To determine the crude and sex- and age-adjusted prevalence rates of atherogenic dyslipidemia (AD) and low HDL-cholesterol levels (low-HDLc), and to assess their associations with cardiovascular risk factors, chronic kidney disease, cardiovascular and cardiometabolic diseases.
MethodsPopulation-based cross-sectional study conducted in Primary Care, with randomly selected adult subjects. The AD was considered if the patients had hypertriglyceridemia (triglycerides ≥150 mg/dL) and low-HDLc (<40 mg/dL [men]; <50 mg/dL [women]). Crude and sex- and age-adjusted prevalence rates were determined, and univariate and multivariate analysis were performed to assess related cardiometabolic factors.
ResultsStudy population with 6,588 adults (55.9% women) with mean age 55.1 (±17.5) years. The mean HDLc levels were 49.2 (±12.6) mg/dL in men and 59.2 (±14.7) mg/dL in women. The crude prevalence rates of low-HDLc and AD were 30.8% (95%CI: 29.7–31.9), and 14.3% (95%CI: 13.5–15.2), respectively. The adjusted prevalence rates of low-HDLc were 28.0% in men and 31.0% in women, and AD were 16.4% in men and 10.6% in women. Seventy-three percent of the population with AD had high or very high cardiovascular risk. The independent factors associated with low HDLc or with AD were diabetes, smoking, abdominal obesity, and obesity. The major factors associated with low HDLc and AD were hypertriglyceridemia and diabetes, respectively.
ConclusionsAlmost a third of the adult population had low HDL-C and half of them met AD criteria. Cardiometabolic factors were associated with low HDL-C and AD, highlighting HTG with low HDLc, and DM with AD
Determinar las prevalencias ajustadas por edad y sexo de concentraciones bajas de colesterol HDL (cHDL-bajo) y de dislipidemia aterogénica (DA), y valorar sus asociaciones con factores de riesgo cardiovascular, enfermedad renal crónica, enfermedades cardiovasculares y cardiometabólicas.
MétodosEstudio observacional transversal de base poblacional realizado en Atención Primaria, con sujetos adultos seleccionados aleatoriamente. Se consideró DA si los pacientes tenían hipertrigliceridemia (triglicéridos ≥150 mg/dL) y cHDL-bajo (<40 mg/dL [hombres]; <50 mg/dL [mujeres]). Se determinaron las tasas de prevalencia crudas y ajustadas por edad y sexo, y se realizó análisis univariado y multivariante para evaluar los factores cardiometabólicos relacionados.
ResultadosPoblación de estudio con 6.588 adultos (55,9% mujeres) con edad media 55,1 (±17,5) años. Las medias de cHDL fueron 49,2 (±12,6) mg/dL en hombres y 59,2 (±14,7) mg/dL en mujeres. Las prevalencias crudas de cHDL-bajo y de DA fueron 30,8% (IC95%: 29,7–31,9), y 14,3% (IC95%: 13,5–15,2), respectivamente. Las prevalencias ajustadas de cHDL-bajo fueron 28,0% en hombres y 31,0% en mujeres, y de DA fueron 16,4% en hombres y 10,6% en mujeres. El 73% de la población con DA tenía riesgo cardiovascular alto o muy alto. Los factores independientes asociados con cHDL-bajo o con DA fueron diabetes, tabaquismo, obesidad abdominal y obesidad. Los principales factores asociados con cHDL-bajo y con DA fueron hipertrigliceridemia y diabetes, respectivamente.
ConclusionesCasi un tercio de la población adulta presentaba cHDL-bajo y la mitad de ellos cumplía criterios de DA. Los factores cardiometabólicos se asociaban con cHDL-bajo y DA, destacando la HTG con el cHDL-bajo, y la DM con la DA.
Atherogenic dyslipidaemia (AD) is characterised by the coexistence of low concentrations of cholesterol bound to high-density lipoproteins (HDL), hypertriglyceridaemia (HTG) and high remnant concentrations of triglyceride-rich lipoproteins (TG), and a predominance of small, dense low-density lipoproteins (LDL).1
AD increases the risk of atherosclerotic cardiovascular disease (ACVD) because of the synergistic action of these 3 factors: decreased antiatherogenic functions due to low concentrations of HDL-C (low HDL-C),2 increased atherogenicity due to increased TG and remnants, and small, dense LDL particles, which penetrate the vascular wall more easily and are more susceptible to oxidation.3
Despite the widespread use of statins in these patients, control of LDL-C is still not sufficient. This is because the synergistic effect of HTG and low HDL-C confers a residual atherogenic risk that persists despite achieving LDL-C control objectives.4–6 There are many studies that assess these effects, either by analysing low HDL-C in isolation, or together with HTG within the concept of AD. Regarding low HDL-C, meta-analyses show a higher incidence of serious cardiovascular events in patients with low HDL-C, even on statin therapy.7,8 In an analysis of patients with high cardiovascular risk (CVR) or coronary heart disease (HD) equivalents, low HDL-C was present in 66%, reaching 79% in patients with controlled LDL-C, regardless of statin therapy.9
With regard to AD, the Spanish Society of Arteriosclerosis (SEA) assessed the importance of the risk of AD, following the initiative of residual risk reduction (R3i),4 showing that the prevalence of AD was higher in patients with high CVR and controlled LDL-C, and in up to one third of patients with diabetes mellitus (DM) or a history of ACVD.6 In the PROCAM study, the risk of myocardial infarction was 5 times higher in patients with HTG or low HDL-C despite having controlled LDL-C.10 In the TNT study, patients with stable HD on high-intensity statin therapy and controlled LDL-C were at greater risk if they also had AD.11 In the ACCORD-Lipid study, DM and AD patients had a 71% increased risk of ACVD.12
This phenotype of the lipid profile of AD is frequently expressed in patients with a history of ACVD, chronic kidney disease (CKD), DM, metabolic syndrome (MS), or familial combined hyperlipidaemia, and therefore it is important to determine TG and HDL-C levels to assess overall CVR, and to consider the presence of AD to assess these patients’ residual risk.1,4,6,13–15
Low HDL-C and AD are clinically relevant as they are associated with an increased risk of ACVD and because they are under-diagnosed, under-treated and uncontrolled.5,15 According to the global plan of action for non-communicable diseases, the prevalence of ACVD risk factors must be evaluated to improve prevention, plan health resources, and to monitor and evaluate established strategies.16
The aim of this study was to determine the age and sex-adjusted prevalence rates of AD and low HDL-C in the adult population and to assess their respective associations with cardiovascular risk factors (CVRF), CKD and cardiometabolic diseases.
