Adequate LDL cholesterol (LDLc) control after an acute coronary syndrome (ACS) is a crucial secondary prevention strategy to minimise the incidence of recurrent myocardial infarction and cardiovascular death. There are tables that predict the necessary dosage of lipid-lowering treatment from the initial LDLc but have not been tested in ACS. Variables associated with optimal LDLc after an ACS were analysed and the therapeutic yield of the use of Masana's recommendations in this setting.
MethodsA total number of 326 ACS-patients were included between January-2015 and May-2016. Baseline LDLc concentration and prescribed hypolipemiant treatment at hospital discharge were registered. We analysed the variables associated with optimal LDLc levels (<70mg/dl) control during follow-up.
ResultsAmong our patient population (72% male, age 66±13 years), the hypolipemiant treatment at hospital discharge fulfilled the Masana's recommendations in 196 (60%) patients. After a follow-up period of 122 [66–184] days the targeted LDLc levels were achieved in 148 (45%) patients, being this percentage greater among those in whom the Masana's recommendations were fulfilled (109/196, 56%), as compared with the remaining (39/130, 30%; p<0.001). The male gender (p<0.001), the absence of prior history of dyslipemia (p<0.001) and the adherence to Masana's recommendations (p=0.007) were independent predictors for the achievement of targeted LDLc levels during follow-up.
ConclusionsIn less than half of ACS-patients adequate mid-term LDLc control is obtained. The dosage of the lipid-lowering therapy according to Masana's recommendations helps to achieve this important therapeutic goal.
El adecuado control lipídico tras un síndrome coronario agudo (SCA) es una estrategia de prevención secundaria crucial para disminuir el riesgo de reinfarto y muerte cardiovascular. Existen tablas que predicen la dosificación necesaria del tratamiento hipolipidemiante según el colesterol LDL (cLDL) inicial pero no han sido probadas en el SCA. Analizamos los factores asociados al control del cLDL tras un SCA y la utilidad de las tablas de Masana y Plana en este contexto.
MétodosEntre enero de 2015 y mayo de 2016 se incluyeron 326 pacientes con SCA. Se registraron las concentraciones basales de cLDL y el tratamiento hipolipidemiante al alta. Se analizaron las variables asociadas a un adecuado control del cLDL (<70mg/dL) en el seguimiento.
ResultadosLa edad media fue 66±13 años, el 72% varones. El tratamiento hipolipidemiante al alta se ajustó a las recomendaciones de Masana en 196 (60%) pacientes. Tras 122 [66-184] días, en 148 (45%) se alcanzó el objetivo de cLDL, siendo este porcentaje mayor (109/196 –56%– vs. 39/130 –30%– pacientes) cuando el tratamiento fue planificado según las tablas de Masana y Plana (p<0,001). En el análisis multivariante, el género masculino (p<0,001), la ausencia de dislipidemia previa (p<0,001) y la aplicación de las tablas de Masana y Plana (p=0,007) fueron predictores independientes para alcanzar el cLDL objetivo.
ConclusionesEl control lipídico adecuado tras un SCA se alcanza en menos de la mitad de casos. La dosificación de la terapia hipolipidemiante según las tablas de Masanay Plana mejora la consecución de este crucial objetivo terapéutico.
Cardiovascular disease is responsible for 3.9 million deaths in Europe every year, 20–30% of which are associated with poor dyslipidaemia control.1 Numerous studies have demonstrated the importance of lowering low-density lipoprotein cholesterol (LDL-C) levels in reducing cardiovascular morbidity and mortality.2–5 Despite this, the degree of LDL-C control in real life is discouraging, particularly in secondary prevention.6–9
Therapeutic inertia, inadequate drug doses, lack of titration in subsequent visits, lack of lipid-lowering drug combinations and poor patient adherence to medication7,9,10 are some of the factors that help to explain inadequate LDL-C control.
In patients with very high cardiovascular risk, both the attainment of the treatment target as well as the time taken to do so are important.4,11,12 Planning a lipid-lowering pharmacological strategy is therefore crucial.
