Type 2 diabetes mellitus (T2D) has acquired epidemic proportions worldwide. In recent years, new oral glucose-lowering drugs (OGLD) have emerged that improve the cardiovascular–kidney–metabolic control in T2D people.
ObjectivesTo compare the baseline clinical–biological characteristics among T2D people to whom had added-on dapagliflozin (DAPA group) or another OGLD (SOC group) second-line hypoglycaemic therapies among the AGORA study population.
MethodsThis is a multicentre cross-sectional observational study of the baseline characteristics of T2D people recruited through competitive sampling among 46 primary care health centres in Spain for the AGORA study. The inclusion and exclusion criteria of participants, and justification of the sample size are reported. After verifying the data necessary to be evaluated and informed consent, 317 subjects were included to the DAPA group and 288 to the SOC group. Both categorical and continuous variables were analysed and compared with the usual statistics. Cohen's d was used to assess the standardised difference in means.
ResultsSix hundred and five patients with T2D were assessed (mean age 63.5 [SD±8.1] years, 61.8% men), whom 17.4% were smokers, 47.6% had obesity, 74.8% hypertension, 87.3% dyslipidaemia, and 41.7% reported physical inactivity, with no significant differences between both comparison groups. The mean (SD) evolution time of T2D was 10.1 (5.6) years. Most baseline clinical–biological characteristics at recruitment were similar in both groups. However, DAPA group was younger (2.9 years), and had lower systolic blood pressure (SBP) (2.8mmHg), higher body weight (BW) (3.7kg), and higher glycated haemoglobin A1c (HbA1c) (0.3%) than SOC group. Only 11.5% of participants had poor glycaemic control (HbA1c>8%) at recruitment, 54.9% had good glycaemic control (HbA1c<7%), being significantly lower in the DAPA group (47.3%) than in the SOC group (63.4%). The percentage of T2D patients with high vascular risk (VR) was 46.3%, and 53.7% with very high VR, being significantly higher in the DAPA group (57.4%) than in the SOC group (49.6%).
ConclusionsMost baseline cardiovascular–kidney–metabolic characteristics were similar in T2D patients whom had added dapagliflozin on second-line hypoglycaemic therapy as those whom had added-on another OGLD. However, patients whom had added-on dapagliflozin had higher VR, lower SBP, higher BW, and slightly worse HbA1c control. Future research is necessary to explain the causes of these differences in cardiometabolic control.
La diabetes mellitus tipo 2 (DM2) ha adquirido proporciones epidémicas en todo el mundo. En los últimos años han surgido nuevos fármacos hipoglucemiantes orales (OGLD) que mejoran el control cardiovascular-renal-metabólico en personas con DM2.
ObjetivosComparar las características clínico-biológicas basales entre personas con DM2 a las que se les había añadido dapagliflozina (grupo DAPA) u otro OGLD (grupo SOC) como terapias hipoglucemiantes de segunda línea, en la población del estudio AGORA.
MétodosEstudio multicéntrico observacional transversal sobre las características basales de personas con DM2 reclutadas mediante muestreo competitivo en 46 centros de atención primaria en España para el estudio AGORA. Se describen los criterios de inclusión y exclusión de los participantes, y la justificación del tamaño de la muestra. Tras comprobar los datos necesarios para ser evaluados y el consentimiento informado, 317 sujetos se incluyeron en el grupo DAPA y 288 en el grupo SOC. Las variables categóricas y cuantitativas se analizaron y compararon con los estadísticos habituales. Se utilizó la d de Cohen para evaluar la diferencia estandarizada de medias.
ResultadosSe evaluaron 605 pacientes con DM2 (edad media 63,5 [DE±8,1] años, 61,8% hombres), de los cuales 17,4% eran fumadores, 47,6% tenían obesidad, 74,8% hipertensión, 87,3% dislipidemia y 41,7% referían inactividad física, sin diferencias significativas entre ambos grupos de comparación. El tiempo medio (DE) de evolución de la DM2 era de 10,1 (5,6) años. La mayoría de las características clínico-biológicas basales al reclutamiento fueron similares en ambos grupos. Sin embargo, el grupo DAPA era más joven (2,9 años) y tenía una presión arterial sistólica (PAS) más baja (2,8mmHg), un mayor peso corporal (3,7kg) y una hemoglobina glicada A1c (HbA1c) más alta (0,3%) que el grupo SOC. Sólo el 11,5% de los participantes tenía un control glucémico deficiente (HbA1c>8%), el 54,9% tenía un buen control glucémico (HbA1c<7%), siendo significativamente menor en el grupo DAPA (47,3%) que en el grupo SOC (63,4%). El porcentaje de pacientes con DM2 con riesgo vascular (RV) alto era del 46,3%, y del 53,7% con RV muy alto, siendo significativamente mayor en el grupo DAPA (57,4%) que en el grupo SOC (49,6%).
ConclusionesLa mayoría de las características cardiovascular-renal-metabólicas basales eran similares en los pacientes con DM2 a los que se les había añadido dapagliflozina en terapia hipoglucemiante de segunda línea como a los que se les había añadido otro OGLD. Sin embargo, los pacientes a los que se les había añadido dapagliflozina tenían un RV más alto, una PAS más baja, un mayor peso corporal y un control de HbA1c ligeramente peor. Son necesarias más investigaciones para explicar las causas de estas diferencias del control cardiometabólico.
