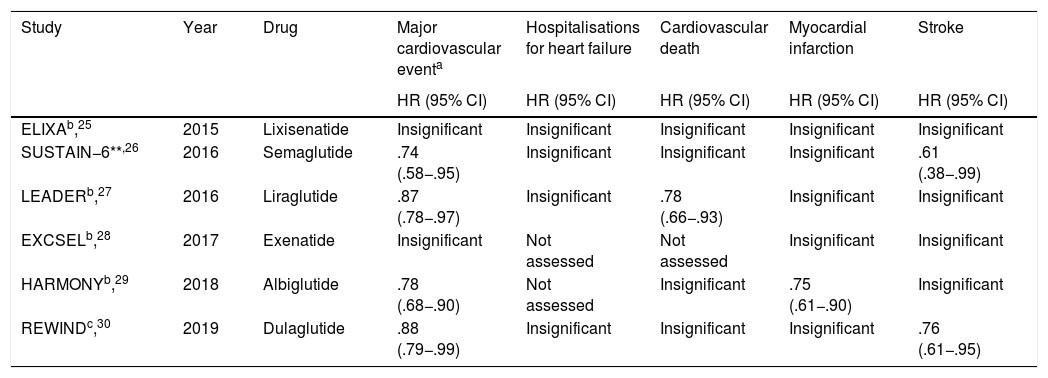
A total of 5 relevant, multicentre, randomised clinical trials have been published to date, with favourable results of these drugs in the reduction of major cardiovascular events (Table 2).
Clinical trials which assessed the impact of GLP-1ª in cardiovascular outcomes.
| Study | Year | Drug | Major cardiovascular eventa | Hospitalisations for heart failure | Cardiovascular death | Myocardial infarction | Stroke |
|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| ELIXAb,25 | 2015 | Lixisenatide | Insignificant | Insignificant | Insignificant | Insignificant | Insignificant |
| SUSTAIN−6**,26 | 2016 | Semaglutide | .74 (.58−.95) | Insignificant | Insignificant | Insignificant | .61 (.38−.99) |
| LEADERb,27 | 2016 | Liraglutide | .87 (.78−.97) | Insignificant | .78 (.66−.93) | Insignificant | Insignificant |
| EXCSELb,28 | 2017 | Exenatide | Insignificant | Not assessed | Not assessed | Insignificant | Insignificant |
| HARMONYb,29 | 2018 | Albiglutide | .78 (.68−.90) | Not assessed | Insignificant | .75 (.61−.90) | Insignificant |
| REWINDc,30 | 2019 | Dulaglutide | .88 (.79−.99) | Insignificant | Insignificant | Insignificant | .76 (.61−.95) |
The results from the ELIXA study were published in 2015. This study assessed lixisenatide in patients diagnosed with type 2 DM and with a history of acute coronary events in the previous 180 days. No reduction in any of the objectives assessed compared with placebo was apparent.25
In contrast to these results, the SUSTAIN–6 and LEADER studies published the following year did however obtain a reduction in major cardiovascular and cerebrovascular events in patients with type 2 DM and high cardiovascular risk. In the case of the LEADER study treatment with semaglutide and liraglutide was compared to placebo. Liraglutide also showed a reduction in the rate of cardiovascular death and in the SUSTAIN–6 trial there was a reduction in the rate of non-fatal stroke.26,27
In a clinical trial published in Cardiovascular Diabetology Lambadiari et al.31 compared the use of metformin and liraglutide, demonstrating significant differences regarding arterial stiffness, global longitudinal strain of the left ventricle, NT–pro BNP values as well as other motility parameters and left ventricular deformation with reduced oxidative stress in patients with newly diagnosed type 2 DM who underwent GLP-1a treatment, providing some evidence for some of the cardiovascular protective effects of these new anti-diabetic drugs.
In contrast to some of these results, research by Kumarathurai et al.32 showed no significant differences in treatment with liraglutide versus placebo regarding neither the increase of the systolic function of the left ventricle in type 2 diabetic patients and stable coronary artery disease undergoing dobutamine, nor any increase in exercise capacity.
