The increase in myocardial fat has been proposed as one of the main precursors of myocardial dysfunction of diabetic aetiology, independent of coronary artery disease. However, biomarkers reflecting the myocardial fat content for the clinical detection of this pathology are currently lacking.
MethodsCorrelations between the content of cardiac triglycerides and plasma levels of the main molecules altered during diabetes and cardiac mRNA levels of genes involved in cardiac metabolism (Cd36 and Pdk4) have been explored in a murine model of insulin resistance induced by a high-fat diet.
ResultsIn insulin-resistant mice, the fatty diet increased myocardial triglyceride levels, compared to control animals fed with a standard diet. Cardiac triglyceride content was found to be directly associated with plasma levels of glucose, triglycerides, VLDL, resistin and leptin In addition, an inverse correlation was observed between triglyceride content and cardiac mRNA levels of Cd36 and Pdk4.
ConclusionsOur data reveal that cardiac triglyceride content is associated with an altered plasma biochemical profile and with a reprogramming of gene expression aimed at attenuating the impact of ectopic lipid accumulation in the myocardium.
El incremento de grasa miocárdica ha sido propuesto como uno de los principales precursores de la disfunción miocárdica de etiología diabética independiente de la enfermedad arterial coronaria. Sin embargo, actualmente se carece de biomarcadores que reflejen el contenido de grasa miocárdica para la detección clínica de esta patología.
MétodosLas correlaciones entre el contenido de triglicéridos cardíacos y los niveles plasmáticos de las principales moléculas alteradas durante la diabetes y los niveles cardíacos de ARNm de genes implicados en el metabolismo cardíaco (Cd36 y Pdk4) han sido exploradas en un modelo murino de resistencia a la insulina inducida por una dieta con alto contenido en grasas.
ResultadosEn ratones resistentes a la insulina, la dieta grasa aumentó los niveles de triglicéridos del miocardio, en comparación con animales controles alimentados con una dieta estándar. El contenido de triglicéridos cardíacos se encontró directamente asociado con los niveles plasmáticos de glucosa, triglicéridos, VLDL, resistina y leptina. Además, se observó una correlación inversa entre el contenido de triglicéridos y los niveles cardíacos de ARNm de Cd36 y Pdk4.
ConclusionesNuestros datos revelan que el contenido cardíaco de triglicéridos se encuentra asociado con un perfil bioquímico plasmático alterado y con una reprogramación de la expresión de genes dirigida a atenuar el impacto de la acumulación ectópica de lípidos en miocardio.
Insulin resistance has been proposed as a significant risk factor for heart failure (HF), the leading cause of death in individuals with diabetes mellitus (DM).1 Diabetic individuals present between two and five times more risk of HF.2 The main myocardial disorder related to this disease is coronary artery disease. However, HF has been associated with metabolic alterations independent of vascular lesion.3 Specifically, an accumulation of triglycerides at the myocardial level has been observed in patients with non-ischaemic cardiomyopathy in the terminal state.4 Lipid accumulation at the cardiac level is commonly known as cardiac steatosis and has been associated independently with an impaired contractile function and with the onset of myocardial dysfunction.5–8 In fact, the increase in myocardial fat is evident in the absence of HF and precedes myocardial dysfunction,6,8 being proposed as one of the main precursors of myocardial dysfunction due to diabetes.4–8
The mechanisms underlying ectopic accumulation of fat in the myocardium are not fully known at present. The increase in circulating fatty acids observed during DM facilitates their use as a preferred energy substrate for the production of energy in heart cells. However, the uptake of fatty acids exceeds the ability of the cell to metabolise them,9 so they accumulate intracellularly as bioactive lipid metabolites, contributing directly to the activation of molecular pathways that lead to the blockage of the insulin signalling pathway.10 In addition, adipose tissue releases a large amount of bioactive molecules called adipokines into circulation, which can participate in the control of energy metabolism, regulating the preference of the substrate used for the production of energy in their target tissues. In this way, adipokines are postulated as key molecules in the communication network between adipose tissue and peripheral tissues, including the heart,11 although their potential role as biomarkers of myocardial fat has not been explored in depth.
Despite the evidence that supports the accumulation of cardiac fat as a potential causative agent of myocardial dysfunction independent of ischaemic disease in diabetic individuals, currently there are no biomarkers that reflect the content of myocardial triglycerides for the diagnosis of this pathology.