Over the last decade, type 2 diabetes mellitus (T2D) has acquired epidemic proportions closely related to obesity, sedentary lifestyle, excessive caloric intake and progressive population ageing. The T2D global prevalence is expected to increase by 72% between 2010 and 2030,1 affecting 462 million people worldwide and reaching 629 million in 2045.2,3 The prevalence rates of diabetes mellitus (DM) and T2D in Spain was 13.8% and 11.5% respectively, in the population aged 18 years or older.4,5
The UKPDS study showed that 50% of T2D patients had micro- and macrovascular complications at the time of diagnosis, affecting quality and life expectancy.6 It is known that decreasing 1% in glycated haemoglobin A1c (HbA1c) is associated with 35% risk reduction of developing microangiopathic complications,7,8 and that intensive glycaemic control reduces major adverse cardiovascular events.9 However, the cardiovascular benefit is only maintained as long as adequate control of HbA1c persists.10 The pathophysiological complexity of T2D relegated the kidney as a target of complications for a long time, forgetting its role in increasing glycaemia.11
Achieving optimal care for T2D patients is an ongoing challenge that requires periodic reassessment to determine the effect of treatment and intensify it when necessary. Furthermore, early achievement of HbA1c targets improves long-term outcomes for T2D patients, reducing the vascular risk (VR) and other DM-related.12–16 Consequently, guidelines recommend initiating treatment with metformin in most T2D patients and adding other glucose-lowering drug (OGLD) on second-line therapy if initial monotherapy does not achieve HbA1c control goals. If dual therapy does not achieve the HbA1c target, another OGLD should be considered (third-line therapy).17,17–19
Over recent years, the evidence accumulation of the benefits achieved beyond glycaemic control by drugs such as sodium-glucose cotransporter-2 inhibitors (SGLT-2i) and glucagon-like peptide 1 receptor agonists (GLP-1ra) has facilitated optimising the comprehensive control of T2D patients. The SGLT-2i competitively, selectively and reversible inhibit sodium-glucose cotransporter 2 (SGLT-2) in the proximal renal tubule, increasing the urinary excretion of glucose by reducing its renal reabsorption, with the consequent glycaemia decrease. The ubiquitous distribution of SGLT-2 beyond the kidney,18 including endothelial19 and cardiac cells,20 allows its inhibition to achieve cardiovascular benefits independently of its effect on glycaemia in both patients with and without DM.
Randomised clinical trials with SGLT-2i and real-world studies have shown decreased risks of cardiovascular and all-cause mortality, hospitalisation for heart failure (HF) and progression of both chronic kidney disease (CKD) and albuminuria, improving quality of life, both in DM and non-DM people.21–26 Dapagliflozin was the first SGLT-2i approved in Europe and the one with the largest market share in Spain.27 An open retrospective cohort study conducted in real-world setting with 22,124 patients showed that patients with T2D exposed to dapagliflozin were a lower risk of death from any cause irrespective of their baseline VR.28 The AGORA study aim was to assess the cardiometabolic control real-world effectiveness in T2D patients comparing add-on dapagliflozin vs. other OGLD as second- or third-line hypoglycaemic therapies during more 12 months. We herein present in first article the differential initial clinical–biological characteristics of the participants included in both comparison arms and the design of the AGORA study.
Material and methodsDesignThis is a multicentre cross-sectional observational study of the baseline characteristics of T2D people recruited by 61 physicians (Acknowledgments) under real-world setting in 46 primary care centres from Spanish Health System for the AGORA study, which is an observational longitudinal study of at least 12 months of ambispective follow-up (retrospective and prospective). The design, statistical analysis, objectives, safety and monitoring of potential adverse events of the AGORA study are reported in eSections 1 and 2 (Suppl. Materials).
Study populationStudy subjects had to meet all of the following inclusion criteria: people aged 18–75 years with T2D who had been recommended lifestyle behaviour modifications for more than 12 months prior the recruitment date, which included smoking cessation, reduction of at least 7% in initial BW through healthy diets (low-fat diet, high-fibre diet), and at least 150min/week of aerobic physical activity and strength training29 T2D people on first-line therapy with metformin or, in case of intolerance or contraindication, another OGLD (except SGLT-2i) for more than twelve months prior the recruitment date; T2D people on treatment with dapagliflozin added-on second-line therapy (or third-line therapy if they were previously on second-line therapy) (DAPA arm) for more than six months prior the recruitment date; T2D people on standard of care with another OGLD (eTable S1 in Suppl. Materials) added-on second-therapy (or third-line therapy if they were previously on second-line therapy) (SOC arm) for more than six months prior the recruitment date. Exclusion criteria were DM people receiving insulin, GLP-1ra or SGLT-2i (except dapagliflozin); people with type 1 DM or with suspected DM of specific aetiology: monogenic diabetes syndromes (neonatal diabetes and maturity-onset diabetes of the young [MODY]), diseases of the exocrine pancreas (cystic fibrosis), and secondary to drugs (glucocorticoids, HIV/AIDS treatment, or organ transplantation); women with gestational diabetes, pregnant women, lactating women, or with a desire to become pregnant; patients with creatinine>2mg/dL; people with uncorrected intellectual, visual or hearing disabilities, moderate or severe cognitive impairment, dementia, schizophrenia or psychosis moderate or severe, residing in nursing homes or social institutions for the elderly, immobilised at home who cannot go to a medical consultation, or terminally ill patients; people who are participating in other clinical studies or decline inclusion in this study.
RecruitmentPeople with T2D diagnosis assigned to the researcher physicians from Spanish Health System were enrolled between April 2018 and June 2023. Study subjects were recruited competitively until reaching the sample size necessary to assess the study aims, with informed consent and the necessary clinical and laboratory data to be evaluated. Initially, 783 people with T2D were recruited, of which 605 subjects had the necessary clinical and laboratory data to be evaluated and met all the inclusion criteria and none of the exclusion criteria.