One of the available tools is the cholesterol-lowering therapy planning tables proposed by Masana andPlana13 in 2005 and updated in 2010 and 2015,14,15 which are based on the baseline concentration of LDL-C, the target LDL-C according to cardiovascular risk and the theoretical potency of the lipid-lowering drugs.16,17 The type and dose of statin and/or the need to add ezetimibe to achieve the treatment target of each patient are established based on these parameters.
Despite their ease of use and widespread dissemination in primary care clinical practice guidelines,18 there is little evidence to support their efficacy. To date, only the results of a computer-based clinical decision support system (HTE-DLP)19 in 77 outpatients with high cardiovascular risk have been published. The applicability and therapeutic performance have not been established in acute coronary syndrome (ACS).
The aims of this study were to evaluate LDL-C control after an ACS and to analyse whether the prescription of lipid-lowering therapy according to the Masana and Plana cholesterol-lowering therapy planning tables improve the attainment of the treatment target in this population.
Patients and methodsStudy populationPatients who attended our centre between January 2015 and May 2016 due to an ACS who underwent appropriate clinical follow-up were consecutively enrolled. Patients who died in the first three months and those without a lipid profile in the first year of follow-up were excluded.
The study was designed in compliance with the ethical principles set forth by the Declaration of Helsinki and was approved by our centre's Independent Ethics Committee.
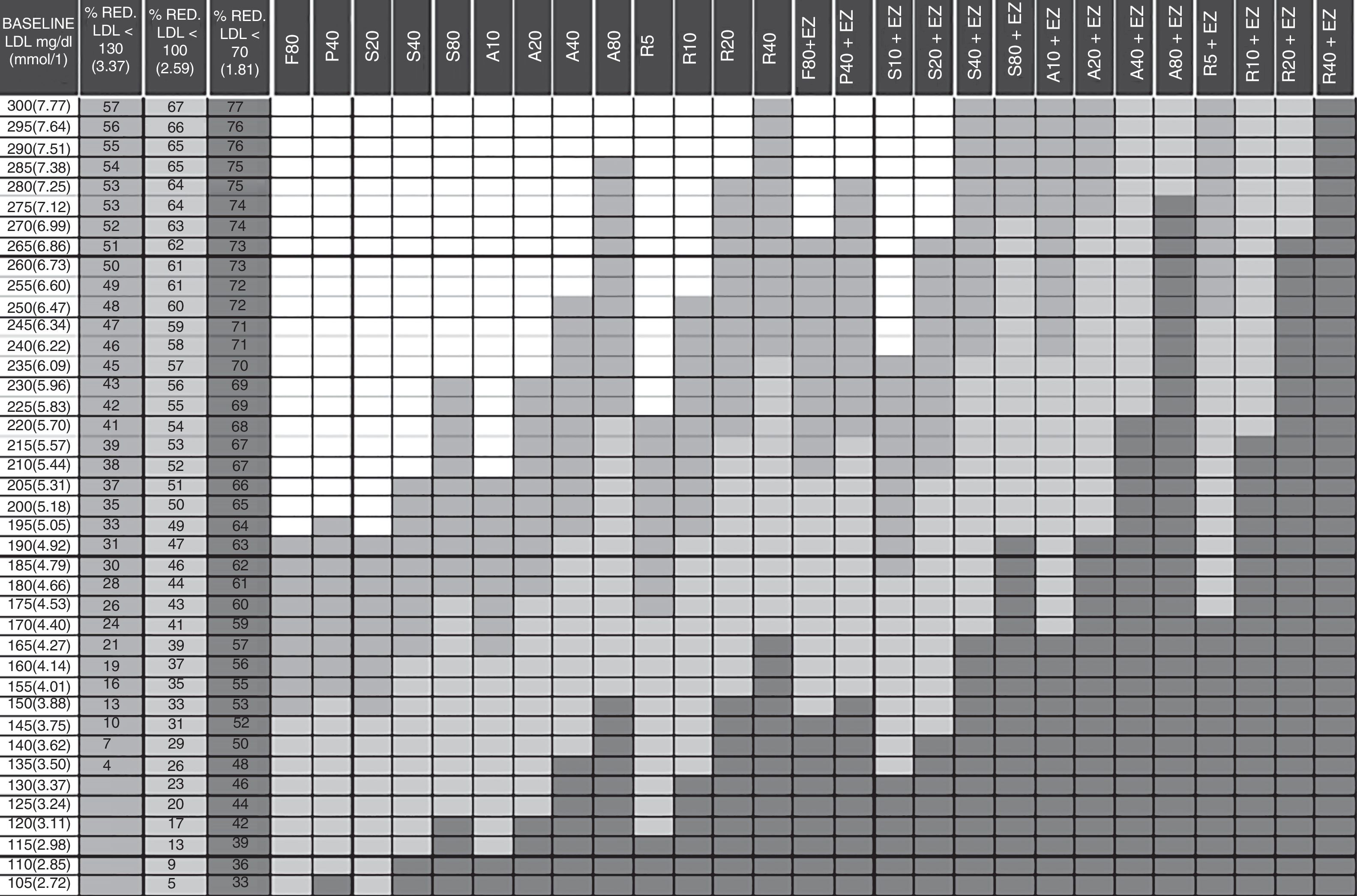
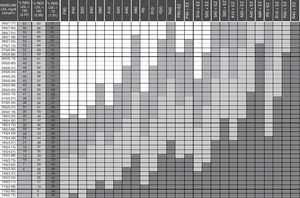
Cholesterol-lowering therapy planning tables proposed by Masana and PlanaThis study used the updated 2010 version of the Masana and Plana Tables14 (Fig. 1). The baseline LDL-C concentration (first column of the table) must be known in order to apply the tables. In patients already receiving lipid-lowering therapy, the theoretical baseline LDL-C concentration is assumed to be 30% higher than the LDL-C level recorded during treatment. The target LDL-C concentration is established based on the