In this work, the associations between cardiac triglyceride content and plasma biochemical profile in mice fed a high-fat diet (HFD) are analysed.
MethodsAnimalsC57BL/6J 6-week-old male mice were kept in standard conditions of light/darkness (12-h cycles) and at a temperature (21±1°C) with access ad libitum to water and food. The animals were randomly distributed into two experimental groups (10 animals/group) and were fed a standard diet (STD) (10% kcal of fat; Panlab; Barcelona, Spain) or an HFD (60% kcal of fat; Panlab; Barcelona, Spain) for 12 weeks.12 At 12 weeks, the animals were sacrificed and the hearts were immediately frozen in liquid nitrogen and stored at −80°C for future analysis. Plasma samples were obtained, where glucose levels (Spinreact; Barcelona, Spain), free fatty acids (FFA) (Wako; Osaka, Japan), total cholesterol and high-density lipoproteins (HDL) were determined) (Spinreact; Barcelona, Spain) using a Cobas Mira Plus autoanalyser (Roche Diagnostics, Barcelona, Spain). Low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL) levels were determined by Friedewald.13 Insulin, resistin, leptin and adiponectin levels were determined using commercial kits (Milliplex®, Millipore; Billerica, MA, USA). Insulin resistance was assessed using the HOMA index using the following equation: (fasting glucose [mmol/l]×fasting insulin [μU/ml]/22.5).14 Animal handling and experiments were performed according to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH publication No. 85–23, revised in 1996). All procedures were approved by the Bioethics Committee of the Universitat Rovira i Virgili, as established in Law 5/12 July 1995, and were approved by the Regional Government of Catalonia.
Lipid content stainingThe lipid content was determined in 5mm sections of frozen mouse hearts using Oil Red O dye (Sigma-Aldrich; Madrid, Spain) following the protocol described by Mehlem et al.15 The images were captured with an Olympus IX71 microscope (Madrid, Spain).
Determination of myocardial triglyceridesThe cardiac lipid content was extracted in 0.1% methanol of formic acid. The sample was fragmented by vortex, inversion in liquid N2 and ultrasonication. Next, three volumes of dichloromethane and one volume of water were added sequentially. The samples were incubated at 4°C for 20min. and centrifuged at 15,000rpm for 15min. at 4°C. The organic phase was collected and dried under a stream of nitrogen. Lipid pellets were resuspended in methanol: toluene (9:1) for liquid chromatography/mass spectrometry (LC/MS) analysis.
The LC/MS analyses were performed using a UHPLC system (1200 series, Agilent Technologies) coupled to an MS 6550 ESI-QTOF (Agilent Technologies, Madrid, Spain) operating in a positive ionisation mode by electrospray (ESI+). The lipids were separated by reverse phase chromatography with an Acquity UPLC C18-RP (ACQUITY UPLC BEH C18 1.7μM, M, Waters, Barcelona, Spain).
