Adequate lifestyle changes significantly reduce the cardiovascular risk factors associated with prediabetes and type 2 diabetes mellitus. Therefore, healthy eating habits, regular physical activity, abstaining from using tobacco, and good sleep hygiene are recommended for managing these conditions. There is solid evidence that diets that are plant-based; low in saturated fatty acids, cholesterol, and sodium; and high in fiber, potassium, and unsaturated fatty acids are beneficial and reduce the expression of cardiovascular risk factors in these subjects. In view of the foregoing, the Mediterranean diet, the DASH diet, a low-carbohydrate diet, and a vegan–vegetarian diet are of note. Additionally, the relationship between nutrition and these metabolic pathologies is fundamental in targeting efforts to prevent weight gain, reducing excess weight in the case of individuals with overweight or obesity, and personalizing treatment to promote patient empowerment.
This document is the executive summary of an updated review that includes the main recommendations for improving dietary nutritional quality in people with prediabetes or type 2 diabetes mellitus. The full review is available on the webpages of the Spanish Society of Arteriosclerosis, the Spanish Diabetes Society, and the Spanish Society of Internal Medicine.
Los cambios adecuados del estilo de vida reducen significativamente los factores de riesgo cardiovascular asociados a la prediabetes y la diabetes mellitus tipo 2, por lo que en su manejo se debe recomendar un patrón saludable de alimentación, actividad física regular, no consumir tabaco, y una buena higiene del sueño. Hay una sólida evidencia de que los patrones alimentarios de base vegetal, bajos en ácidos grasos saturados, colesterol y sodio, con un alto contenido en fibra, potasio y ácidos grasos insaturados, son beneficiosos y reducen la expresión de los factores de riesgo cardiovascular en estos sujetos. En este contexto destacan la dieta mediterránea, la dieta DASH, la dieta baja en hidratos de carbono y la dieta vegano-vegetariana. Adicionalmente, en la relación entre nutrición y estas enfermedades metabólicas es fundamental dirigir los esfuerzos a prevenir la ganancia de peso o a reducir su exceso en caso de sobrepeso u obesidad, y personalizar el tratamiento para favorecer el empoderamiento del paciente.
Este documento es un resumen ejecutivo de una revisión actualizada que incluye las principales recomendaciones para mejorar la calidad nutricional de la alimentación en las personas con prediabetes o diabetes mellitus tipo 2, disponible en las páginas web de la Sociedad Española de Arteriosclerosis, la Sociedad Española de Diabetes y la Sociedad Española de Medicina Interna.
Prediabetes and type 2 diabetes mellitus (DM2) are illnesses whose growing increase in the population has become a serious public health problem. From the clinical point of view, controlling these metabolic alterations directly affects cardiovascular morbidity and mortality, which makes it necessary to provide effective strategies for prevention and treatment.
Adequate changes in lifestyle significantly reduce cardiovascular risk (CVR) factors associated with prediabetes and DM2. Consequently, it is necessary to recommend a healthy diet, regular physical activity, abstention from smoking and good sleep hygiene in managing these 2 illnesses.1
There is solid evidence that following eating patterns based on vegetables, low in saturated fatty acids, cholesterol and sodium, with high fibre, potassium and unsaturated fatty acid content, is beneficial and reduces the expression of CVR factors in these individuals.2 In this context, the Mediterranean diet,3 the low carbohydrate (CH) diet4 and the vegan–vegetarian diet5 stand out.
In addition, in the relationship between nutrition and these metabolic illnesses, it is crucial to attempt to prevent weight gain or reduce excess weight if individuals are overweight or obese. It is also fundamental to individualise the treatment to favour patient empowerment.
In this paper, we present an up-dated review that provides useful evidence and hierarchical recommendations by levels. We use evidence from clinical trials (when available), observational studies on clinical evidence or substitution markers and expert consensus. Based on the preceding, 3 types of recommendations are given: (1) strong, based on clinical trials and meta-analyses incorporating criteria of quality; (2) moderate, supported by prospective cohort studies and by studies of cases and controls; and (3) weak, justified by consensuses and expert opinions or based on extensive clinical practice.