baseline LDL-C concentration and the patient's cardiovascular risk. The following columns define the recommended drug and dose, or combination of lipid-lowering drugs in accordance with the target. The cells in dark grey represent the various therapeutic options to achieve secondary prevention objectives after an ACS (LDL-C<70mg/dl).
Guidelines for the prescription of lipid-lowering therapy aimed at achieving the treatment targets. A: atorvastatin; EZ: ezetimibe; F: fluvastatin; LDL-C: low-density lipoprotein cholesterol; P: pravastatin; R: rosuvastatin; S: simvastatin; %RED: percentage reduction. Column 1: baseline low-density lipoprotein (LDL) value of the patient to be treated. Columns 2–4: percentage reduction of LDL required to achieve the treatment targets in primary prevention (column 2) and in secondary prevention, diabetes, metabolic syndrome and high overall cardiovascular risk (column 3). Column 4: in accordance with new recommendations LDL<70mg/dl. Columns 5–31: drugs or drug combinations that facilitate the necessary LDL reduction to achieve the treatment targets. In green for primary prevention. In orange for secondary prevention. In red the new recommendations LDL<70mg/dl. How to use: 1. Establish the baseline LDL value. 2. Calculate the reduction required to achieve the target of LDL<130mg/dl, <100mg/dl or <70mg/dl in the adjacent columns. 3. Follow the row. 4. The cells in light grey indicate the suitable treatments for achieving the targets in primary prevention. 5. The cells in medium grey indicate the suitable treatments for achieving the targets in secondary prevention, diabetes, metabolic syndrome and high overall cardiovascular risk, etc. 6. The cells in dark grey indicate the new recommendations of LDL=70mg/dl.
Demographic data, cardiovascular risk factors and the lipid-lowering therapy prior to hospitalisation were collected upon admission. The cardiovascular risk factors were defined as follows: (1) Active smoker: current smokers or former smokers who have quit in the last 12 months; (2) Hypertension, diabetes mellitus or dyslipidaemia: if reported by the patient or recorded in the medical record, or if the patient received pharmacological treatment for the condition.