Through the analysis by MS/MS, 36 triglycerides increased by HFD were identified. The data from LC/MS were processed using the XCMS software (version 1.34.0, SCIEX, Madrid, Spain).16 The sum of the intensities of the individual species was performed as an indicator of the total triglyceride content identified in each experimental group. Platform performance was assessed by calculating the relative standard deviation of these characteristics in pooled samples (CVQC).17
Preparation of ribonucleic acid and analysis of gene expression by real-time quantitative polymerase chain reactionRelative levels of messenger ribonucleic acid (mRNA) were analysed by reverse transcriptase real-time polymerase chain reaction (RT-real time PCR), as previously described.18,19 Total RNA was isolated using the TRI™ reagent (Sigma-Aldrich; Madrid, Spain), according to the manufacturer's recommendations. RNA integrity was determined by agarose gel electrophoresis and quantified with a NanoDrop 1000 Spectrophotometer (Thermo Scientific; Madrid, Spain); 1mg of total RNA was retrotranscribed using the PrimeScript RT Reagent Kit (Takara Bio; Saint-Germain-en-Laye, France). mRNA levels were determined by real-time PCR on an ABI PRISM 7900 device (Applied Biosystems; Waltham, Massachusetts, USA). Mouse TaqMan probes (IDT; Leuven, Belgium) were used for the detection of gene expression of Cd36 (Mm.PT.58.7548967) and Pdk4 (Mm.PT.58.9453460). The level of expression of TBP (TATA-binding protein) was analysed as a reference for the normalisation of the results.19–21
Statistical analysisThe results are expressed as the median and interquartile range. Significant differences were established using the Mann-Whitney U test. The associations between the variables were analysed using the Spearman correlation test. Statistical analyses were performed using SPSS software (IBM SPSS Statistics, version 22.0; New York, USA). The differences were considered significant from p<0.05
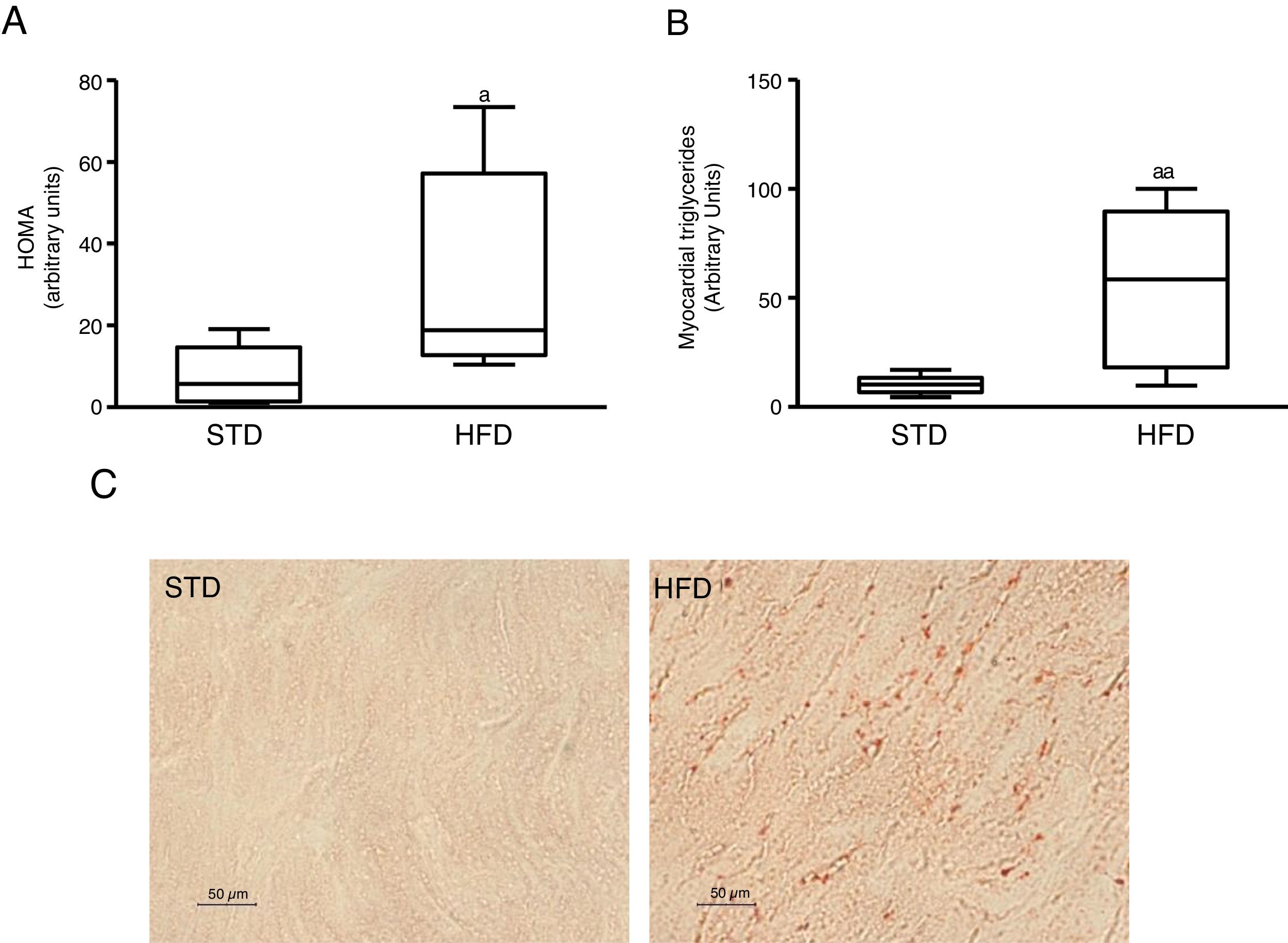
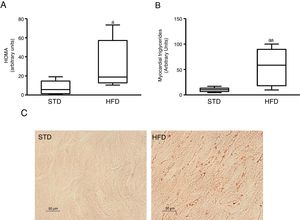
ResultsInsulin resistance induced by a fatty diet increases myocardial lipid contentTo induce insulin resistance we fed mice with an HFD. Animals fed this diet showed increased plasma glucose levels (median 360.00; interquartile range 243.00–487.00mg/dl) and insulin (median 70.50; interquartile range 23.79–158.29pg/dl) compared to control animals fed an STD (glucose: median 185.00; interquartile range 127.00–223.00mg/dl) (insulin: median 23.79; interquartile range 7.12–68.52pg/dl) (p<0.05). As a consequence, animals fed with an HFD had a HOMA-insulin resistance index superior to control animals (Fig. 1A). These animals showed a higher lipid myocardial content than the control animals (Fig. 1C). Through a metabolomic approach, it was observed that animals fed with HFD had a myocardial triglyceride content increased ∼six times compared to animals fed the control diet (Fig. 1B).