In short, there is evidence revealing that a high rate of adults present prediabetes or DM2, with the consequent short- and medium-term increase in CVR. Lifestyle, especially the diet, constitutes the basis of treatment to improve blood pressure, lipid and blood glucose control, and to reduce the elevated cardiovascular morbidity and mortality that appear in these individuals.1
This document is an executive summary of an updated review that includes the principal recommendations for improving the nutritional quality of the dietary intake of individuals with prediabetes or DM2. It is available at the websites of the Spanish Arteriosclerosis Society [Sociedad Española de Arteriosclerosis] (https://www.se-arteriosclerosis.org/guias-documentos-consenso), the Spanish Diabetes Society [Sociedad Española de Diabetes] (https://www.sediabetes.org/grid/?tipo=cursos_formacion&categoria=consensos-guias-y-recommendations) and the Spanish Internal Medicine Society [Sociedad Española de Medicina Interna] (https://www.fesemi.org/actualizacion-en-el-tratamiento-de-la-prediabetes-y-la-diabetes-tipo-2-consenso-semi-sed-y-sea).
Objectives of the diet in the population with prediabetes or type 2 diabetesThe general objective of dietary treatment for individuals with prediabetes or DM2 is to help them to modify nutritional habits to prevent and/or delay the illness, to improve their metabolic control, to treat the complications and the associated processes or comorbidities, and to maintain or improve their quality of life. Within this general objective, there are specific objectives that are applicable to the majority of people with DM2 or at risk of developing it, objectives that are backed by solid information.1,6–8
The specific dietary objectives for the population with prediabetes or DM2 are as follows:
- □
Prevent and/or delay the progression to DM2 for individuals with prediabetes. Programmes that combine healthy diets and physical activity are effective in reducing the incidence of DM2 and improving CVR factors, with the most intensive programmes being the most effective.8(Strong evidence).
- □
Achieve and maintain the objective of individualised blood glucose control. Different nutritional interventions for individuals with DM2, including the reduction of energy intake, make it possible to achieve absolute decreases in glycosylated haemoglobin (HbA1c) of .3%–2.0% at 3−6 months, and this can be maintained or improved at > 12 months. This improvement is better in those with a recent diagnosis and/or more elevated initial levels of HbA1c. Likewise, decreasing the dose and/or quantity of antidiabetic drugs is also prossible.8(Strong evidence).
- □
Reach and maintain the personalised weight objective. In adults with DM2, nutritional therapy makes it possible to reduce weight (2.4–6.2 kg), or it does not modify it.8(Moderate evidence).
- □
Achieve and maintain the personalised objectives for control of lipids and blood pressure. Nutritional interventions with individuals with normal or near-normal values improve or do not modify the lipid profile and blood pressure.8(Moderate evidence).
- □
Maintain or improve quality of life. For individuals with DM2, implementing nutritional therapy significantly improves the self-perception of the state of health, increases knowledge and motivation, and reduces emotional stress.8(Strong evidence).
The objective of the calories provided by the diet is to achieve and maintain a reasonable body weight (understanding reasonable to mean a more realistic weight than the ideal, one that can be reached and maintained in the short and long term).1
The majority (approximately 80%) of the people with DM2 are obese, so weight reduction is initially the main therapeutic objective. Obesity, particularly abdominal, constitutes the principal factor for the development of DM2 in genetically-predisposed subjects, and preventing obesity represents the principal course of action in reducing the incidence of DM2. There is solid evidence on the effectiveness of a moderate weight loss (5%–10%) in preventing or delaying the progression from prediabetes to DM2.9,10
Moderate calorie reduction (≥500 kcal/day), together with physical exercise, modification of dietary habits and provision of psychological support, are the most used effective measures to obtain and maintain a gradual, moderate weight loss. However, the clinical benefits of weight loss are progressive and increase as greater weight loss reductions are achieved.11,12
An alternative for people with DM2 and obesity who cannot achieve weight loss in a structured programme, and for specific selected individuals, is the very low calorie diet (<800 kcal/day), generally a diet of liquids, for short periods (<3 months) and with progressive reintroduction of foods. This very low calorie diet achieves a marked loss of weight and the remission of DM2 at the end of a year in 50% of the subjects.12
Healthy dietary models for the treatment of diabetesThere are several models or patterns of diet considered to be healthy.13 The best known models are the Mediterranean diet, the Dietary Approaches to Stop Hypertension (DASH) diet, the low CH diet and the vegetarian diet.
- □
The Mediterranean diet is based on the consumption of vegetables, fruits, legumes, nuts and seeds, whole grains; moderate to high consumption of olive oil (as the main source of fat); low to moderate consumption of dairy, fish and poultry; and low consumption of red meat.