A baseline blood test was performed within the first 24h of admission, recording total cholesterol, cholesterol fractions and triglycerides. The LDL-C test was performed using the Friedewald formula or enzymatic method if the triglyceride concentration was >400mg/dl.
For the purposes of the study, the therapy planning tables were deemed to have been followed if the chosen lipid-lowering therapy was the same as, or superior to that recommended by the tables based on the patient's baseline LDL-C. Given that the Masana tables encompass the use of combination therapy with ezetimibe, high-intensity lipid-lowering therapy (including statins in monotherapy or statins with ezetimibe in combination) was defined as treatment capable of achieving 50–60% LDL-C reductions, and very-high intensity therapy as capable of achieving reductions in excess of 60%.20
Follow-upThe clinical follow-up of patients after discharge was performed at the primary care centres of our healthcare network. The lipid profile was monitored using the Historia Clínica Compartida de Cataluña [Shared Medical Records Network of Catalonia], which also collected data from the clinical follow-up.
To assess LDL-C control, the first lipid profile performed after the ACS was taken, provided that it was conducted between one and 12 months after the ACS. Patients with an LDL-C concentration <70mg/dl were deemed to be adequately controlled, consistent with the European clinical practice guidelines.21,22
Statistical analysisA descriptive analysis of the data was performed in which the results of the normal quantitative variables were expressed as mean±standard deviation, the non-normal quantitative variables as median [interquartile range] and the qualitative variables as frequency and percentage. The Student's t-test was used to compare the normal quantitative variables in two independent groups, the Kruskal–Wallis test for non-normal quantitative variables and the Chi-squared test for qualitative variables. A multiple logistic regression model, which included as independent variables those with a p-value<0.05 according to the univariate analysis, was applied to evaluate the factors associated with the treatment target of an LDL cholesterol level <70mg/dl (dependent variable). The analyses were performed using R statistical software, version 3.4.1. (R: A language and environment for statistical computing, Vienna, Austria.)
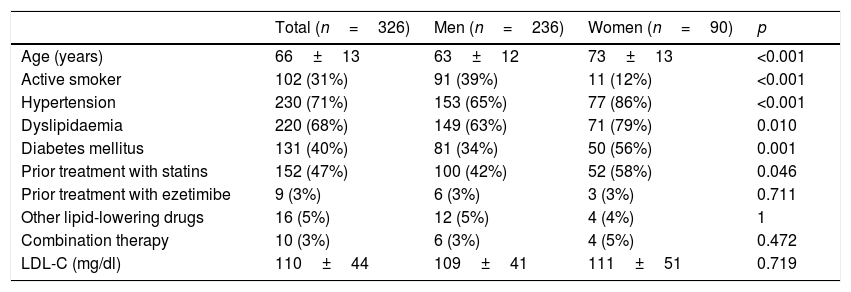
ResultsStudy populationIn total, 396 patients were consecutively enrolled at our centre between January 2015 and May 2016 due to an ACS. Eighteen (18) patients were excluded having died in the first three months and 52 due to a lack of LDL-C blood test in the first year, leaving a study population of 326 patients. The baseline clinical characteristics, blood tests and treatment prior to admission are shown in Table 1. Noteworthy findings include the fact that women were older (p<0.001) and had a higher prevalence of hypertension (p<0.001), diabetes mellitus (p=0.001), dyslipidaemia (p=0.01) and prior statin use (p=0.046).
Baseline characteristics of the study population distributed by gender.
| Total (n=326) | Men (n=236) | Women (n=90) | p | |
|---|---|---|---|---|
| Age (years) | 66±13 | 63±12 | 73±13 | <0.001 |
| Active smoker | 102 (31%) | 91 (39%) | 11 (12%) | <0.001 |
| Hypertension | 230 (71%) | 153 (65%) | 77 (86%) | <0.001 |
| Dyslipidaemia | 220 (68%) | 149 (63%) | 71 (79%) | 0.010 |
| Diabetes mellitus | 131 (40%) | 81 (34%) | 50 (56%) | 0.001 |
| Prior treatment with statins | 152 (47%) | 100 (42%) | 52 (58%) | 0.046 |
| Prior treatment with ezetimibe | 9 (3%) | 6 (3%) | 3 (3%) | 0.711 |
| Other lipid-lowering drugs | 16 (5%) | 12 (5%) | 4 (4%) | 1 |
| Combination therapy | 10 (3%) | 6 (3%) | 4 (5%) | 0.472 |
| LDL-C (mg/dl) | 110±44 | 109±41 | 111±51 | 0.719 |
The results are expressed as a number (%) or mean±standard deviation.
