After an ischemic cerebrovascular event the risk of new ischemic events is high, therefore antithrombotic therapy are indicated to prevent stroke recurrence.
DiscussionDespite its clear benefit, these therapies increase the risk of bleeding. Therefore, it is essential to identify high hemorrhagic risk patients.
There are different predictive models of hemorrhage, in particular of intracranial hemorrhage, associated with the use of antiaggregants in patients who have presented an ischemic stroke or TIA, such as the CCSC, intracranial scales – B2LEED3S score or S2TOP-BLEED. However, though main international guidelines recommend the use of scales, in particular, the HAS-BLED score, to assess the risk of bleeding in anticoagulated patients, there is no specific recommendation in the case of the use of antiplatelet drugs.
ConclusionsIn this review we present the main models currently available for the prediction of bleeding of antithrombotic therapy in patients who have had a stroke or TIA.
Tras un evento cerebrovascular isquémico el riesgo de recurrencias es elevado, por lo que se hace necesario el uso de terapia antitrombótica para disminuir nuevos eventos.
DesarrolloA pesar de su beneficio, estas terapias aumentan el riesgo de sangrado. Por tanto, determinar qué pacientes presentan mayor riesgo de hemorragia es fundamental. Existen diferentes modelos predictores de hemorragia y, en particular, de hemorragia intracraneal, asociados al uso de antiagregantes en pacientes con ictus isquémico o AIT, como las escalas CCSC, Intracranial-B2LEED3S score o la S2TOP-BLEED. No obstante, mientras que las principales guías internacionales recomiendan el uso de escalas, como HAS-BLED, para evaluar el riesgo de sangrado en pacientes anticoagulados, no existe una recomendación específica en el caso del uso de antiagregantes.
ConclusionesEn esta revisión se presentan los principales modelos disponibles en la actualidad para la predicción de sangrado de la terapia antitrombótica en pacientes con ictus o AIT.
In Spain, stroke is a major problem, since it is one of the main causes of morbidity, mortality and hospitalization, constituting the second leading cause of death in the general population, the first in women, and the leading cause of disability.1 In addition, in the year 2015 alone, 119,353 strokes and 14,940 transient ischemic accidents (TIA) were admitted to the hospitals of the National Health System.2 Therefore, the care of patients with a previous stroke is a frequent problem in daily clinical practice.
After an ischemic stroke or TIA the risk of occurrence of new ischemic events is high, up to 30% in 5 years,3 therefore, it is necessary to use a specific treatment that prevents recurrence. At present, antithrombotic therapy is the cornerstone in the secondary prevention of stroke, either through the use of platelet antiaggregants or anticoagulants mainly in patients with cardioembolic strokes. Both therapies reduce the risk of stroke recurrence; thus antiplatelet therapy does so by 25%, while anticoagulation in the context of non-valvular atrial fibrillation (AF) (NVAF) by approximately 60%.4
Despite their clear benefit, these therapies increase the risk of bleeding, some of special severity—such as intracranial hemorrhage (ICH)—, which often limits the use of these drugs, especially in patients with previous bleeding.
On average, the overall risk of bleeding is doubled in patients treated with aspirin compared to placebo, or even more so if we use dual antiplatelet therapy (DAPT), especially by increasing the risk of severe bleeding such as ICH.5 On the other hand, anticoagulation with vitamin K antagonists (VKA) in NVAF increases the risk of major bleeding by 0.3–0.5% per year and, in particular, the risk of ICH, which is the main cause of death and disability associated with treatment with VKA, increases by approximately 0.2% per year compared to controls.4
In fact, although extracranial haemorrhages, predominantly of gastrointestinal (GI) origin, are much more common than ICHs, intracranial hemorrhages are usually more severe. Only 5.1% of extracranial hemorrhages with warfarin cause death at 30 days, compared with a mortality rate close to 50% in patients with ICH associated with warfarin.6
Therefore, it is essential to make an adequate risk/benefit assessment of antithrombotic treatment based on the characteristics of the patient, so that the prediction of bleeding risk can help physicians make clinical decisions, not only in relation to the use of antithrombotic agents, but also in the use of gastroprotective agents, among others.
ProcedureSince antithrombotic therapy is key in the secondary prevention of stroke, the main models that we currently have for the prediction of bleeding risk in patients who have presented an ischemic stroke or TIA and initiate antithrombotic therapy are presented below.