This diet has been shown to be effective in improving both glycaemic control and CVR factors.14 Compared with the diet based on the recommendations of the American Diabetes Association, both the traditional Mediterranean diet and the low-CH Mediterranean diet reduce HbA1c and triglyceridemia, while only the low-CH Mediterranean diet improves plasma levels of cholesterol associated with low-density and high-density lipoproteins in subjects with overweight and DM2 after a year of treatment.15
In a systematic review, the low-CH diet was more effective than the low-fat diet in reducing HbA1c in the short term, but not at 2 years.16 In the randomised multicentre study carried out in Spain, Prevención con la dieta mediterránea (Predimed) [Prevention with the Mediterranean diet], with individuals at high CVR, almost half of the subjects diagnosed with DM2 at the beginning of the study showed benefits in the reduction of cardiovascular events with the Mediterranean diet supplemented with extra-virgin olive oil or varied nuts and seeds, in comparison with another fat-limited diet recommended by the American Heart Association.17
- □
The DASH diet, discussed in the 2020 recommendations of the American Diabetes Association,1 emphasises the consumption of fruits, vegetables, non-fat dairy products, whole grain products, poultry, fish and nuts and seeds. The recommendations also include reducing the intake of saturated fat, red meal, sugar and sugar-sweetened drinks, as well as reducing sodium. However, only 1 short-term (8 weeks) study,18 randomised and controlled, of those that the American Diabetes Association mentions, is carried out on patients with DM2; it shows a significant improvement in weight, basal blood glucose, blood pressure, cholesterol associated with high- and low-density lipoproteins and HbA1c. A systematic review and meta-analysis of prospective cohort studies that included individuals with DM2 showed that following the DASH diet was associated with a reduction in cardiovascular events.19
- □
The model of vegetarian diet includes the lacto-ovo-vegetarian, lacto-vegetarian, ovo-vegetarian and vegan diets. Observational studies find lower prevalence of DM2 in vegetarian subjects than in the general population, while intervention studies on people with DM2 observe that vegetarian diets lead to a greater reduction in weight, basal blood glucose and HbA1c and better lipid control than a conventional hypocaloric diet, with less need for antidiabetic medicines.20 Another systematic review of controlled randomised studies shows that vegetarian and vegan diets improve HbA1c and basal blood glucose in DM2.21