p<0.05 was considered to be statistically significant.
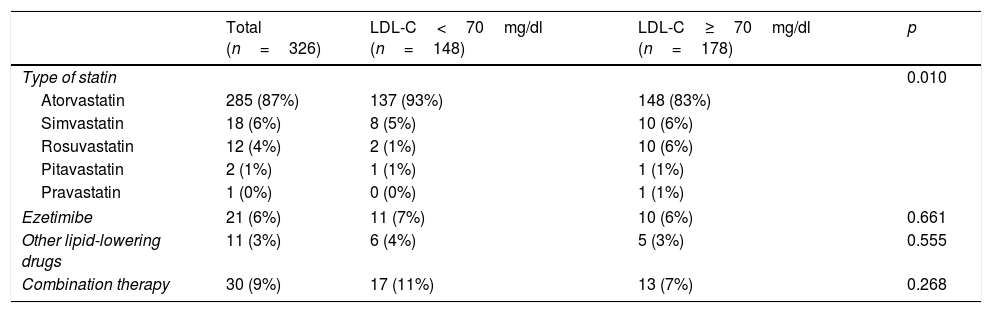
In total, 98% of patients (318/326) received treatment with statins. Table 2 shows the lipid-lowering therapy indicated upon discharge following an ACS. High-intensity lipid-lowering therapy was prescribed in 80% of patients (260/326) and very high-intensity therapy in 6% (19/326). The combination of statins and ezetimibe was used in 9% (30/326).
Achievement of the target LDL-C (LDL<70mg/dl) during follow-up by lipid-lowering therapy at discharge following an acute coronary syndrome.
| Total (n=326) | LDL-C<70mg/dl (n=148) | LDL-C≥70mg/dl (n=178) | p | |
|---|---|---|---|---|
| Type of statin | 0.010 | |||
| Atorvastatin | 285 (87%) | 137 (93%) | 148 (83%) | |
| Simvastatin | 18 (6%) | 8 (5%) | 10 (6%) | |
| Rosuvastatin | 12 (4%) | 2 (1%) | 10 (6%) | |
| Pitavastatin | 2 (1%) | 1 (1%) | 1 (1%) | |
| Pravastatin | 1 (0%) | 0 (0%) | 1 (1%) | |
| Ezetimibe | 21 (6%) | 11 (7%) | 10 (6%) | 0.661 |
| Other lipid-lowering drugs | 11 (3%) | 6 (4%) | 5 (3%) | 0.555 |
| Combination therapy | 30 (9%) | 17 (11%) | 13 (7%) | 0.268 |
The results are expressed as a number (%).
p<0.05 was considered to be statistically significant.
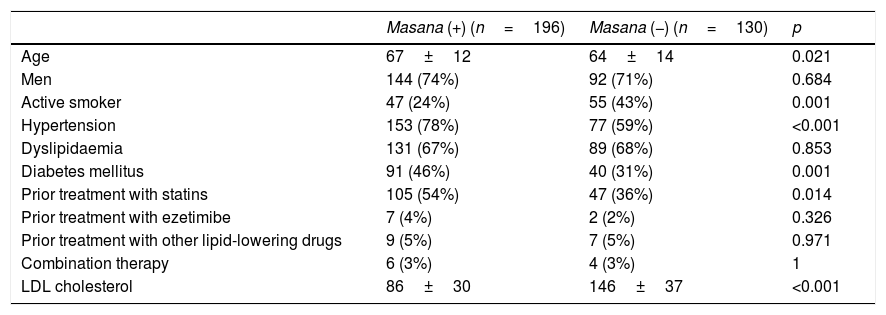
The recommendations of Masana were only adhered to in 196 patients (60%), while in the remaining 40% (130/326), lipid-lowering therapy below that recommended was prescribed.