For this, a narrative review of the current medical literature has been carried out through an advanced search using the PubMed web platform.
Risk of bleeding with antiplatelet agentsAs we have mentioned, chronic antiplatelet therapy in the post-acute phase of non-cardioembolic ischemic stroke is limited by the risk of bleeding complications, particularly ICH. Thus, for example, in relation to the annual risk of major bleeding, in a meta-analysis of 25 randomized trials of antiplatelet therapy in the primary or secondary prevention of cardiovascular disease,7 aspirin increased the absolute annual risk of severe bleeding by 0.13% (number needed to harm 769). In particular, and focusing on ICH, in the Swedish Aspirin Low dose Trial (SALT) study,8 in patients with ischemic stroke or TIA randomized against placebo, the 2-year risk of ICH was 0.34% versus 1.14% of those treated with aspirin. In the Clopidogrel Aspirin in Prevention of Recurrent Ischemic Event (CAPRIE) trial,9 the 2-year risk of ICH varied based on the antiplatelet agent used: clopidogrel 0.37%, aspirin 0.52% or 1.41% with DAPT (clopidogrel plus aspirin).
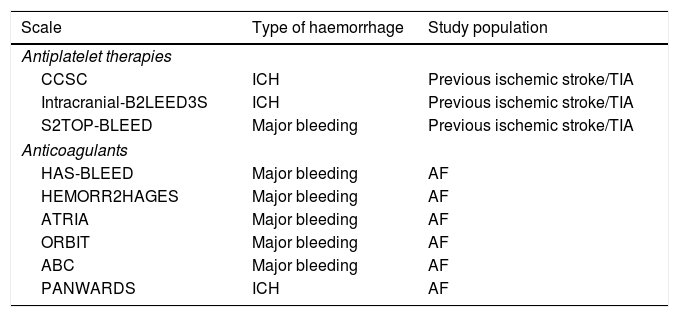
There are different predictive models of hemorrhage and, in particular, of ICH associated with the use of antiplatelet therapy in patients who have presented an ischemic stroke or TIA (Table 1).
Main bleeding risk scales in patients with antithrombotic treatment.
| Scale | Type of haemorrhage | Study population |
|---|---|---|
| Antiplatelet therapies | ||
| CCSC | ICH | Previous ischemic stroke/TIA |
| Intracranial-B2LEED3S | ICH | Previous ischemic stroke/TIA |
| S2TOP-BLEED | Major bleeding | Previous ischemic stroke/TIA |
| Anticoagulants | ||
| HAS-BLEED | Major bleeding | AF |
| HEMORR2HAGES | Major bleeding | AF |
| ATRIA | Major bleeding | AF |
| ORBIT | Major bleeding | AF |
| ABC | Major bleeding | AF |
| PANWARDS | ICH | AF |
TIA: transient ischemic attack; AF: atrial fibrillation; ICH: intracranial hemorrhage.
The first was based on the database of 8 cohorts and 12,648 patients, the Cerebrovascular Cohort Studies Collaboration (CCSC).10 In this study, the following predictors related to the risk of ICH were identified in the Cox multivariate regression analysis: age (>60 years, Hazard ratio [HR] – 2.07), systolic blood pressure (≥140mmHg, HR 2.17), use of antihypertensive drugs (HR 1.53) and, as a protector, blood glucose levels (≥7mmol/l, HR 0.33). The highest risk quartile was associated with 5 times greater risk of ICH than the lowest quartile.
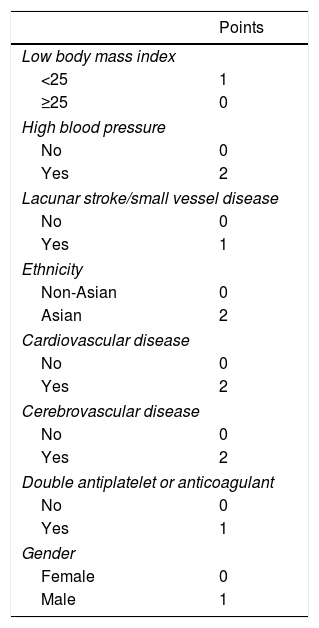
Later, the Intracranial-B2LEED3S score (body mass index, blood pressure, lacune, elderly, Asian ethnicity, coronary artery or cerebrovascular disease history, dual antithrombotic agent or oral anticoagulant, sex) was performed11 (Table 2), based on the cohort of patients of the PERFORM study,12 which included 19,100 patients with TIA or non-cardioembolic ischemic stroke, and which was also validated in a trial of similar characteristics, the PRoFESS study,13 which included 20,332 subjects who had had a non-cardioembolic ischemic stroke in the last 120 days. Where age, blood pressure and having a low body mass index were the greatest predictors of HIC. The scale is based on 9 items, with a score between 0 and 13 points. In PERFORM, the observed risk of ICH at 2 years varied from 0.75% in those at low risk (score ≤2) to 2.44% in high-risk patients (score ≥5) with an acceptable calibration but low discrimination in both PERFORM (C statistic: 0.64; 95% confidence interval [CI]: 0.61–0.68) and in external validation in PRoFESS (0.58; 95% CI: 0.55–0.62). And, though the score of Intracraneal-B2LEED3S helps identify patients who have a high risk of bleeding, the authors themselves point out that other variables would need to be incorporated to improve the score, such as, for example, the presence of microhemorrhages on magnetic resonance imaging.