Study aimsTo compare the baseline clinical–biological characteristics at recruitment among T2D people to whom dapagliflozin or another OGLD were added-on second-line hypoglycaemic therapies, and to describe the design of the AGORA study.
Variables evaluatedThe criteria defining the variables assessed, clinic events, medical conditions and comorbidities are show in eTable S2 (Suppl. Materials). VR was estimated for T2D patients according to 2016 European Guidelines on cardiovascular disease prevention in clinical practice,30 which were subsequently endorsed in 2021.31
Statistical analysisBased on previous studies on the reduction of HbA1c≥0.5%, systolic blood pressure (SBP)≥2mmHg and body weight (BW)≥2kg as the primary composite endpoint for the evaluation of cardiometabolic control in T2D people,32–34 we consider that the magnitude of the difference in potential cardiometabolic control achievement between the group adding-on dapagliflozin (20% in the DAPA arm) vs. standard of care (SOC) group adding-on another OGLD (10% in SOC arm) was assumed for AGORA study design would be 10%. The minimum sample size of 572 study subjects was calculated for a confidence level of 95% (α error 0.05) and a statistical power of 90% (β error 0.10), using the Chi-square test in a bilateral contrast.35 Initially, 744 study subjects would be needed assuming 30% non-response, loss of follow-up data, and dropouts. Qualitative variables indicated the number and percentage of each category, calculating the lower and upper limits of the bilateral 95% confidence interval (CI), and were compared using Chi-square or Fisher exact tests, depending on their distribution. Continuous variables were analysed with mean, standard deviation (±SD), median, interquartile range (IQR), and compared using Student's T, Levene and ANOVA. Cohen's d was used to assess the standardised effect size for measuring the difference between two groups means. All tests were 2-sided, and p-value<0.05 was considered significant. Statistical analysis was performed using the SPSS and/or SAS/STAT statistical packages.