Insulin-resistant mice have increased myocardial lipid levels. The animals were fed a standard (STD) or high-fat (HFD) diet for 12 weeks. A) HOMA-insulin resistance index. B) Levels of myocardial triglycerides. C) Representative images of lipid staining by Oil Red O in hearts of mice fed with STD or HFD (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Data are expressed as the median and interquartile range.
*p<0.05.
**p<0.01 vs. STD.
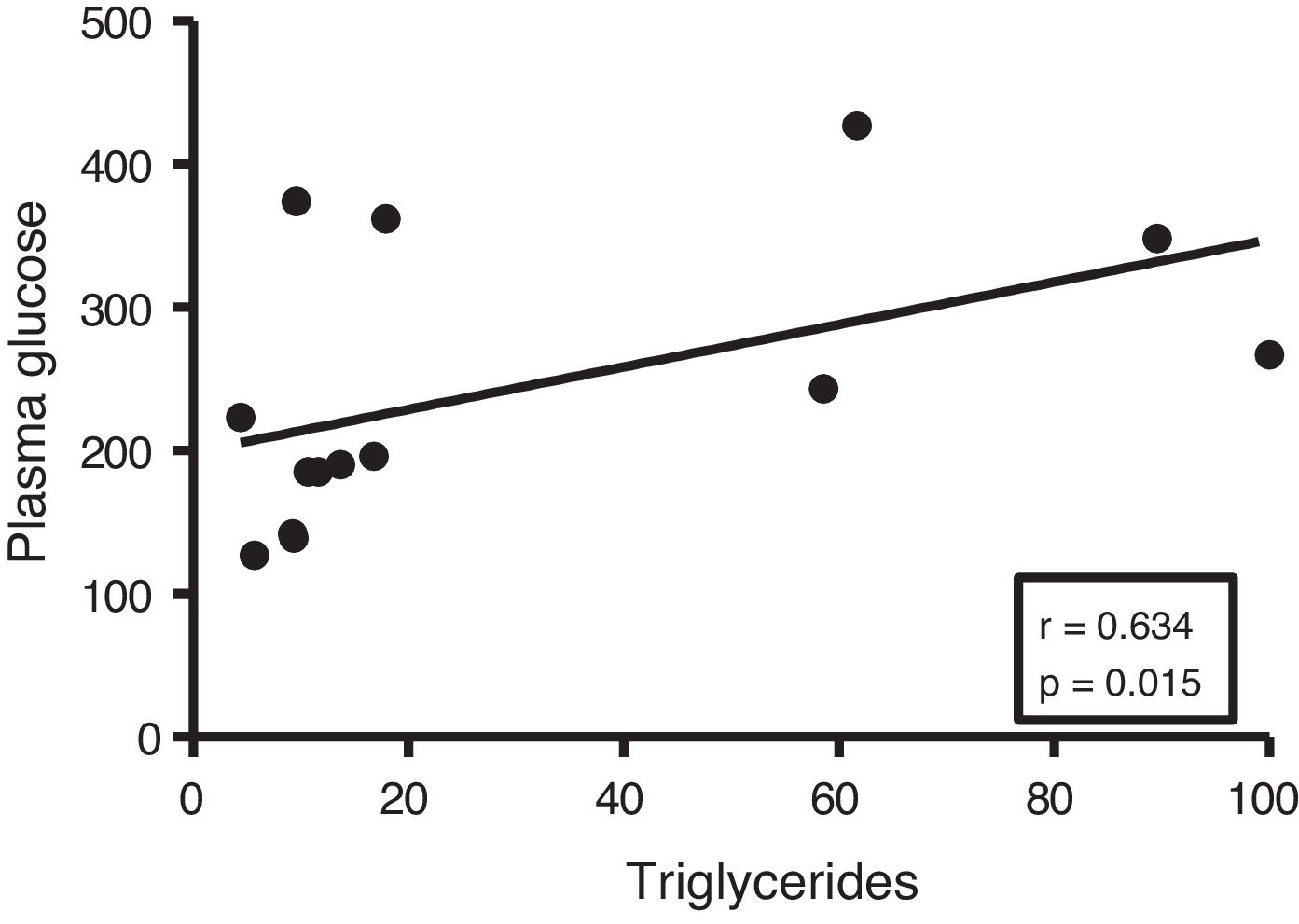
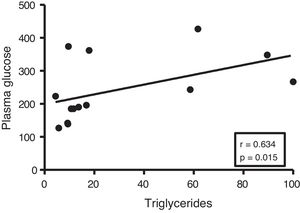
Given the close relationship between DM and the accumulation of ectopic fat in the myocardium, we first analyse the potential associations between myocardial triglyceride content and glucose metabolism. Although no correlations were