The studies that have attempted to determine the effects of meal frequency on health do not offer conclusive evidence, independently of energy and nutrient intake.22 However, other more recent studies would be in agreement with reducing intake frequency.
In a randomised, crossover study in patients with DM2 treated with oral glucose-lowering medication, distributing the food into 2 bigger meals a day (breakfast and supper) offers benefits in terms of weight and blood glucose control compared with distributing the food into 6 daily meals.23 Focusing on people with DM2 treated with insulin in a non-defined regime, Jakubowicz et al.,24 in their randomised study comparing the use of a 3-meal and 6-meal isocaloric diets, show that the diet with 3 meals/day offers benefits in weight loss, lowered HbA1c and glycaemia in general, and also in reduced appetite and insulin need.
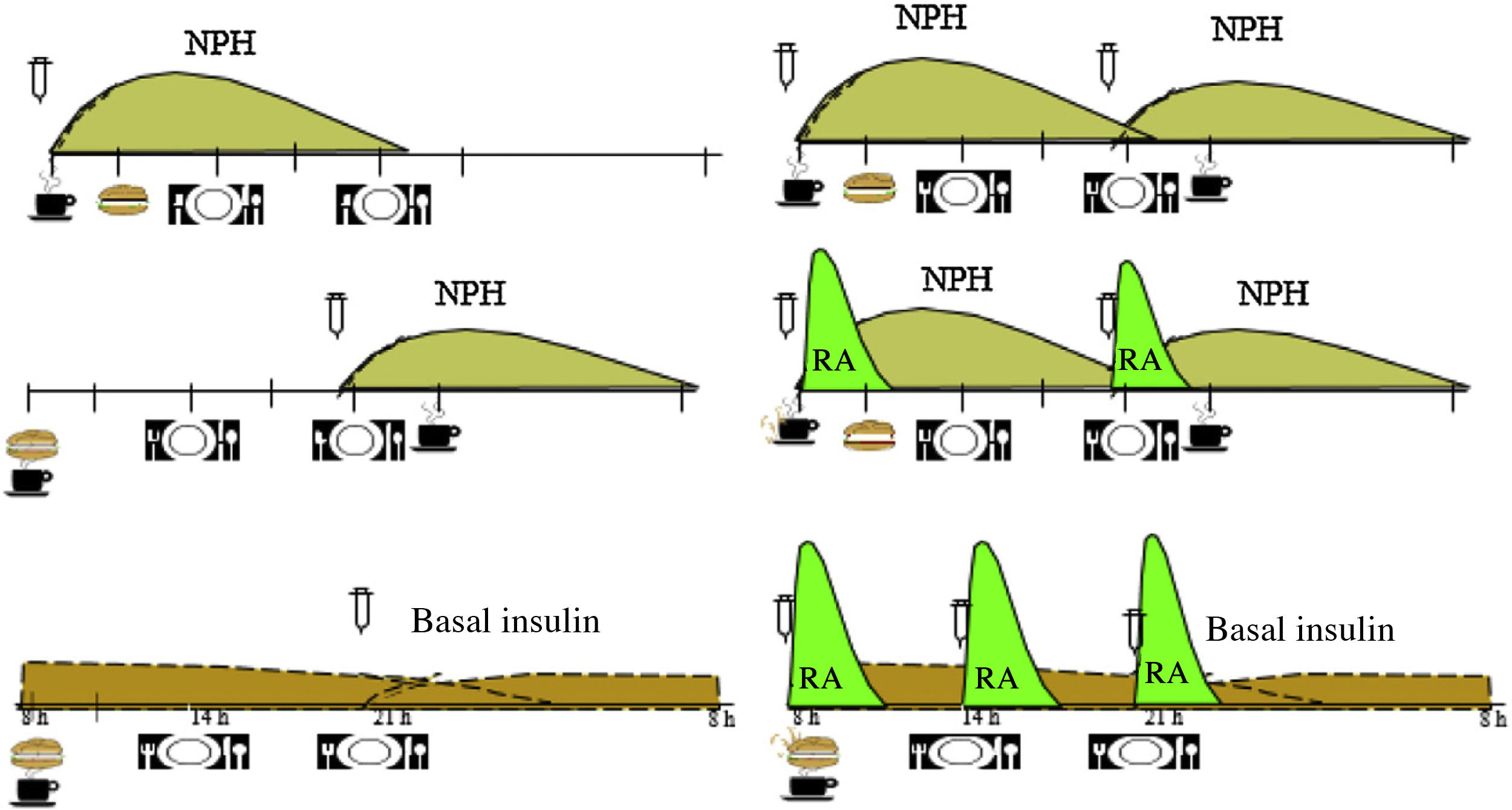
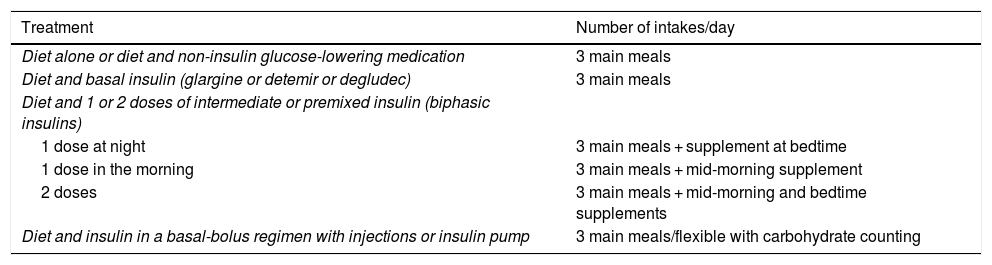
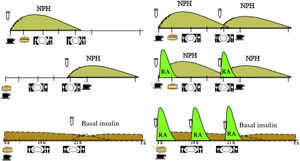
Based on the evidence available, we consider that the distribution of the CHs should depend on the type of glucose-lowering pharmacological treatment, the glycaemic profile and the patient’s habits and, after that, on adjustments arising from the results of blood glucose control monitoring. In Table 1, an example of meal distribution depending on DM2 treatment is proposed.25
Initial distribution of carbohydrates based on diabetes treatment.
| Treatment | Number of intakes/day |
|---|---|
| Diet alone or diet and non-insulin glucose-lowering medication | 3 main meals |
| Diet and basal insulin (glargine or detemir or degludec) | 3 main meals |
| Diet and 1 or 2 doses of intermediate or premixed insulin (biphasic insulins) | |
| 1 dose at night | 3 main meals + supplement at bedtime |
| 1 dose in the morning | 3 main meals + mid-morning supplement |
| 2 doses | 3 main meals + mid-morning and bedtime supplements |
| Diet and insulin in a basal-bolus regimen with injections or insulin pump | 3 main meals/flexible with carbohydrate counting |
Fig. 1 shows the distribution of the CHs throughout the day, according to the guidelines and the profile of insulin action. Table 2 presents recommendations about dietary treatment of prediabetes and DM2, with the level of evidence on which they are based.