ResultsThe mean (SD) age of 605 adults included was 63.5 (±8.1) years (median [IQR] 64.0 years [58.0–70.0]), of whom 61.8% were men. Three hundred and seventeen subjects were assigned to the group that had added dapagliflozin (DAPA arm), and 288 to the standard of care (SOC) group that had added another OGLD (SOC arm). The median (IQR) age were 62.0 (62.0–57.0) years in the DAPA group, and 67.0 (59.5–71.0) years in the SOC group. The proportion of men assigned to the DAPA arm (60.6% [95% CI 55.2–65.9]) was similar (p=0.508) to that assigned to the SOC arm (63.2% [95% CI 57.6–68.8]). The baseline social characteristics of both groups are shown in eTable S3 (Suppl. Materials).
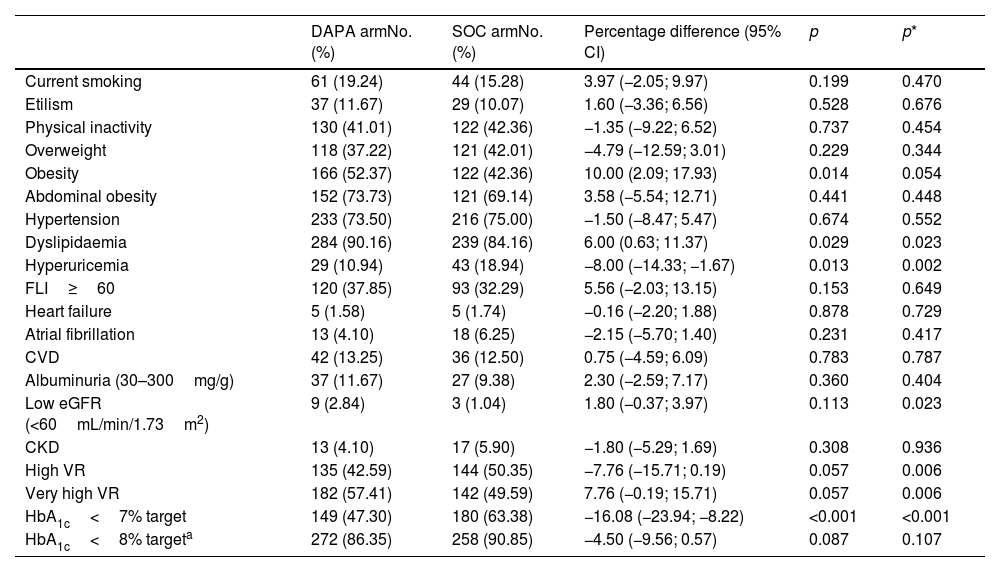
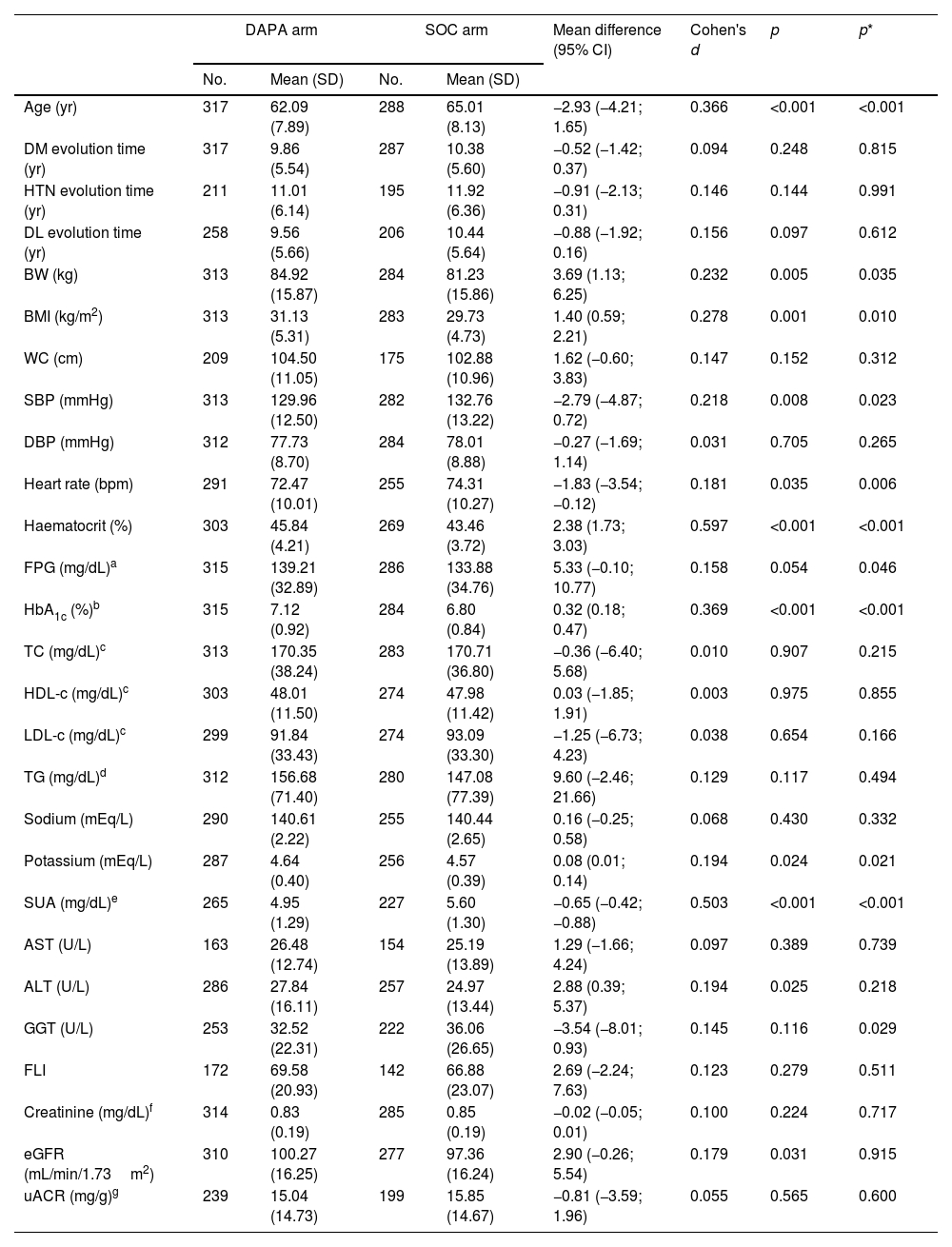
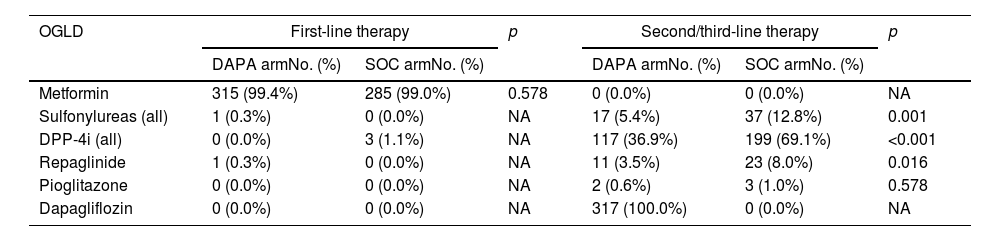
The categorical and quantitative clinical–biological baseline characteristics of participants included in both comparison groups are shown in Tables 1 and 2, respectively. The prevalence rates of comorbidities related to cardiovascular–kidney–metabolic (CKM) syndrome36 as overweight, abdominal obesity, arterial hypertension (HTN), albuminuria, CKD, probable steatotic liver disease (SLD), cardiovascular disease, HF, and atrial fibrillation had no significant differences between comparison groups (Table 1). Likewise, the evolution times of DM, HTN and dyslipidaemia, waist circumference (WC), diastolic blood pressure, and the most biochemical parameters such as serum sodium, lipid and liver profiles, fatty liver index (FLI), creatinine and albuminuria were similar in both comparison groups. However, despite adjusting for age and sex, BW, body mass index (BMI), haematocrit, HbA1c and serum potassium were higher in the DAPA group, and conversely, SBP, heart rate, and serum uric acid were higher in the SOC arm (Table 2). The study subjects had high- (46.3% [95% CI 42.3–50.2]) or very high-VR (53.7% [95% CI 49.8–57.7]). The percentage of T2D patients with very high-VR was significantly higher in the DAPA group when adjusted for age and sex. Finally, 54.9% (95% CI 50.9–58.9) of patients had good glycaemic control (HbA1c<7%) at recruitment, with a significantly lower percentage in the DAPA group (47.3%) than in the SOC group (63.4%) (Table 1). Drugs used in baseline first- and second/third-line therapies are summarised in Table 3.