found between cardiac triglycerides and plasma insulin levels, they were found to be positively associated with plasma glucose levels (r=0.634, p=0.015) (Fig. 2).
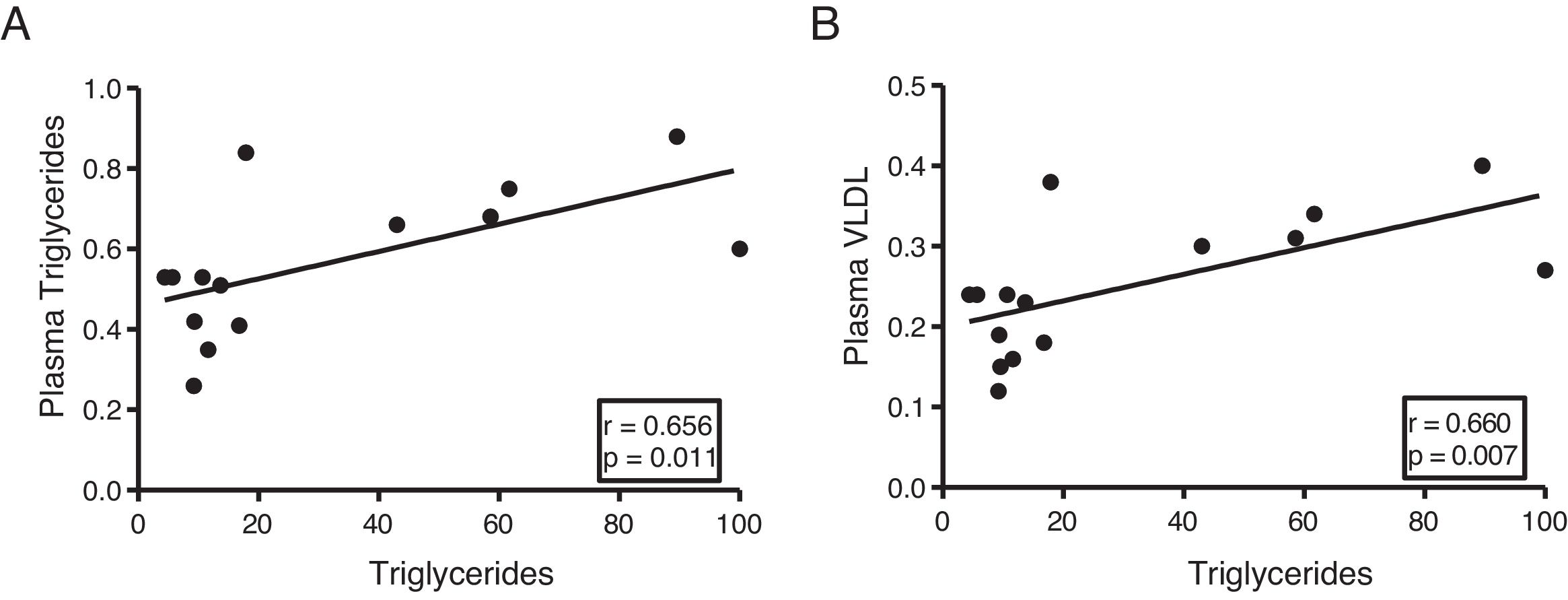
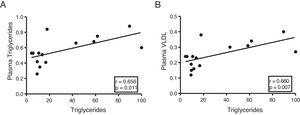
The myocardial content of triglycerides is associated with plasma levels of triglycerides and very low-density lipoproteinsNext, we determine the association of cardiac triglycerides with lipid and cholesterol metabolism. As Fig. 3 shows, cardiac triglycerides were directly associated with plasma triglyceride levels (r=0.656, p=0.011) (Fig. 3A) and VLDL (r=0.660, p=0.007) (Fig. 3B). However, no significant associations were found between these molecules and plasma levels of AGL, total cholesterol, LDL or HDL.