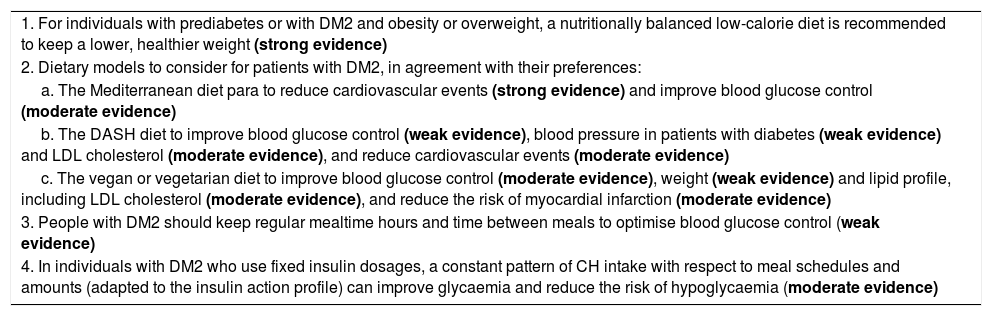
Dietary treatment of prediabetes and type 2 diabetes. Recommendations.
| 1. For individuals with prediabetes or with DM2 and obesity or overweight, a nutritionally balanced low-calorie diet is recommended to keep a lower, healthier weight (strong evidence) |
| 2. Dietary models to consider for patients with DM2, in agreement with their preferences: |
| a. The Mediterranean diet para to reduce cardiovascular events (strong evidence) and improve blood glucose control (moderate evidence) |
| b. The DASH diet to improve blood glucose control (weak evidence), blood pressure in patients with diabetes (weak evidence) and LDL cholesterol (moderate evidence), and reduce cardiovascular events (moderate evidence) |
| c. The vegan or vegetarian diet to improve blood glucose control (moderate evidence), weight (weak evidence) and lipid profile, including LDL cholesterol (moderate evidence), and reduce the risk of myocardial infarction (moderate evidence) |
| 3. People with DM2 should keep regular mealtime hours and time between meals to optimise blood glucose control (weak evidence) |
| 4. In individuals with DM2 who use fixed insulin dosages, a constant pattern of CH intake with respect to meal schedules and amounts (adapted to the insulin action profile) can improve glycaemia and reduce the risk of hypoglycaemia (moderate evidence) |
Table 3 presents a summary of the recommendations for the intake of different foods in the prevention and treatment of prediabetes and DM2, with the level of evidence existing for them.
Food recommendations in the prevention and treatment of prediabetes and type 2 diabetes.
| Edible fats |
| Substitute unsaturated fat food sources for saturated fats to improve the lipid profile, blood glucose control and insulin resistance26 |
| Moderate evidence |
| The Mediterranean dietary regimen shows that it can reduce basal and postprandial glycaemia, improve metabolic control and body weight, as well as prevent cardiovascular disease27,28 |
| Moderate evidence |
| The most recommendable fat for salad dressing and cooking purposes is virgin olive oil13,17 |
| Moderate evidence |
| At high temperatures, soybean, corn and sunflower oils undergo oxidative phenomena, producing free radicals and other pro-inflammatory molecules, so they should not be used for frying foods29 |
| Weak evidence |
| Meats |
| Eating meat (no more than 4 servings a week) does not appear to be prejudicial for CVR and DM2.30–33 However, to improve dietary sustainability, it is a good idea for the world population to reduce consumption of meat and increase that of vegetables.34 It is better to choose lean pieces and remove the skin and visible fat before cooking |
| Weak evidence |
| Eating processed meat is linked to the risk of cardiovascular events, colorectal cancer, DM2 and mortality from any cause.30–33 Consumption of sausages and other processed meats is not recommended |
| Moderate evidence |
| Eggs |
| Consuming eggs is not prejudicial and can be part of a heathy diet. There do not seem to be sufficient grounds to restrict their consumption in order to reduce CVR or improve metabolic control.35–38 However, some series limit their intake to a maximum of 3 eggs a week13 |
| Weak evidence |
| Fish |
| Greater consumption of fish has positive cardiovascular effects. Eating fish or shellfish at least 3 times a week is recommendable (2 of these consumptions should be in the form of oily fish such as salmon and trout)39,40 |
| Moderate evidence |
| Dairy products |
| Consumption of cheese is not linked with increased CVR41,42 |
| Moderate evidence |
| Consumption of dairy products shows a reduction of the risk of DM2,43–45 especially yogurt41,46 |
| Moderate evidence |
| Consuming dairy products, no matter their fat content, does not increase CVR.41,42 It does not seem appropriate to limit the consumption of full-fat dairy products in order to reduce the incidence of DM2 or cardiovascular events. Consuming at least 2 daily servings of whole or skimmed dairy products is recommendable, although the intake of dairy products with added sugar is not recommended. If reducing the calorie content of the diet is a goal, low-fat or non-fat dairy products should be chosen preferentially |
| Moderate evidence |
| Grains |
| The consumption of whole grains reduces the risk of DM2 and of cardiovascular mortality.47–49 Consuming whole grains instead of refined grains is recommended |
| Moderate evidence |
| Eating white or brown rice is not associated with higher CVR50,51 or with an increased risk of DM2, although it is linked to a greater risk of metabolic syndrome51 |
| Weak evidence |
| Legumes |
| In the context of the Mediterranean diet, greater intake of legumes (especially lentils) seems to be associated with a lower risk of DM252 |
| Weak evidence |