Baseline categorical clinical characteristics of participants.
| DAPA armNo. (%) | SOC armNo. (%) | Percentage difference (95% CI) | p | p* | |
|---|---|---|---|---|---|
| Current smoking | 61 (19.24) | 44 (15.28) | 3.97 (−2.05; 9.97) | 0.199 | 0.470 |
| Etilism | 37 (11.67) | 29 (10.07) | 1.60 (−3.36; 6.56) | 0.528 | 0.676 |
| Physical inactivity | 130 (41.01) | 122 (42.36) | −1.35 (−9.22; 6.52) | 0.737 | 0.454 |
| Overweight | 118 (37.22) | 121 (42.01) | −4.79 (−12.59; 3.01) | 0.229 | 0.344 |
| Obesity | 166 (52.37) | 122 (42.36) | 10.00 (2.09; 17.93) | 0.014 | 0.054 |
| Abdominal obesity | 152 (73.73) | 121 (69.14) | 3.58 (−5.54; 12.71) | 0.441 | 0.448 |
| Hypertension | 233 (73.50) | 216 (75.00) | −1.50 (−8.47; 5.47) | 0.674 | 0.552 |
| Dyslipidaemia | 284 (90.16) | 239 (84.16) | 6.00 (0.63; 11.37) | 0.029 | 0.023 |
| Hyperuricemia | 29 (10.94) | 43 (18.94) | −8.00 (−14.33; −1.67) | 0.013 | 0.002 |
| FLI≥60 | 120 (37.85) | 93 (32.29) | 5.56 (−2.03; 13.15) | 0.153 | 0.649 |
| Heart failure | 5 (1.58) | 5 (1.74) | −0.16 (−2.20; 1.88) | 0.878 | 0.729 |
| Atrial fibrillation | 13 (4.10) | 18 (6.25) | −2.15 (−5.70; 1.40) | 0.231 | 0.417 |
| CVD | 42 (13.25) | 36 (12.50) | 0.75 (−4.59; 6.09) | 0.783 | 0.787 |
| Albuminuria (30–300mg/g) | 37 (11.67) | 27 (9.38) | 2.30 (−2.59; 7.17) | 0.360 | 0.404 |
| Low eGFR (<60mL/min/1.73m2) | 9 (2.84) | 3 (1.04) | 1.80 (−0.37; 3.97) | 0.113 | 0.023 |
| CKD | 13 (4.10) | 17 (5.90) | −1.80 (−5.29; 1.69) | 0.308 | 0.936 |
| High VR | 135 (42.59) | 144 (50.35) | −7.76 (−15.71; 0.19) | 0.057 | 0.006 |
| Very high VR | 182 (57.41) | 142 (49.59) | 7.76 (−0.19; 15.71) | 0.057 | 0.006 |
| HbA1c<7% target | 149 (47.30) | 180 (63.38) | −16.08 (−23.94; −8.22) | <0.001 | <0.001 |
| HbA1c<8% targeta | 272 (86.35) | 258 (90.85) | −4.50 (−9.56; 0.57) | 0.087 | 0.107 |
DAPA arm: subjects with dapagliflozin added as second- or third-line therapy; SOC arm: subjects on standard of care with OGLD added-on second- or third-line therapy; No. (%): cases number (percentage); CI: confidence interval; p: p-value of the difference in percentages.
CKD: chronic kidney disease; CVD: cardiovascular disease; eGFR: estimated glomerular filtration rate; FLI: fatty liver index; HbA1c: glycated haemoglobin A1c; VR: vascular risk.
HbA1c<8% goal for patients with long-duration DM, elderly, frail, limited life expectancy, hypoglycaemias, microvascular or macrovascular complications, comorbid conditions, or in whom the goal is difficult to achieve despite self-management education about DM, adequate glucose monitoring, and effective doses of various hypoglycaemic agents.13,16,34
p: age- and sex-adjusted p-value.
The definitions of the comorbidities or medical conditions are shown in eTable S2 (Suppl. Materials).
Baseline quantitative clinical characteristics of participants.
| DAPA arm | SOC arm | Mean difference (95% CI) | Cohen's d | p | p* | |||
|---|---|---|---|---|---|---|---|---|
| No. | Mean (SD) | No. | Mean (SD) | |||||
| Age (yr) | 317 | 62.09 (7.89) | 288 | 65.01 (8.13) | −2.93 (−4.21; 1.65) | 0.366 | <0.001 | <0.001 |
| DM evolution time (yr) | 317 | 9.86 (5.54) | 287 | 10.38 (5.60) | −0.52 (−1.42; 0.37) | 0.094 | 0.248 | 0.815 |
| HTN evolution time (yr) | 211 | 11.01 (6.14) | 195 | 11.92 (6.36) | −0.91 (−2.13; 0.31) | 0.146 | 0.144 | 0.991 |
| DL evolution time (yr) | 258 | 9.56 (5.66) | 206 | 10.44 (5.64) | −0.88 (−1.92; 0.16) | 0.156 | 0.097 | 0.612 |