These data show how diverse the findings in multiple studies have been which deal with the use of GLP-1a in diabetic patients with cardiovascular diseases.
In 2017 the EXCSEL study assessed the drug exenatide administered weekly (vs. placebo) in type 2 diabetic patients with or without a background of previous cardiovascular disease. No significant differences were found in the primary composite of cardiovascular death, non fatal MI and stroke. The trial did not assess the incidence of HF hospitalisations.28
Parallel to this, in another study, Scalzo et al.24 reported favourable results with the use of exenatide versus placebo in type 2 uncomplicated diabetic patients, with an improvement in diastolic function and reduction of arterial stiffness, but they were not accompanied by an increase in the ability to do exercise.
Although similar primary objectives were not assessed in the two clinical trials, the results were contrasted to a certain extent, because it is well-known that there is an association between arterial stiffness and the majority of cardiovascular risk factors, and therefore its reduction and the improvement of heart function should lead to a lower rate of major cardiac events and a better quality of life, reducing the amount of acute coronary events long-term. A higher capacity for physical exercise and consequently better functional capacity would hypothetically imply a reduction in HF hospitalizations but this was not assessed in either of these studies.
The HARMONY trial determined the superiority of the drug albiglutide versus placebo in the reduction of major cardiovascular events without assessing HF hospitalisations and showed no superiority on assessing cardiovascular death alone, but did find a reduction in the incidence of fatal or non-fatal MI.29
One clinical trial conducted by Lepore et al.33 and published in the Journal of American College of Cardiology revealed that compared with placebo there was no increase in the cardiac function through echocardiography nor reduction of the myocardial oxygen consumption or use of glucose by cardiac muscle in individuals treated with albiglutide.
Similarly to the HARMONY study, the REWIND multicentre randomised study demonstrated the superiority of dulaglutide compared with placebo in diabetic patients above 50 years old with a previous diagnostic of cardiovascular disease or risk factors, concerning the reduction of major cardiac events, with no significant differences regarding cardiovascular death or HF hospitalization, although it did show a reduction in non-fatal stroke when the variable was assessed independently.30
Large studies involving GLP-1a drugs in the treatment of type 2 DM have shown mixed results and have been shown to be safe in the treatment of patients with concomitant cardiovascular disease or high risk factors for cardiovascular disease. The SUSTAIN-6, LEADER, HARMONY and REWIND clinical trials showed a reduction in major cardiovascular and cerebrovascular events, with neutral effects on cardiovascular mortality (except the LEADER study), all-cause death, non-fatal MI, non-fatal stroke, as well as HF hospitalisations.
Dipeptidyl peptidase-4inhibitorsDPP-4i are anti-diabetic drugs which act by inhibiting the dipeptidyl peptidase-4 enzyme. They increase the levels and enhance the action of endogenous incretins, leading to increased insulin secretion and decreased glucagon secretion.2
Inhibition of this enzyme enhances the actions of GLP1, and also modulates the activities of several vascular peptides associated with cardiac protective effects.2
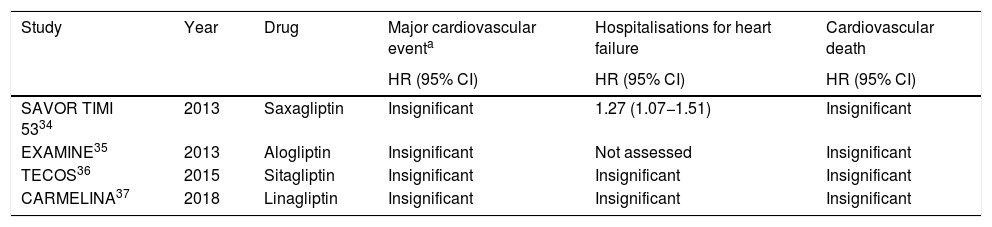
However, none of the randomised clinical trials conducted have demonstrated any real benefit in reducing major cardiovascular events, HF hospitalisations and cardiovascular death (Table 3).