| Eating legumes is associated with a lower total CVR and ischemic cardiopathology risk.53 Eating a serving of legumes at least 4 times a week is recommended13 |
| Moderate evidence |
| Root vegetables |
| Moderate consumption of root vegetables, from 2 to 4 servings a week (preferably baked or boiled), is recommended; the intake of commercially-processed potatoes or those with added salt should be limited to very occasional servings.13 Daily consumption of potatoes (especially if they are fried) may cause an increased risk of DM254,55 |
| Moderate evidence |
| Nuts and seeds |
| Eating nuts and seeds in moderate amounts (30 g/day) has been associated with lower cardiovascular mortality and morbidity.17,56 In people with DM2, regular intake of nuts and seeds reduces the risk of cardiovascular mortality and total mortality57 |
| Habitual intake of nuts and seeds can be recommended for the general population and for people with hypercholesterolemia or high blood pressure, obesity and/or DM2.13 Eating a handful of raw nuts or seeds (equivalent to approximately 30 g) frequently (every day or at least 3 times a week) is recommended, although they should not be salted |
| Strong evidence |
| Fruits y vegetables |
| Consuming more fruits and vegetables is a habit that helps to prevent cardiovascular events58 |
| Eating at least 5 servings a day of vegetables and fruits is recommended. Intake should be varied, avoiding preparations that contain added sugars or fats |
| Moderate evidence |
| Chocolate and cocoa |
| Eating dark chocolate with ≥70% cocoa is associated with a reduced risk of myocardial infarction, stroke, DM2 and mortality from cardiovascular events59,60 |
| The majority of the products derived from cocoa that are found on the market have sugar and other fats added, and are not recommended |
| Dark chocolate with ≥70% cocoa can be consumed in moderate amounts (up to 30 g/day) |
| Weak evidence |
| Processed foods |
| Eating ultra-processed foods is associated with a greater risk of DM2, total cardiovascular, coronary and cerebrovascular disease, and mortality from any cause61–63 |
| The intake of ultra-processed foods in the diet should be avoided. Instead, consumption of fresh food products (unprocessed or minimally processed) should be encouraged |
| Moderate evidence |
| Salt |
| The intake of excess sodium is associated with the presence of chronic renal disease, obesity, high blood pressure and cardiovascular mortality64 |
| The consumption of up to 2.3 g a day of sodium is recommended.6 An alternative to salt is lemon juice, herbs, spices or garlic for use in cooking. It is necessary to limit the intake of precooked foods, tinned foods, cured or salted products, carbonated drinks, sausages and deli meats, which all normally have a high sodium content |
| Moderate evidence |
| Coffee y tea |
| Regular consumption of up to 5 cups a day of coffee (filtered or instant, normal or decaffeinated) or tea (green or black) is beneficial for cardiovascular health65,66 and the consumption of coffee is inversely associated with the risk of DM267 |
| The intake of coffee or tea with added sugar has to be limited as much as possible |
| Moderate evidence |
| Alcoholic drinks |
| In comparison with abstinence or excessive consumption of alcoholic drinks, moderate intake is associated with a reduction of CVR and of DM268,69 |
| Moderate evidence |
| The maximum acceptable intake is up to 1 fermented drink a day for women and up to 2 for men (with the unit being equivalent to a 330-ml beer or a 150-ml glass of wine)1 |
| Weak evidence |
| Drinking alcohol should not be encouraged in people who do not normally drink, and any consumption is prohibited for people who present a history of illnesses that contraindicate alcohol intake (liver disease, hypertriglyceridemia, history of addictions, etc.)70 |
| Moderate evidence |
| Beverages with added sugar and artificial sweeteners |
| Frequent intake of beverages with added sugar is associated with an increase in the risk of obesity, metabolic syndrome, prediabetes and DM271 |
| Strong evidence |
| Substituting water or sugarless herb tea for beverages with added sugar reduces energy consumption and the risk of obesity, metabolic syndrome, prediabetes and DM21,13,70 |
| Moderate evidence |
| Herbal, vitamin or mineral supplements |
| There is insufficient evidence for recommending the use of herbal, vitamin or mineral supplements by patients with DM2 that have no associated deficit6 |
| Weak evidence |
Dietary adherence of individuals with DM2 is very low. In general, just as for pharmacological treatment, the factors that contribute most to the lack of adherence are the lack of education about health, the perception of the illness itself, the complexity of the treatment, economic limitations, psychological factors and the lack of social support. In Table 4, we summarise the main factors that make following a diet difficult for people with DM2.