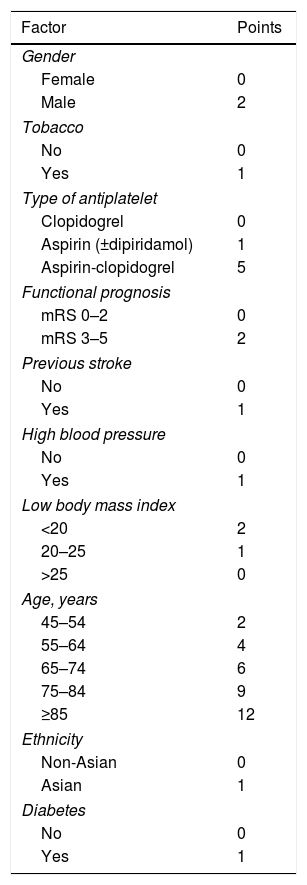
S2TOP-BLEED scale.
| Factor | Points |
|---|---|
| Gender | |
| Female | 0 |
| Male | 2 |
| Tobacco | |
| No | 0 |
| Yes | 1 |
| Type of antiplatelet | |
| Clopidogrel | 0 |
| Aspirin (±dipiridamol) | 1 |
| Aspirin-clopidogrel | 5 |
| Functional prognosis | |
| mRS 0–2 | 0 |
| mRS 3–5 | 2 |
| Previous stroke | |
| No | 0 |
| Yes | 1 |
| High blood pressure | |
| No | 0 |
| Yes | 1 |
| Low body mass index | |
| <20 | 2 |
| 20–25 | 1 |
| >25 | 0 |
| Age, years | |
| 45–54 | 2 |
| 55–64 | 4 |
| 65–74 | 6 |
| 75–84 | 9 |
| ≥85 | 12 |
| Ethnicity | |
| Non-Asian | 0 |
| Asian | 1 |
| Diabetes | |
| No | 0 |
| Yes | 1 |
mRS: modified Rankin scale.
Recently, another scale has been published that includes the risk of major hemorrhage in patients with ischemic stroke or TIA treated with antiplatelet agents, the S2TOP-BLEED score (male sex, smoking, type of antiplatelet agents, outcome on mRS, prior stroke, high blood pressure, lower BMI, elderly, Asian ethnicity, and diabetes)14 (Table 3). The scale calculates the 3-year risk of major bleeding. This scale is based on data from 6 randomized clinical trials (CAPRIE, ESPS-2, MATCH, CHARISMA, ESPRIT and ProFESS) which include 43,112 patients and which has subsequently been validated with the patients of the PERFORM study. The S2TOP-BLEED has a C statistic of 0.63 (95% CI: 0.60–0.64). The risk of major bleeding ranged from 2% in patients 45–54 years without additional risk factors to more than 10% in patients 75–84 years with multiple risk factors. In external validation, the model showed a C statistic of 0.61 (95% CI: 0.59–0.63), with a slightly underestimated risk of major bleeding.