Clinical trials which assessed the impact of DPP-4i in cardiovascular outcomes.
| Study | Year | Drug | Major cardiovascular eventa | Hospitalisations for heart failure | Cardiovascular death |
|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| SAVOR TIMI 5334 | 2013 | Saxagliptin | Insignificant | 1.27 (1.07−1.51) | Insignificant |
| EXAMINE35 | 2013 | Alogliptin | Insignificant | Not assessed | Insignificant |
| TECOS36 | 2015 | Sitagliptin | Insignificant | Insignificant | Insignificant |
| CARMELINA37 | 2018 | Linagliptin | Insignificant | Insignificant | Insignificant |
The 2013 SAVOR-TIMI 53 study, which assessed saxagliptin against placebo in type 2 DM patients, showed an increase in HF hospitalisations, but no significant differences in the incidence of the primary composite of stroke, MI and cardiovascular death.34
A recent meta-analysis assessed diabetic patients treated with DPP-4i. This showed an increase in HF hospitalisations, in keeping with the SAVOR TIMI 53.38
In contrast, the other major clinical trials with DPP-4i did not reveal any significant differences regarding the rate of HF hospitalizations.36,37
The EXAMINE, TECOS and CARMELINA studies assessed alogliptin, sitagliptin and linagliptin respectively in diabetic patients. They did not find any differences compared with placebo in the composite primary objective of cardiovascular death, MI or stroke.35–37
A meta-analysis conducted in 2017 assessed the effect of DPP-4i in HF, with no significant increase in hospitalisations from this in patients treated with vildagliptin, sitagliptin or saxagliptin, but alogliptin was associated with a higher risk of this event. Findings were in contrast to those obtained in the SAVOR TIMI 53 y EXAMINE studies.39
However, the ESPECIAL-ACS study assessed the effect of sitagliptin on coronary plaque changes in diabetic patients with acute coronary syndrome treated by coronary intervention. Using serial intravascular ultrasound, treatment with sitagliptin, diet and exercise versus diet and exercise alone was compared. The trial showed a significant reduction in atheroma plaque lipid volume in patients treated with sitagliptin, but no significant difference in total coronary plaque volume reduction was found between the two groups.40
The PROLOGUE study assessed progression of carotid atherosclerosis by carotid intima-media ratio in patients with type 2 DM treated with sitagliptin vs. conventional therapy by carotid Doppler imaging, revealing no significant difference in the reduction of atherosclerosis progression between the two sclerosis subgroups.41
These results indicate a probable pathophysiological benefit of these drugs on HF, although in practice this has not been corroborated.
A sub-analysis of PROLOGUE assessed over 2 years the progression of arterial stiffness in diabetic patients treated with sitagliptin vs. conventional antidiabetics, based on the measurement of pulse wave velocity. Changes in this parameter were similar in patients in both groups, showing that patients with poor glycaemic control experienced a greater increase in this quantification.42
Another sub-analysis of PROLOGUE assessed diastolic function using the E/e′ ratio showing a significant decrease in this ratio in patients treated with sitagliptin at 2 years of follow-up.43
Corresponding to these studies, a sub-analysis of the REASON trial evaluated the effects of anagliptin and sitagliptin on the concentration of the adipokine FABP4, whose elevation is associated with obesity, insulin resistance and atherosclerosis. The study was conducted in type 2 diabetic patients at high risk of cardiovascular events. Plasma FABP4 levels decreased by 7.9% in anagliptin-treated patients, with no significant decrease in patients who received sitagliptin.44
The latter sub-analysis calls into question whether the likely pathophysiological benefits of some DPP-4i in cardiovascular conditions have a class effect and are therefore not attributable to the other drugs in the group.
Comparison between pharmacological groupsAll of the above demonstrates the clear benefit of SGLT2i and GLP-1a in reducing major cardiovascular events, as well as the apparent lack of utility of DPP-4i for this purpose. However, there are clinical contexts in which it is useful to select between one or the other pharmacological group, based on the specific benefit desired.