| BW (kg) | 313 | 84.92 (15.87) | 284 | 81.23 (15.86) | 3.69 (1.13; 6.25) | 0.232 | 0.005 | 0.035 |
| BMI (kg/m2) | 313 | 31.13 (5.31) | 283 | 29.73 (4.73) | 1.40 (0.59; 2.21) | 0.278 | 0.001 | 0.010 |
| WC (cm) | 209 | 104.50 (11.05) | 175 | 102.88 (10.96) | 1.62 (−0.60; 3.83) | 0.147 | 0.152 | 0.312 |
| SBP (mmHg) | 313 | 129.96 (12.50) | 282 | 132.76 (13.22) | −2.79 (−4.87; 0.72) | 0.218 | 0.008 | 0.023 |
| DBP (mmHg) | 312 | 77.73 (8.70) | 284 | 78.01 (8.88) | −0.27 (−1.69; 1.14) | 0.031 | 0.705 | 0.265 |
| Heart rate (bpm) | 291 | 72.47 (10.01) | 255 | 74.31 (10.27) | −1.83 (−3.54; −0.12) | 0.181 | 0.035 | 0.006 |
| Haematocrit (%) | 303 | 45.84 (4.21) | 269 | 43.46 (3.72) | 2.38 (1.73; 3.03) | 0.597 | <0.001 | <0.001 |
| FPG (mg/dL)a | 315 | 139.21 (32.89) | 286 | 133.88 (34.76) | 5.33 (−0.10; 10.77) | 0.158 | 0.054 | 0.046 |
| HbA1c (%)b | 315 | 7.12 (0.92) | 284 | 6.80 (0.84) | 0.32 (0.18; 0.47) | 0.369 | <0.001 | <0.001 |
| TC (mg/dL)c | 313 | 170.35 (38.24) | 283 | 170.71 (36.80) | −0.36 (−6.40; 5.68) | 0.010 | 0.907 | 0.215 |
| HDL-c (mg/dL)c | 303 | 48.01 (11.50) | 274 | 47.98 (11.42) | 0.03 (−1.85; 1.91) | 0.003 | 0.975 | 0.855 |
| LDL-c (mg/dL)c | 299 | 91.84 (33.43) | 274 | 93.09 (33.30) | −1.25 (−6.73; 4.23) | 0.038 | 0.654 | 0.166 |
| TG (mg/dL)d | 312 | 156.68 (71.40) | 280 | 147.08 (77.39) | 9.60 (−2.46; 21.66) | 0.129 | 0.117 | 0.494 |
| Sodium (mEq/L) | 290 | 140.61 (2.22) | 255 | 140.44 (2.65) | 0.16 (−0.25; 0.58) | 0.068 | 0.430 | 0.332 |
| Potassium (mEq/L) | 287 | 4.64 (0.40) | 256 | 4.57 (0.39) | 0.08 (0.01; 0.14) | 0.194 | 0.024 | 0.021 |
| SUA (mg/dL)e | 265 | 4.95 (1.29) | 227 | 5.60 (1.30) | −0.65 (−0.42; −0.88) | 0.503 | <0.001 | <0.001 |
| AST (U/L) | 163 | 26.48 (12.74) | 154 | 25.19 (13.89) | 1.29 (−1.66; 4.24) | 0.097 | 0.389 | 0.739 |
| ALT (U/L) | 286 | 27.84 (16.11) | 257 | 24.97 (13.44) | 2.88 (0.39; 5.37) | 0.194 | 0.025 | 0.218 |
| GGT (U/L) | 253 | 32.52 (22.31) | 222 | 36.06 (26.65) | −3.54 (−8.01; 0.93) | 0.145 | 0.116 | 0.029 |
| FLI | 172 | 69.58 (20.93) | 142 | 66.88 (23.07) | 2.69 (−2.24; 7.63) | 0.123 | 0.279 | 0.511 |
| Creatinine (mg/dL)f | 314 | 0.83 (0.19) | 285 | 0.85 (0.19) | −0.02 (−0.05; 0.01) | 0.100 | 0.224 | 0.717 |
| eGFR (mL/min/1.73m2) | 310 | 100.27 (16.25) | 277 | 97.36 (16.24) | 2.90 (−0.26; 5.54) | 0.179 | 0.031 | 0.915 |
| uACR (mg/g)g | 239 | 15.04 (14.73) | 199 | 15.85 (14.67) | −0.81 (−3.59; 1.96) | 0.055 | 0.565 | 0.600 |
DAPA arm: subjects with dapagliflozin added-on second- or third-line therapy; SOC arm: subjects on standard of care with OGLD added-on second- or third-line therapy; SD: standard deviation; CI: confidence interval; Cohen's d; standardised mean difference; p: p-value of the difference in means.
ALT: alanine aminotransferase; AST: aspartate aminotransferase; BMI: body mass index; BW: body weight; DBP: diastolic blood pressure; DL: dyslipidaemia; DM: diabetes mellitus; eGFR: estimated glomerular filtration rate; FGP: fasting plasma glucose; FLI: fatty liver index; GGT: gamma-glutamyl transferase; HbA1c: glycated haemoglobin A1c; HDL-c: high-density lipoprotein cholesterol; HTN: arterial hypertension; LDL-c: low-density lipoprotein cholesterol; SBP: systolic blood pressure; SUA: serum uric acid; TC: total cholesterol; TG: triglyceride; uACR: urine albumin-creatinine ratio; WC: waist circumference.
p: age- and sex-adjusted p-value.
The definitions of the variables are shown in eTable S2 (Suppl. Materials).