Intracranial-B2LEED3S scale.
| Points | |
|---|---|
| Low body mass index | |
| <25 | 1 |
| ≥25 | 0 |
| High blood pressure | |
| No | 0 |
| Yes | 2 |
| Lacunar stroke/small vessel disease | |
| No | 0 |
| Yes | 1 |
| Ethnicity | |
| Non-Asian | 0 |
| Asian | 2 |
| Cardiovascular disease | |
| No | 0 |
| Yes | 2 |
| Cerebrovascular disease | |
| No | 0 |
| Yes | 2 |
| Double antiplatelet or anticoagulant | |
| No | 0 |
| Yes | 1 |
| Gender | |
| Female | 0 |
| Male | 1 |
In our experience, the S2TOP-BLEED scale is the most useful for various reasons: because it includes in the prognosis all types of major hemorrhages—not just HIC—and due to its methodology (it is based on a larger number of patients and populations of different randomized clinical trials). The predefined risk groups include low risk (0–10 points in the S2TOP-BLEED score), medium risk (11–15 points) and high risk (>15 points).14 In addition, it has recently been validated in 2072 patients with ischemic stroke or TIA in treatment with antiplatelet agents in an English population (the OXVASC [Oxford Vascular Study]) with acceptable statistical performance—C of 0.69 (95% CI: 0.64–0.73).15
Risk of bleeding with anticoagulantsAt the end of the 1990s, at the same time that different schemes of stratification of stroke risk appeared in patients with AF, the development of scoring systems that assessed the risk of bleeding in those same patients began.
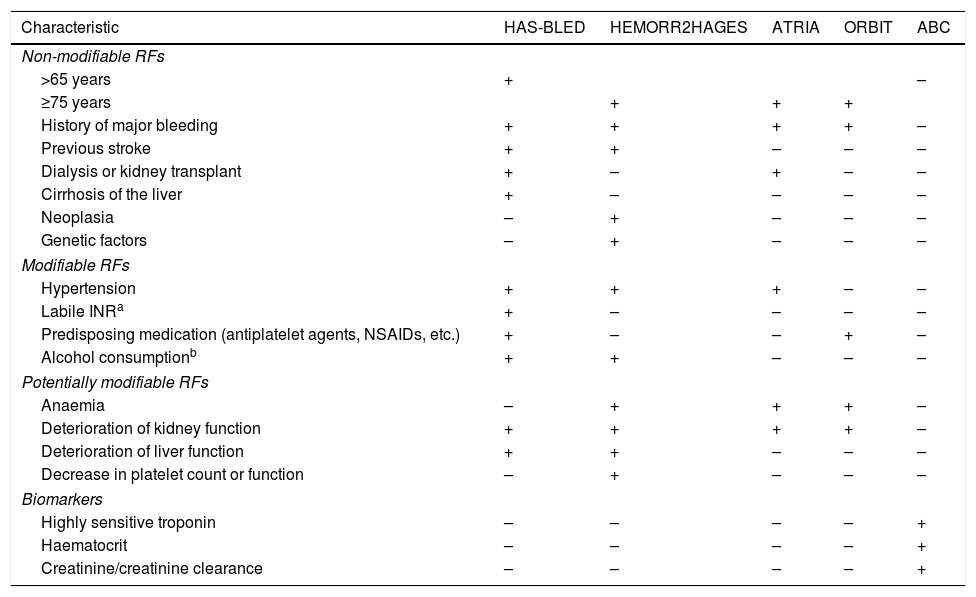
Currently, there are several validated scoring systems that can be used to assess the risk of bleeding in anticoagulated patients for AF, including the HAS-BLED score16 (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly – >65 years –, drugs/alcohol concomitantly, available online at: https://www.mdcalc.com/has-bled-score-major-bleeding-risk; in an app: https://play.google.com/store/apps/details?id=com.gumptionmultimedia.hasbledcalculator&hl=es;https://itunes.apple.com/us/app/has-bled/id734518338?mt=8), the HEMORR2HAGES scale17 (hepatic or renal disease, ethanol abuse, malignancy, older age, reduced platelet count or function, re-bleeding, hypertension, anemia, genetic factors, excessive fall risk and stroke, available online at: https://www.mdcalc.com/hemorr2hages-score-major-bleeding-risk), ATRIA18 (Anticoagulation and Risk Factors in Atrial Fibrillation, available online at: https://www.mdcalc.com/atria-bleeding-risk-score), the ORBIT scale19 (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation) and, more recently, the ABC bleeding scale20 (age, biomarkers, clinical history), which also makes use of selected biomarkers.
These scores were obtained and validated in different study cohorts, ranging from clinical trial cohorts to “real world” populations. Thus, for example, the HAS-BLED scale, which was published in 2010, was designed from only 53 major bleeding events in a sample of 3978 patients of the Euro Heart Survey of AF.16
Therefore, the performance and accuracy of classification of the different scales vary depending on their derivation cohort.21
As with antiplatelet therapy, risk factors for stroke and hemorrhage overlap on most scales. For example, advanced age is one of the most important predictors for both stroke and bleeding in patients with AF (Table 4).