The patients who adhered to the recommendations of Masana were older (p=0.021), had a history of hypertension (p<0.001) and diabetes mellitus (p=0.007) and had received prior treatment with statins (p=0.014), which meant that their LDL-C concentrations at admission were lower (p<0.001; Table 3).
Factors associated with adherence to the Masana criteria in the prescription of lipid-lowering drugs following an acute coronary syndrome.
| Masana (+) (n=196) | Masana (−) (n=130) | p | |
|---|---|---|---|
| Age | 67±12 | 64±14 | 0.021 |
| Men | 144 (74%) | 92 (71%) | 0.684 |
| Active smoker | 47 (24%) | 55 (43%) | 0.001 |
| Hypertension | 153 (78%) | 77 (59%) | <0.001 |
| Dyslipidaemia | 131 (67%) | 89 (68%) | 0.853 |
| Diabetes mellitus | 91 (46%) | 40 (31%) | 0.001 |
| Prior treatment with statins | 105 (54%) | 47 (36%) | 0.014 |
| Prior treatment with ezetimibe | 7 (4%) | 2 (2%) | 0.326 |
| Prior treatment with other lipid-lowering drugs | 9 (5%) | 7 (5%) | 0.971 |
| Combination therapy | 6 (3%) | 4 (3%) | 1 |
| LDL cholesterol | 86±30 | 146±37 | <0.001 |
The results are expressed as a number (%) or mean±standard deviation.
p<0.05 was considered to be statistically significant.
Masana (+)=adherence to the Masana recommendations.
Masana (−)=lack of adherence to the Masana recommendations.
In total, 45% of the 326 patients included (148/326) achieved the target LDL-C concentration <70mg/dl. This percentage increased to 56% (109/196 patients) for those patients who followed the Masana tables, and fell to 30% (39/130 patients) when the lipid-lowering therapy did not adhere to these recommendations (p<0.001).
The mean time to the first blood test after the ACS was 122 [66–184] days, with no differences found between patients who achieved adequate LDL-C control and those who did not (p=0.893).
Atorvastatin was the most commonly prescribed lipid-lowering drug of choice among patients who achieved their LDL-C objective (93% vs 83%, p=0.010; Table 2). However, no differences in the attainment of the target LDL-C concentration were found between patients receiving high- or very high-intensity lipid-lowering therapy (p=0.866). The choice of lipid-lowering therapy intensity was not influenced by gender (p=0.285).
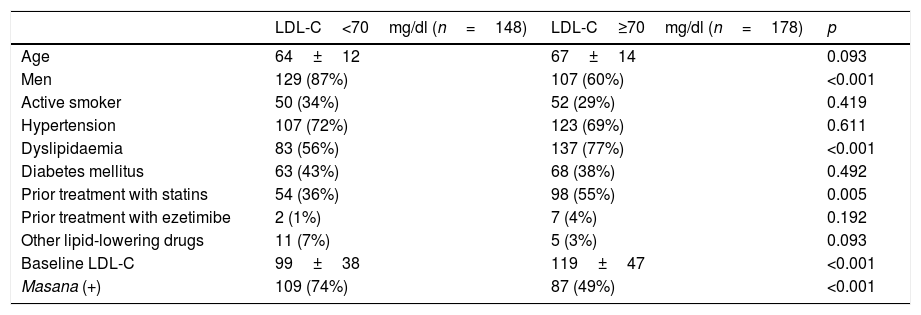
The population of patients that attained the treatment target (LDL-C<70mg/dl) was primarily male (p<0.001), had a lower prevalence of dyslipidaemia (p<0.001), lower baseline LDL-C concentrations (p<0.001) and lower prior use of statins (p<0.005). It also had greater adherence to Masana's recommendations (74% vs 49%, p<0.001; Table 4).