In a meta-analysis of 9 clinical trials Kramer et al.45 compared the 3 groups based on the risk of HF hospitalisations. SGLT2i compared with placebo reduced this event by 44%, unlike the other 2 drug classes, which was to be expected, bearing in mind the results of the available clinical trials.
Consistent with this, another meta-analysis showed that both SGLT2i and GLP-1a were associated with decreased mortality when compared with placebo and a DPP-4i.46
Another systematic review and meta-analysis evaluating GLP-1a and DPP-4i concluded that both do not lead to an increased risk of cardiovascular events, although there is no significant reduction in them.47 This result contrasts with that observed in most clinical trials that have studied GLP-1a for this purpose (Table 2).
The study by Fei et al.48 compared the three groups of drugs on the basis of risk of cardiovascular events. SGLT2i reduced cardiovascular deaths compared to placebo and DPP-4i. Both SGLT2i and GLP-1a reduced the incidence of major cardiovascular events, renal events and HF hospitalisations, with superiority of SGLT2i in the latter 2 categories. In discordance, in the study by MacKee et al.49 both groups reduced the incidence of renal events.
Another meta-analysis evaluating SGLT2i and GLP-1a obtained results similar to those of Fei et al. in terms of the superiority of the former in preventing renal events and HF hospitalisations. Notably, both groups decreased the incidence of the primary composite (cardiovascular death, MI and cerebrovascular disease) by a similar magnitude, with no difference in the subgroup with established atherosclerotic cardiovascular disease either.50
These results could be related to various effects on endothelial function and arterial stiffness shown by these drugs, with SGLT2i being associated with a predominant effect on endothelial function while GLP-1a has a predominant action on arterial stiffness.51
The 2021 American Diabetes Association clinical practice guideline recommends in the pharmacological treatment of patients with type 2 DM to consider the presence of HF, CKD and IC. For patients with established HF or CKD, it recommends starting with SGLT2i as a first step. However, in patients with IC or any other established atherosclerotic disorder, GLP-1a and SGLT2i are shown as the 2 initial alternatives with no obvious priority in favour of one or the other group.1
The authors of this article agree with these recommendations as both pharmacological groups have demonstrated a significant reduction in these events in the composite primary endpoints, although when evaluated individually there is no evidence in clinical trials of a reduction in the incidence of MI or stroke with the use of SGLT2i, and some results suggest that LPG1-a may have these benefits. There are no differences for these parameters in the meta-analyses reviewed (except for stroke in the Fei et al. article). It would be desirable to evaluate this issue in a clinical trial comparing both pharmacological groups.
The EMPRISE study compared empaglifozin vs. Sitagliptin in patients with type 2 DM, showing a reduction of HF hospitalisations with the first drug.52
This result (Table 1) is not surprising given the clear benefit of SGLT2i in reducing this event as opposed to DPP-4i which has even been associated in some cases with an increase in this event.
In agreement with the EMPRISE results, the CVD-REAL 2 study included individuals with type 2 DM, comparing SGLT2i with other antidiabetic therapies, determining that the former reduced the risk of death from cardiovascular causes, HF hospitalisations, and a composite of these two variables plus MI and stroke.53
ConclusionsIn individuals with DM, the use of SGLT2i and GLP-1a is safe in terms of major cardiovascular events. The use of these two groups of drugs is associated with a significant reduction in major cardiovascular events. SGLT2i (especially dapaglifozin and empaglifozin) show a decrease in cardiovascular mortality and HF hospitalisations. Current evidence regarding the effect of DPP-4i is mixed, although an increased risk of HF hospitalisations is observed with the use of some drugs in this class (saxagliptin). There is little difference in the reduction of cardiac events between GLP-1a and SGLT2i, except for HF hospitalisations, where the latter is clearly superior.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Rojas-Velázquez JM, Giralt-Herrera A, Leiva-Enríquez J, Leiva-Enríquez J. Rol de nuevos fármacos antidiabéticos en prevención cardiovascular e insuficiencia cardiaca. Clin Invest Arter. 2021;33:314–322.