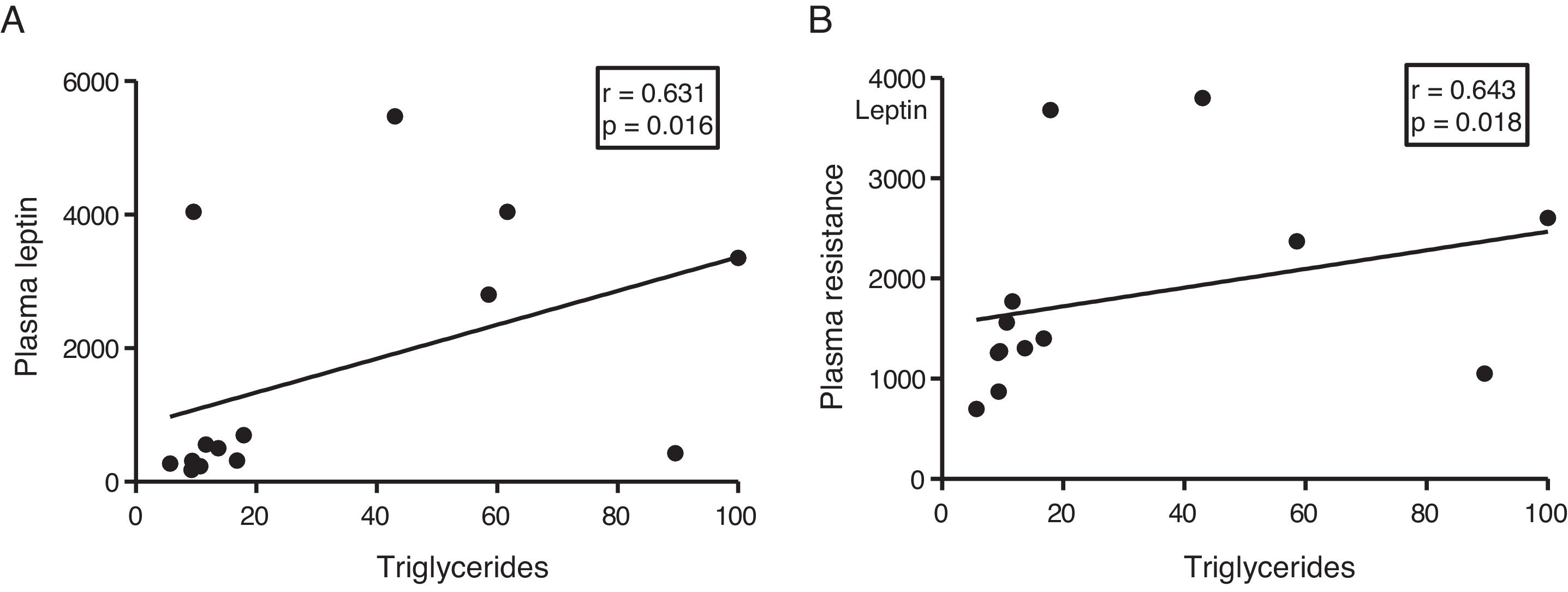
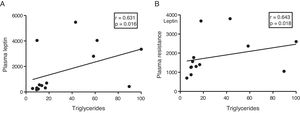
The myocardial content of triglycerides is associated with plasma levels of leptin and resistinGiven the role of adipokines as potential regulators of energy metabolism in the heart, we then analyse the associations between cardiac triglycerides and these molecules. Cardiac triglycerides were not associated with plasma adiponectin levels. However, a direct correlation was found between these molecules and plasma leptin levels (r=0.631, p=0.016) (Fig. 4A) and resistin (r=0.643, p=0.018) (Fig. 4B).