Factors that make it more difficult to follow a diet.
| Related with diet design and prescription |
| Using recommendations whose usefulness has not be established; for example, glycaemic indexes, diets «in fashion», etc. |
| Using standard diets that are strict, monotonous and not adapted to the patient’s characteristics |
| Proposing unrealistic objectives |
| Neglecting to implicate the patient in designing the diet and not including individual preferences |
| Related with the health professionals |
| Prescribing by professionals that are not involved or connected with the diabetes team |
| Having a lack of knowledge and, above all, convictions about the importance and the feasibility of the diet |
| Giving insufficient instructions to the patient about the importance, goals and management of the diet |
| Related with the patient and the social environment (emotional aspects) |
| Having a habit of using food to face problems |
| Resisting temptations in relation to social engagements and special meals |
| Finding it hard to control amounts and ingredients |
| Lacking the desire to follow the diet |
| Feeling unable to eat in the same way as people who do not have diabetes |
| Being tempted to abandon the diet temporarily (taking a vacation) |
| Putting other aspects that the patient considers of higher priority above the diet |
| Feeling a lack of support/understanding from the family and friends |
| Having a lack of information |
There are 2 types of interventions that permit greater dietary adherence among individuals with DM272: ones in which the health professionals take the cultural beliefs, the family and the social environment of the patient into consideration, and multifactor interventions that include various elements related to knowledge and perception of the illness and the diet, along with support and follow-up. Table 5 presents the strategies that must be considered to improve dietary adherence and the level of evidence on which they are grounded.1,7,26
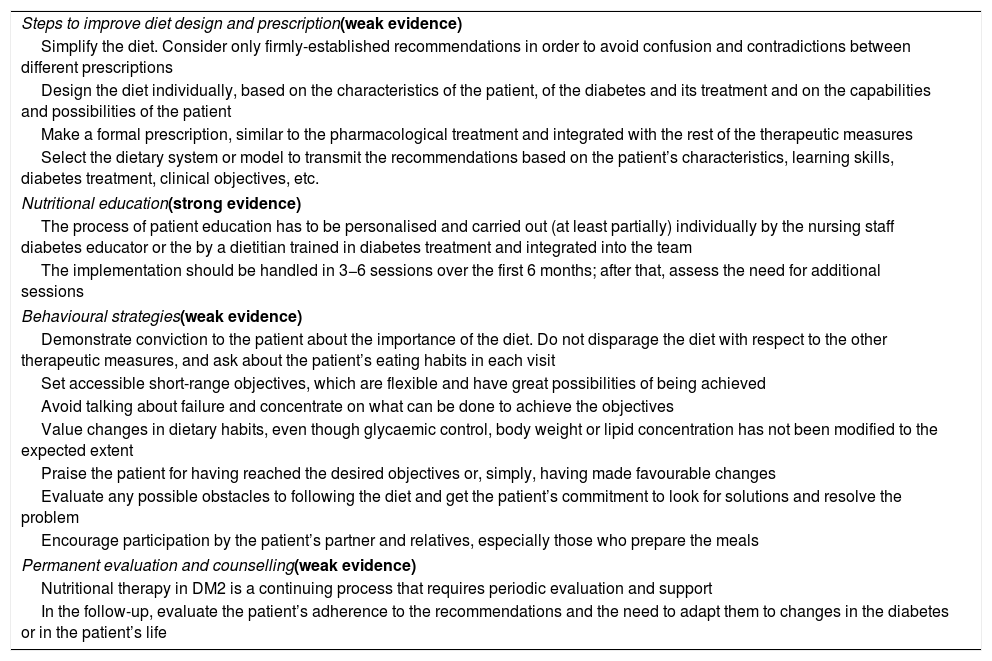
Strategies to improve adherence to the diet.