RF of bleeding in patients anticoagulated for AF according to the different risk scales.
| Characteristic | HAS-BLED | HEMORR2HAGES | ATRIA | ORBIT | ABC |
|---|---|---|---|---|---|
| Non-modifiable RFs | |||||
| >65 years | + | – | |||
| ≥75 years | + | + | + | ||
| History of major bleeding | + | + | + | + | – |
| Previous stroke | + | + | – | – | – |
| Dialysis or kidney transplant | + | – | + | – | – |
| Cirrhosis of the liver | + | – | – | – | – |
| Neoplasia | – | + | – | – | – |
| Genetic factors | – | + | – | – | – |
| Modifiable RFs | |||||
| Hypertension | + | + | + | – | – |
| Labile INRa | + | – | – | – | – |
| Predisposing medication (antiplatelet agents, NSAIDs, etc.) | + | – | – | + | – |
| Alcohol consumptionb | + | + | – | – | – |
| Potentially modifiable RFs | |||||
| Anaemia | – | + | + | + | – |
| Deterioration of kidney function | + | + | + | + | – |
| Deterioration of liver function | + | + | – | – | – |
| Decrease in platelet count or function | – | + | – | – | – |
| Biomarkers | |||||
| Highly sensitive troponin | – | – | – | – | + |
| Haematocrit | – | – | – | – | + |
| Creatinine/creatinine clearance | – | – | – | – | + |
NSAIDs: non-steroidal anti-inflammatory drugs; AF: atrial fibrillation; RF: risk factors; INR: International Normalized Ratio.
Regarding the superiority of one scale with respect to another, the results of the different comparative studies are variable.22
However, a recent meta-analysis23 concludes that the HAS-BLED scale should be the optimal option to assess the risk of major bleeding in clinical practice in anticoagulated patients with AF. In addition, other scales, such as HEMORR2HAGES or ABC, are used less in clinical practice because, for example, the former involves numerous factors and requires the use of genetic studies, and the second requires blood biomarkers such as troponin C, haematocrit or creatinine clearance.
Due to its simplicity and the inclusion of modifiable risk factors (e.g., uncontrolled hypertension, use of concurrent antiplatelet agents, nonsteroidal anti-inflammatory drugs or excessive alcohol consumption), the European and Canadian guidelines incorporate in their guidelines and recommend the use of the HAS-BLED score to assess the risk of bleeding in patients with AF.21,24
Therefore, in patients with cardioembolic stroke in the context of a NVAF, once the indication for treatment with oral anticoagulants has been established, the hemorrhagic risk should be calculated with the HAS-BLED scale. A HAS-BLED score ≥3 indicates “high risk” of bleeding with dicumarinics.
However, a high risk of bleeding for a patient does not mean that the use of anticoagulants should be avoided. Instead, in most cases, an anticoagulant should be used with due caution. Regular follow-up appointments should be scheduled and measures taken to resolve any modifiable risk factor that contributes to the patient's high risk.25
The possibility of other therapeutic alternatives, such as the new direct anticoagulants (DOAC) or auricular occlusion with implantable devices should also be assessed.26
The safety and efficacy of DOACs (rivaroxaban, dabigatran, apixaban and edoxaban) are well established from phase III studies in patients with NVAF. However, since its marketing, multiple studies and meta-analyses have appeared assessing the hemorrhagic risk of DOACs.
In general, DOACs are just as or more effective as warfarin for the prevention of systemic stroke and embolism, and the rates of major bleeding were similar or lower in patients treated with DOACs compared to patients treated with warfarin. However, rivaroxaban, the highest doses of dabigatran (150mg twice daily) and edoxaban (60mg once daily) appear to be associated with significantly higher rates of higher GI bleeding.27
Another more recent meta-analysis,28 for example, which assessed the risk of GI and intracranial bleeding revealed that only aspirin + clopidogrel increased the risk of GI bleeding compared to placebo (OR 0.33, 95% CI: 0.01–0.92), and no statistically significant differences were found in patients with AF treated with aspirin, warfarin or DOAC (dabigatran, edoxaban, rivaroxaban, apixaban) compared with placebo or among them. Regarding ICH, although no significant differences were found between any anticoagulant and placebo, warfarin conferred a significantly higher risk of ICH compared to edoxaban 30mg (OR 3.42, 95% CI: 1.22–7.24) and dabigatran 110mg (OR 3.56, 95% CI: 1.10–8.45). No DOAC increased the risk of ICH compared to standard treatment or placebo treatment.