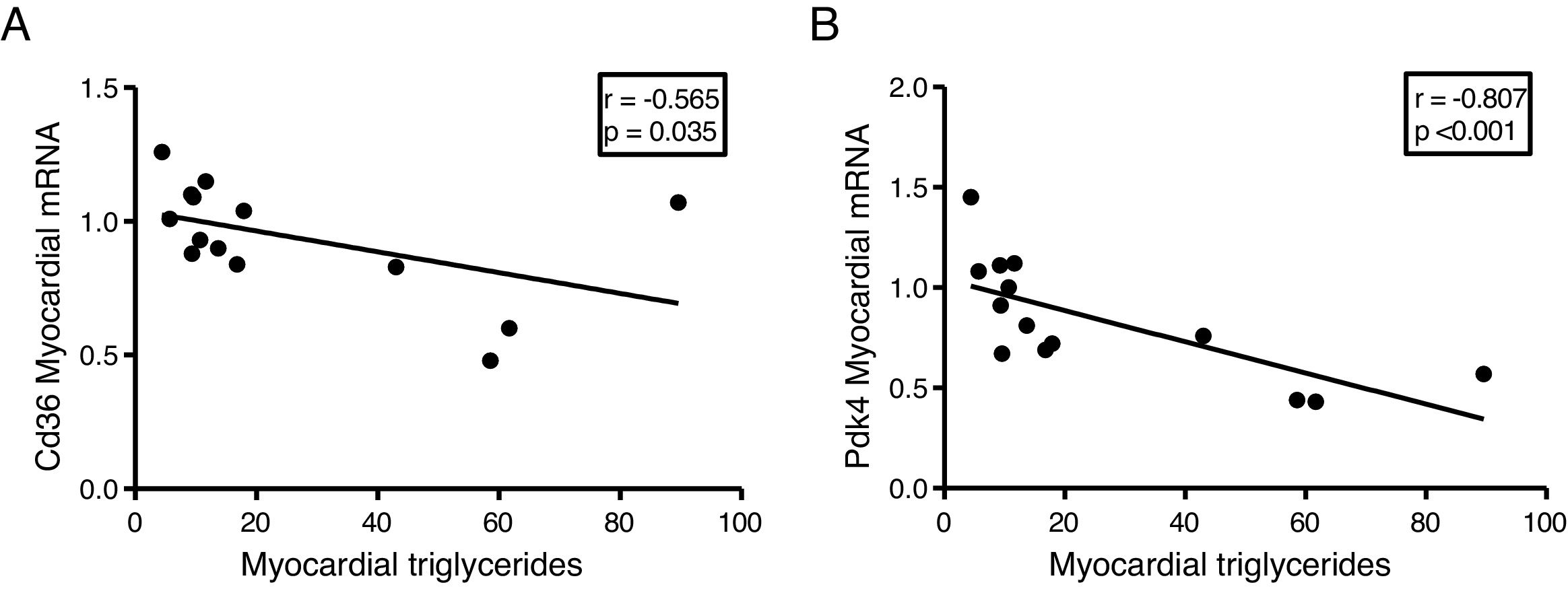
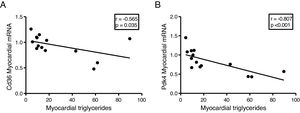
The myocardial content of triglycerides is inversely associated with the expression of genes that regulate cardiac energy metabolismSince the ectopic accumulation of fat in the myocardium is finely regulated by the preferential use of metabolised substrates for energy production, we finally analyse the content of cardiac triglycerides with the expression of two key genes in the regulation of these processes. Cardiac triglycerides were found to be inversely related to the cardiac expression of the fatty acid transporter Cd36 (r=–0.565, p=0.035) (Fig. 5A) and to pyruvate dehydrogenase kinase 4 (Pdk4) (r=–0.807, p<0.001) (Fig. 5B).