Factors associated with the achievement of the LDL-C target<70mg/dl during follow-up.
| LDL-C<70mg/dl (n=148) | LDL-C≥70mg/dl (n=178) | p | |
|---|---|---|---|
| Age | 64±12 | 67±14 | 0.093 |
| Men | 129 (87%) | 107 (60%) | <0.001 |
| Active smoker | 50 (34%) | 52 (29%) | 0.419 |
| Hypertension | 107 (72%) | 123 (69%) | 0.611 |
| Dyslipidaemia | 83 (56%) | 137 (77%) | <0.001 |
| Diabetes mellitus | 63 (43%) | 68 (38%) | 0.492 |
| Prior treatment with statins | 54 (36%) | 98 (55%) | 0.005 |
| Prior treatment with ezetimibe | 2 (1%) | 7 (4%) | 0.192 |
| Other lipid-lowering drugs | 11 (7%) | 5 (3%) | 0.093 |
| Baseline LDL-C | 99±38 | 119±47 | <0.001 |
| Masana (+) | 109 (74%) | 87 (49%) | <0.001 |
The results are expressed as a number (%) or mean±standard deviation.
p<0.05 was considered to be statistically significant.
Masana (+)=adherence to the Masana recommendations.
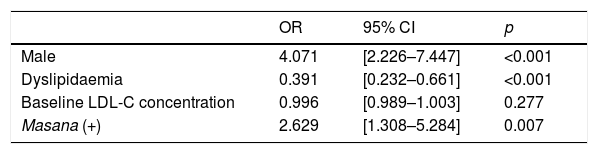
In the multivariate analysis, the male gender (OR=4.071; p<0.001), a lack of dyslipidaemia prior to admission (OR=0.391; p<0.001) and treatment upon discharge in line with the Masana tables (OR=2.629; p=0.007) were independent predictors for achieving the target LDL-C concentration during follow-up (Table 5).
Factors associated with the achievement of the LDL cholesterol target<70mg/dl: Logistic regression model (multivariate analysis).
| OR | 95% CI | p | |
|---|---|---|---|
| Male | 4.071 | [2.226–7.447] | <0.001 |
| Dyslipidaemia | 0.391 | [0.232–0.661] | <0.001 |
| Baseline LDL-C concentration | 0.996 | [0.989–1.003] | 0.277 |
| Masana (+) | 2.629 | [1.308–5.284] | 0.007 |
95% CI: 95% confidence interval; OR: odds ratio.
Masana (+)=adherence to the Masana recommendations.
This study found that the application of the Masana therapy planning tables were associated with the attainment of the target LDL-C levels<70mg/dl as defined in the current clinical practice guidelines (p<0.001). However, these recommendations were only applied to 60% of patients following an ACS, which had a negative impact on the attainment of target LDL-C levels (p<0.001). Our results highlight the importance of planning lipid-lowering therapy immediately after an ACS and, in this regard, the application of the Masana tables represents a significant aid in the attainment of this crucial treatment target.
Suboptimal low-density lipoprotein cholesterol control following an acute coronary syndromeDespite the fact that lower LDL-C levels significantly reduce cardiovascular morbidity and mortality in patients with coronary heart disease,2–5 in our study, less than half of the patients (45%) achieved LDL-C concentrations<70mg/dl. Although low, these dyslipidaemia control rates are actually higher than those published in previous studies6–9 and are consistent with the most up-to-date registers in Spain, such as the LIPICERES study.23
Attainment of the treatment target was poor despite the widespread prescription of high-intensity statins as recommended by the clinical practice guidelines.20,21,24 It would therefore appear that the use of high-intensity statins is not an effective strategy for attaining target LDL-C levels.