First- and second/third-line therapies in DAPA vs. SOC groups.
| OGLD | First-line therapy | p | Second/third-line therapy | p | ||
|---|---|---|---|---|---|---|
| DAPA armNo. (%) | SOC armNo. (%) | DAPA armNo. (%) | SOC armNo. (%) | |||
| Metformin | 315 (99.4%) | 285 (99.0%) | 0.578 | 0 (0.0%) | 0 (0.0%) | NA |
| Sulfonylureas (all) | 1 (0.3%) | 0 (0.0%) | NA | 17 (5.4%) | 37 (12.8%) | 0.001 |
| DPP-4i (all) | 0 (0.0%) | 3 (1.1%) | NA | 117 (36.9%) | 199 (69.1%) | <0.001 |
| Repaglinide | 1 (0.3%) | 0 (0.0%) | NA | 11 (3.5%) | 23 (8.0%) | 0.016 |
| Pioglitazone | 0 (0.0%) | 0 (0.0%) | NA | 2 (0.6%) | 3 (1.0%) | 0.578 |
| Dapagliflozin | 0 (0.0%) | 0 (0.0%) | NA | 317 (100.0%) | 0 (0.0%) | NA |
OGLD: oral glucose-lowering drugs; DAPA arm: subjects on treatment with dapagliflozin added as second- or third-line therapy; SOC arm: subjects on standard of care with OGLD added-on second- or third-line therapy; DPP-4i: dipeptidyl peptidase-4 inhibitors.
p: p-value of the difference in percentages; NA: not applicable.
Because T2D is a progressive disease, maintenance of HbA1c targets usually requires prompt combination therapy that should involve agents with complementary mechanisms of action.13,17 An effectiveness meta-analysis suggested that each new class of non-insulin agents added to initial metformin therapy generally reduced HbA1c by approximately 0.7–1.0%.37 Strict and early control of HbA1c achieves both macro- and microvascular benefits, especially when reductions are achieved with an HbA1c target below 7%.16,38 The ADA 2024 recommendations on the treatment of T2D patients recommend early drugs-combination therapy added to healthy lifestyle behaviours.17 Furthermore, it points out the importance of addressing the rest of the factors to minimise VR.39
SGLT-2i are drugs that reduce cardiovascular events in addition to HbA1c levels in T2D patients.14,17 Dapagliflozin is a SGLT-2i with moderate to high HbA1c-lowering efficacy, which can be safely combined with metformin or other therapies for T2D,14,17,40,41 and is associated with moderate BW loss, and reductions in blood pressure and oxidative stress,42 and has demonstrated cardiovascular and renal benefits.43 It is common to use the reduction of HbA1c≥0.5%, SBP≥2mmHg and BW≥2kg as the primary composite endpoint for the assessment of cardiometabolic control in T2D patients.29–31 A study carried out in Germany with 1169 patients with T2D initiating therapy with dapagliflozin showed a significant reduction in HbA1c, SBP, and BW after a 6-month follow-up.29 In another study conducted in Italy with 2298 patients with T2D, the cardiometabolic control assessed with this composite endpoint was also achieved in a higher proportion of patients receiving dapagliflozin (17.6%) compared to dipeptidyl peptidase-4 inhibitors (11.7%) after a 7.2-month follow-up.31 The prevalence of obesity among AGORA study participants differed depending on the anthropometric measurement performed, varying between 47.6% among those with BMI≥30kg/m2, and 45.1% when abdominal obesity was assessed using WC was measured. In addition, 95.9% of the T2D patients in our study had increased adiposity. Although WC was similar in both comparison groups, the DAPA group had slightly higher BW and BMI. These differences in the qualification of obesity and adiposity, together with the risk posed by excess fat, mainly at the expense of visceral fat, require us to delve deeper beyond the simple measurement of BMI.44
The subjects in both comparison groups in our study had similar evolution time of DM, HTN and dyslipidaemia, lipid and hepatic profiles, and prevalence rates of comorbidities related to CKM syndrome.37 However, age, heart rate, and SBP were lower in the DAPA group subjects, and conversely, BW, BMI, haematocrit, and HbA1c were higher, probably because random sampling was not performed. Other hypothesis that could be raised is that physicians would have added-on dapagliflozin prior to enrollment in those T2D patients with higher BW and worse HbA1c control because higher BW is related to HbA1c increasing, and because of the beneficial effect that dapagliflozin had showed on these variables in some studies.29–31
Previous data published in 2019 from 373,185 patients with T2D in Spain showed that 55.5% had HbA1c<7%, 71.6% had BP<140/90mmHg and 68% had LDL-c<100mg/dL, but only 23.2% of all of them were on treatment with OGLD combination.45 The present study shows a baseline slight worsening of good glycaemic control (53.9%), probably because the follow-up period of the study subjects coincided with that of the COVID-19 pandemic, during which there were worse patient follow-up and glucometabolic worsening caused by SARS-CoV-2 infection.46,47
The expression of SGLT-2 in the liver justifies its hepatic benefits.48 SGLT-2i reduces adiposity and prevents the progression of liver fibrosis in T2D patients with SLD, improving markers such as alanine aminotransferase or FLI by attenuating hepatic lipid accumulation.49,50 SLD is usually found in more than 50% of T2D patients, reaching almost all of them when it coexists with obesity.51 Among the patients included in our study, 67.0% had FLI≥60 as SLD marker, and were no significant differences for aspartate aminotransferase, gamma-glutamyl transferase and FLI between both comparison groups.
Atherosclerotic vascular disease (ASVD) is responsible for 65% of deaths among T2D patients.52 The VR of T2D patients is strongly modulated by age, T2D evolution time, presence of ASVD, CKD, target organ damage, and concomitant VR factors, doubling the risk of death and shortening life expectancy between 5 and 10 years.53 The confluence and high prevalence of these factors in the T2D population of our study justifies that the entire population had a high (46.1%) or very high VR (53.9%).
The present study has the well-known limitations of observational research. Analysis of non-comparable groups of patients initiating dapagliflozin or OGLD may reveal differences in some clinical characteristics, which may lead to strong confounding by indication. Another important bias was the exclusion of patients with high creatinine because the health authorities did not allow it at the time of authorisation of the study protocol. Finally, we recognise the bias of using only dapagliflozin as a comparator drug because it is the most widely used SGLT-2i in primary care setting.