DiscussionNumerous evidence proposes the ectopic accumulation of fat in the heart as one of the main precursors of myocardial dysfunction of diabetic aetiology.4–8 However, the molecular mechanisms that underlie the intramyocellular accumulation of lipids in diabetic individuals are not fully known and, so far, none of the molecules proposed as potential biomarkers of myocardial fat content has been used for the clinical diagnosis of this pathology. In this work, we study the potential associations between the myocardial content of triglycerides and different plasma components in a murine model of HFD-induced insulin resistance.
As previously described, HFD induced insulin resistance in our animals, evidenced by an increase in plasma glucose and insulin levels, as well as an increased HOMA index, compared to animals fed an STD.22 Insulin-resistant animals had a characteristic plasma biochemical profile of DM, characterised by elevated plasma levels of AGL, triglycerides, resistin and leptin, and reduced levels of plasma adiponectin.22 In addition, these animals showed increased plasma levels of VLDL, without showing changes in the levels of total cholesterol, LDL and HDL. Interestingly, animals fed with HFD had the increased cardiac triglyceride content, suggesting that the accumulation of lipids observed in these animals could predispose to an increased risk of HF in more advanced stages of the pathology.
In order to identify potential plasma biomarkers related to the ectopic accumulation of fat in the heart, we conducted association studies between the cardiac triglyceride content and the main plasma components that are altered in DM. First, we explore the association between these molecules and plasma insulin and glucose levels. Despite not finding significant associations between cardiac triglycerides and insulin, these were found directly associated with plasma glucose, underlining the relationship between DM and myocardial steatosis. In addition, cardiac triglycerides were found directly associated with plasma triglyceride and VLDL levels, indicating that these molecules could transport triglycerides to the myocardium.
Since adipose tissue participates in the regulation of different metabolic processes, such as insulin resistance in peripheral tissues, we decided to explore whether this tissue could also participate in the ectopic accumulation of fat in the heart, through the use of different adipokines such as agents involved in communication between both tissues. Our results showed a positive correlation between the plasma levels of leptin and resistin with the cardiac triglyceride content, suggesting a direct participation of these molecules in the ectopic accumulation of fat in the heart.
Finally, to explore the molecular mechanisms involved in the accumulation of cardiac lipids, we analyse the potential associations of cardiac triglycerides with the expression of two of the main genes involved in the regulation of the substrate used for the production of energy in the heart, the transporter of fatty acids Cd36 and Pdk4. While Cd36 facilitates the uptake of fatty acids by the cardiomyocyte and, therefore, the use thereof, Pdk4 inhibits the entry of pyruvate into the mitochondria through the inhibition of pyruvate dehydrogenase, thereby blocking the obtaining of energy from glucose. Our results show that the expression of both genes is inversely related to the cardiac triglyceride content, suggesting that a high content of these molecules could be related to a metabolic adaptation at the transcriptional level aimed at preparing the cell to reduce the uptake of fatty acids and enhance the use of glucose.
In summary, our results show that cardiac triglyceride content is associated with an altered biochemical profile and with a reprogramming of gene expression aimed at attenuating the impact of ectopic lipid accumulation in the myocardium. However, more studies are needed to validate our results in humans and explore whether transcriptional adaptation is sufficient to revert/attenuate the impact of insulin resistance in terms of substrate utilisation and intracellular lipid accumulation.
FundingThis study has been funded by a FEA/SEA (Spanish Foundation of Atherosclerosis/Spanish Society of Atherosclerosis) grant for Basic Research on basic aspects of atherosclerosis and its risk factors (2015 call), the Instituto de Salud Carlos III [Carlos III Health Institute] (ISCIII) (PI15/00627), CIBER of Diabetes and Associated Metabolic Diseases (CIBERDEM) (CB07/08/0028 and CB07/08/0014) and the European Regional Development Fund (ERDF).
Conflicts of interestNone.
Please cite this article as: Rodríguez-Calvo R, Samino S, Guaita-Esteruelas S, Martínez-Micaelo N, Heras M, Girona J, et al. Niveles plasmáticos de glucosa, triglicéridos, VLDL, leptina y resistina como potenciales biomarcadores de la grasa miocárdica en ratones. An Pediatr (Barc). 2020. https://doi.org/10.1016/j.arteri.2019.05.001