| Steps to improve diet design and prescription(weak evidence) |
| Simplify the diet. Consider only firmly-established recommendations in order to avoid confusion and contradictions between different prescriptions |
| Design the diet individually, based on the characteristics of the patient, of the diabetes and its treatment and on the capabilities and possibilities of the patient |
| Make a formal prescription, similar to the pharmacological treatment and integrated with the rest of the therapeutic measures |
| Select the dietary system or model to transmit the recommendations based on the patient’s characteristics, learning skills, diabetes treatment, clinical objectives, etc. |
| Nutritional education(strong evidence) |
| The process of patient education has to be personalised and carried out (at least partially) individually by the nursing staff diabetes educator or the by a dietitian trained in diabetes treatment and integrated into the team |
| The implementation should be handled in 3−6 sessions over the first 6 months; after that, assess the need for additional sessions |
| Behavioural strategies(weak evidence) |
| Demonstrate conviction to the patient about the importance of the diet. Do not disparage the diet with respect to the other therapeutic measures, and ask about the patient’s eating habits in each visit |
| Set accessible short-range objectives, which are flexible and have great possibilities of being achieved |
| Avoid talking about failure and concentrate on what can be done to achieve the objectives |
| Value changes in dietary habits, even though glycaemic control, body weight or lipid concentration has not been modified to the expected extent |
| Praise the patient for having reached the desired objectives or, simply, having made favourable changes |
| Evaluate any possible obstacles to following the diet and get the patient’s commitment to look for solutions and resolve the problem |
| Encourage participation by the patient’s partner and relatives, especially those who prepare the meals |
| Permanent evaluation and counselling(weak evidence) |
| Nutritional therapy in DM2 is a continuing process that requires periodic evaluation and support |
| In the follow-up, evaluate the patient’s adherence to the recommendations and the need to adapt them to changes in the diabetes or in the patient’s life |
There is currently solid evidence that eating patterns based on vegetables (fundamentally the Mediterranean diet, the vegan–vegetarian diet, the DASH diet and the low-CH diet) constitute the substantive basis for treatments to improve control of risk factors and to reduce the elevated cardiovascular morbidity and mortality of individuals with prediabetes or DM2.
Over the last few years, there have been proposals considering the urgent need to transform the food system, adopting a new model that, in addition to the traditional concept, would be healthy for the human population and also for the planet itself. Willet et al., in line with the recommendations from many corporations and backed scientifically by the Lancet Commission, have proposed a model of «healthy planetary eating» capable of conserving the planetary ecosystem and reducing non-transmissible diseases, with DM2 among them.34 Such a diet would be flexi-vegetarian, based on foods of vegetable origin, with fruits, varied vegetables, legumes, whole grains, nuts and seeds and only small amounts of animal protein. Red meat and its derivatives contribute considerably to global warming, land overuse and water consumption. Likewise, ultra-processed foods (whether meat-based or not) and almost all precooked foods contain products such as added sugar or trans fats and they have to be kept from our diet. For that reason, they must be avoided, and the consumption of foods rich in vegetable proteins must be increased.
The contribution of foods to global warming depends as much on their production as their transportation. Consequently, we must eat local foods that are in season, avoiding ones from farther away. Your lifestyle (which includes regular, maintained physical activity and a diet that follows recommended guidelines) constitutes the main therapeutic tool that there is for improving the control of blood glucose, lipids and blood pressure and reducing the elevated cardiovascular morbidity and mortality in people with prediabetes and DM2.
FundingNone.
Conflict of interestsThe authors declare that they have no conflicts of interest with respect to the content of this work.
Please cite this article as: Pascual Fuster V, Pérez Pérez A, Carretero Gómez J, Caixàs Pedragós A, Gómez-Huelgas R, Pérez-Martínez P. Resumen ejecutivo: actualización en el tratamiento dietético de la prediabetes y la diabetes mellitus tipo 2. Clin Investig Arterioscler. 2021;33:73–84.