ConclusionsParticipants of our study had a high- or very high-VR probably due to the time elapsed since the T2D diagnosis (10 years), and the frequent presence of comorbidities and VR factors. Overall, T2D people in both comparison groups had similar biochemical profiles and prevalence rates of comorbidities related to CKM syndrome. However, subjects whom had added dapagliflozin on second-line hypoglycaemic therapy had higher VR, higher BW, and slightly higher HbA1c levels and, in contrast, were slightly younger and had lower SBP than those whom had added-on another OGLD. Future research should shed light on the causes for these differences in cardiometabolic control factors among T2D patients.
Research ethics committeeThe institutions and health authorities that supervised and approved the AGORA study were the following: Drug Research Ethics Committee of the Health Research Institute of the San Carlos University Clinical Hospital (Madrid, Spain), National Validation Commission of SEMERGEN, Research Foundation of SEMERGEN, and Research Ethics Committees of the Autonomous Communities of Madrid, Galicia, Extremadura, Valencia, and Castilla La Mancha. The Drug Research Ethics Committee of the Health Research Institute of the San Carlos University Clinical Hospital (Madrid, Spain) authorised the performance of this study (internal code: 18/208-O_SP) on May 16, 2018, in accordance to the study protocol VF6 05/08/2018, and whose promoter is the SEMERGEN Foundation (promoter code ESR-17-12871). The Department of Medicines for Human Use of the Spanish Agency of Medicines and Medical Devices (AEMPS, according to its initials in Spanish) of the Ministry of Health, classified the study as “post-authorisation prospective follow-up study” on November 17, 2017.
The AGORA study was carried out in accordance with the ethical principles established by the 18th World Medical Assembly (Helsinki, 1964) and its amendments, and that are consistent with the International Conference of Harmonization (ICH) Guideline for Good Clinical Practice (GCP) E6(R3), as well as with national and international ethical standards and anti-corruption legislation contained in the Organisation for Economic Co-operation and Development (OECD) convention adopted on November 21, 1997, also included in the Foreign Corrupt Practices Act (FCPA).
FundingSEMERGEN Foundation received unconditional grants from AstraZeneca (code ESR-17-12871) and the Spanish Atherosclerosis Society Foundation (code BAP 01/19) to partially finance the AGORA study.
Authors’ contributionsConceptualization and study design: VP-C, AR-G, AS-C, AS-F, SC-S.
Data collection: VP-C, AR-G, AS-C, MIC-P, FJA-M, EA-M, AB-G, DR-A, JP-G, SC-S.
Data analysis and interpretation: SC-S, VP-C, AR-G, AS-C, AS-F, SC-S, VF-P, BS-S.
Original draft preparation: VP-C, AR-G, AS-C, AS-F, SC-S, VF-P, BS-S.
Manuscript review and editing: VP-C, AR-G, AS-C, AS-F, MIC-P, FJA-M, EA-M, AB-G, DR-A, VF-P, BS-S, JP-G, SC-S.
All authors read and approved the final version of this manuscript.
Conflict of interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The effort, dedication, and collaboration of the following physicians who have participated in the AGORA-AP Study Research Group are most appreciated: Alonso-Moreno Francisco Javier, Alonso-Verdugo Antonio, Ariño-Miquel Iris, Arranz-Martínez Ezequiel, Baeza-López Jose María, Baltuille-Aller María del Camino, Barrios-Rueda Elena, Benito-González María de la Vega, Calderín-Morales María del Pino, Castellote-Baylach Amparo, Cervera-Pérez María Inmaculada, Cinza-Sanjurjo Sergio, Cotillas-Soria Juan Antonio, Díaz-González Isabel María, Domínguez-Ávila Julián, Fernández-Escalada Eugenio, Fernández-García José Manuel, García-Álvarez Juan Carlos, García-Fernández María Eugenia, García-García Elena Concepción, García-Pliego Rosa Ana, Gómez-Calvo Ana María, Gómez-Díaz Esther, González-Gamarra Amelia, González-Mohino Loro Belén, Gutiérrez-López María, Heras-Hitos Julio Antonio, Javierre-Miranda Ana Pilar, Juárez-Gonzálvez Paula, Kyrychuk Nikitina Zhanna, Lahoz-Vela Pilar, Macho-del-Barrio Ana Isabel, Manzano-Martín María José, Marañón-Henrich Nuria, Martínez-Irazusta Juncal, Martínez-Torres Juan Ángel, Miguel-Garzón Marta, Migueláñez-Valero Alfonso, Minguela-Puras María Esther, Montero-Costa Alejandra, Morales-Cobos Luis Enrique, Moyá-Amengual Ana, Nimo-Pérez María Belén, Ortuño-Pascual Elia María, Pallarés-Carratalá Vicente, Pérez-Aradas Verónica, Pérez-Vázquez María Estrella, Redondo-de-Pedro Sonia, Rivas-Plasencia María José, Ruiz-García Antonio, Ruiz-Huertas Rocío, Sanz-García Francisco Javier, Sanz-Pozo Blanca, Sanz-Velasco Carmelina, Scavone-Rabeya Sandra Liliana, Simonaggio-Stancampiano Paula Fabiana, Turégano-Yedro Miguel, Vieira-Pascual María Carmen, Villafañe-Sanz Victoria Fátima, Vizcaíno-Batllés Amparo, Vizcaíno-Pinedo Roberto.