These results have also been proven in patients with previous stroke. In a recent meta-analysis on 20,500 patients with FA and previous stroke or TIA29 It is shown that, compared to warfarin, DOACs are associated with a significant reduction not only in stroke (relative risk reduction: 13.1%), but also in ICHs (relative risk reduction: 46.1%, absolute risk reduction: 0.88%, number needed to treat [NNT]: 113 patients for 1.8–2.8 years).
In fact, in the latest recommendations of the European Society of Cardiology, DOACs are recommended in preference to VKA or aspirin in patients with AF and previous stroke.21
However, it is important to note that these bleeding risk scales in anticoagulated patients have multiple limitations.
Thus, in general, the performance of the clinical prediction scores for hemorrhage is low (C statistic 0.65), being similar for the different scales, 0.65 for HAS-BLED, 0.63 for HEMORR2HAGES and 0.63 for ATRIA.23
The predictive value of the different bleeding scales also changes if we refer specifically to the risk of ICH. So, for example, the ability to predict the ICH of the HEMORR2HAGES and HAS-BLED scales in the Swedish population were similar, with an approximate C statistic of 0.629. Although other authors demonstrate a greater predictive power of ICH of the HAS-BLED scale with respect to the HEMORR2HAGE and ATRIA scales,30 and, more recently, of ABC with respect to HAS-BLED and ORBIT,20 probably nuanced by the population where it is applied.
In general, all these prognostic scales are biased towards the prediction of GI hemorrhages, which are the most common.
On the other hand, a specific scale has also been developed for predicting the risk of ICH in anticoagulated patients, such as the PANWARDS scale31 (platelets, albumin, no congestive heart failure, warfarin, age, race, diastolic blood pressure, stroke), derived from a cohort of 14,264 patients from the ROCKET AF study.
Importantly, the risk factors for major bleeding and ICH are often different. In particular, the most important risk factors for ICH associated with anticoagulant therapy are: older patients, presenting a previous ischemic stroke, concomitant antiplatelet therapy and the use of warfarin (vs. a DOAC).32
Thus, for example, in a cohort of patients in Sweden, the history of previous stroke or thromboembolism, severe hemorrhage events (ICH or major hemorrhage) and hypertension were significant predictors of ICH and major hemorrhage, while heart failure, diabetes, renal failure, liver disease, anaemia or platelet/clotting defect and cancer were significant predictors for major hemorrhage, but not ICH.33
ConclusionsAfter an ischemic cerebrovascular event, the risk of new vascular events is high, so it is necessary to use antithrombotic, antiplatelet or anticoagulation therapy, to reduce the risk of new ischemic episodes. However, despite their clear benefit, these therapies increase the risk of bleeding, which often limits the use of these drugs. Due to this fact, when indicating antithrombotic treatment it is essential to make an adequate risk/benefit assessment of the treatment based on the clinical characteristics of the patient. In that sense, various stratification scales have been developed that allow the risk of bleeding associated with antithrombotic treatment to be calculated.
There are different predictor models for hemorrhage and, in particular, ICH associated with the use of antiplatelet agents in patients who have presented an ischemic stroke or TIA, such as the CCSC, Intracranial-B2LEED3S score or the S2TOP-BLEED scales. However, while the main international guidelines recommend the use of scales, in particular the HAS-BLED score, to assess the risk of bleeding in anticoagulated patients, there is no specific recommendation in the case of the use of antiplatelet agents. In any case, the application of these risk scales will be very useful, not only when choosing the best therapeutic alternative for a specific patient, but also to identify and control modifiable bleeding risk factors, such as poor control of blood pressure, concomitant use of anti-inflammatories or excessive alcohol, among others.
We believe that a better knowledge of these scales and their greater application in clinical practice will result in an improvement in the care of our stroke patients.
Conflict of interestsNone.
Please cite this article as: Castilla-Guerra L, Fernández-Moreno MC, de la Vega-Sánchez JM, Leon Jimenez D. Evaluación del riesgo hemorrágico de la terapia antitrombótica en pacientes con ictus. Clín Investig Arterioscler. 2019;31:282–288.