After the publication of the IMPROVE-IT study,25 the addition of ezetimibe is recommended in patients with LDL-C levels>70mg/dl despite administration of a high-intensity statin at the maximum tolerated dose. Only 9% of patients in this study were receiving combination therapy with ezetimibe. Other authors14,15 like Masana recommend the early use of combination therapy to increase attainment of the treatment target, reduce the number of side effects caused by high-dose statin regimens and promote treatment adherence.26 Planning lipid-lowering therapy using the Masana tables prevents the initial underdosing of statins and assesses the need for adjuvant therapy with ezetimibe from the outset, thereby promoting the rapid attainment of the treatment target.
Predictive factors for achieving the low-density lipoprotein cholesterol target of <70mg/dl. Usefulness of the tables proposed by MasanaIn our multiple regression model, the male gender (OR=4.071; p<0.001), a lack of prior dyslipidaemia (OR=0.391; p<0.001) and the planning of lipid-lowering therapy in accordance with the Masana therapy planning tables (OR=2.629; p=0.007) were predictive factors for achieving the target LDL-C of <70mg/dl within 122 [66–184] days of an ACS.
The predominance of the male gender in achieving target LDL-C levels has been found in several studies.27–32 Lower use of statins and high-intensity statins among women, a potentially reduced response to statins and greater statin intolerance seem to be plausible explanations for these gender differences. Our study found that atorvastatin was more frequently prescribed in men (p=0.016), although no differences between the two intensities of lipid-lowering therapy were observed (p=0.285).
A lack of dyslipidaemia prior to admission for ACS should also be analysed. It is important to note that patients with a prior diagnosis of dyslipidaemia had higher baseline LDL-C concentrations and most were already receiving statins. As such, they represent a subgroup of patients in whom achieving the target LDL-C concentration is particularly challenging.
Finally, the prescription of lipid-lowering therapy guided by the Masana and Plana therapy planning tables was associated with a higher target achievement rate. The results of our study highlight the efficacy of using this clinical tool to prescribe the most effective lipid-lowering therapy to patients with an ACS and to achieve the target LDL-C levels.
Limitations of the studyThe main limitation of the study derives from its observational, non-randomised design, with the resulting potential selection bias in patients in whom the Masana tables were applied. Despite this, our study presents results from actual clinical practice and suggests a positive influence of adhering to the recommendations of Masana.
As the study was being conducted, the updated 2015 Masana tables were published, which primarily introduced changes to the population of patients in primary prevention and to patients who had previously received lipid-lowering therapy, and added two new statins.17
In our study, the duration of follow-up during which the reference LDL-C values were collected after the ACS was relatively short (122 [66–184] days), leaving little time for appropriate titration of the lipid-lowering therapy. Nevertheless, previous studies have found disappointing long-term lipid-lowering therapy titration rates.27 In this regard, the results of our study support the efficacy of titration guided by the Masana and Plana tables.
ConclusionsDyslipidaemia control has seen a gradual improvement over the last few decades. Despite this, fewer than half of our patients achieved appropriate LDL-C levels (<70mg/dl) after an ACS. The use of clinical tools like the tables proposed by Masana and Plana to plan lipid-lowering therapy upon discharge following an ACS improves the attainment rates of this crucial treatment target.
AuthorshipThe manuscript was conceived and designed by Núria Ribas, Lluís Recasens, Silvia Pérez and Roberto Elosua. The data were collected by Núria Ribas, Lluís Recasens, Paula Poveda and Sonia Ruiz. The data were analysed and interpreted by Silvia Pérez, Víctor Bazán, Juan Pedro-Botet, Roberto Elosua and Cosme García-García. The manuscript was drafted by Núria Ribas, Lluís Recasens, Víctor Bazán and Juan Pedro-Botet. All the authors reviewed and approved the submitted manuscript.
Conflicts of interestThe authors of the manuscript declare that they have no conflicts of interests.
Please cite this article as: Ribas N, Recasens L, Pérez S, Bazán V, Pedro-Botet J, Ruiz S, et al. Una nueva estrategia para alcanzar los niveles objetivos de colesterol LDL tras un síndrome coronario agudo. Clin Investig Arterioscler. 2019;31:93–100.
This study was carried out within the framework of the Doctoral Programme in Medicine of the Universidad Autónoma de Barcelona [Autonomous University of Barcelona].