Material and methodsThe SIMETAP-AD study is part of the SIMETAP project, approved by the Research Commission for Primary Care Management of the Madrid Regional Health System (SERMAS). The present study is a cross-sectional observational research study conducted by 121 family doctors interested in participating in the SIMETAP research project, whose objective was to assess the prevalence of CVRF, MS and related cardiovascular or cardiometabolic diseases. The doctors were working in 64 primary care centres under SERMAS (25% of the SERMAS health centres). The participating doctors were selected competitively until the necessary sample size was achieved. The details of the material and methods (design, sampling, recruitment, criteria for inclusion and exclusion of study subjects, data collection, statistical analysis and criteria defining the variables and categories of CVR) of the SIMETAP study have been previously detailed in this journal.17 For the purposes of this study, HTG was considered if TG≥150 mg/dL or if this diagnosis was recorded in the clinical history; low HDL-C if HDL-C<40 mg/dL in males or <50 mg/dL in females; AD: HTG with low HDL-C. The study population was obtained by simple random sampling of the population over 18 years of age assigned to SERMAS primary care physicians participating in the study. As per the study protocol, informed consent was obtained from all subjects in the study and terminal, institutionalised, cognitively impaired, pregnant patients or subjects without information on biochemical variables were excluded. The final sample was 10084 study subjects, whose response rate was 65.8%.
The Statistical Package for the Social Sciences was used for the statistical analysis. The descriptive analysis determined range, median and interquartile range (IQR) (25th percentile; 75th percentile) of the age variable and mean and standard deviation (±SD) of other continuous variables. Qualitative variables were analysed using percentages in each category, presented with lower and upper limits of the 95% confidence interval (CI). Prevalence rates were determined as crude rates and age- and sex-adjusted rates. The adjustment of rates by age and sex was performed using ten-year age groups standardized with those of the Spanish population by direct method.18 Information on the Spanish population for January 2015 was obtained from the National Institute of Statistics database.19 The rate adjustment was performed according to the Spanish population rather than the population of the Community of Madrid, because there were no significant differences in the results of the adjusted prevalence rates between either population and to facilitate the comparison of results with other populations. Comparisons of continuous variables were made using the Student’s t-test or the analysis of variance (ANOVA). The analysis of categorical variables was performed using the chi-square test. Odds ratios (OR) were determined with a 95% CI. Logistic regression multivariate analysis with the introductory method was used to assess the effect on the respective dependent variables (low HDL-C and AD) of those independent variables (CVRF and comorbidities) that the univariate analysis performed beforehand would have shown a statistically significant association with the dependent variables. The MS20 variable was not included in the multivariate analysis. All tests were considered statistically significant if the 2-tailed p-value was less than .05. To compare the prevalence rates of low HDL-C and AD determined in the present study, a literature search was conducted in PubMed, Medline, Embase, Google Scholar and Web of Science of the main studies related to these rates, published between 2005 and 2015.
ResultsStudy population. The mean (±SD) age was 55.1 (±17.5) years, the median (IQR) was 54.7 (41.7–68.1) years, and the range was 18–102.8 years. The percentage difference between males (44.1% [42.9%–45.3% CI]) and females (55.9% [54.7%–57.1% CI]) was significant (p < .001). The difference in mean [±SD] ages between the male (55.3 [±16.9] years) and female (55 [±18] years) populations was not significant (p = .634).
The percentage difference in the male population between populations with low HDL-C (46.5% [44.2%–48.8% CI]) and without low HDL-C (43.2% [41.8%–44.6% CI]) was significant (p = .014). The difference of the means [±SD] of age between the populations with low HDL-C (56.4 [±16.9] years) and without low HDL-C (54.7 [±17.7] years) was significant (p < 001).
The percentage difference in the male population between populations with AD (54.6% [51.4%–57.8%]) and without AD (42.3% [41%–43.6%]) was significant (p < .001). The difference of the means [±SD] of age between populations with AD (58.7 [±15.1] years) and without AD (54.5 [±17.8] years) was significant (p < .001).
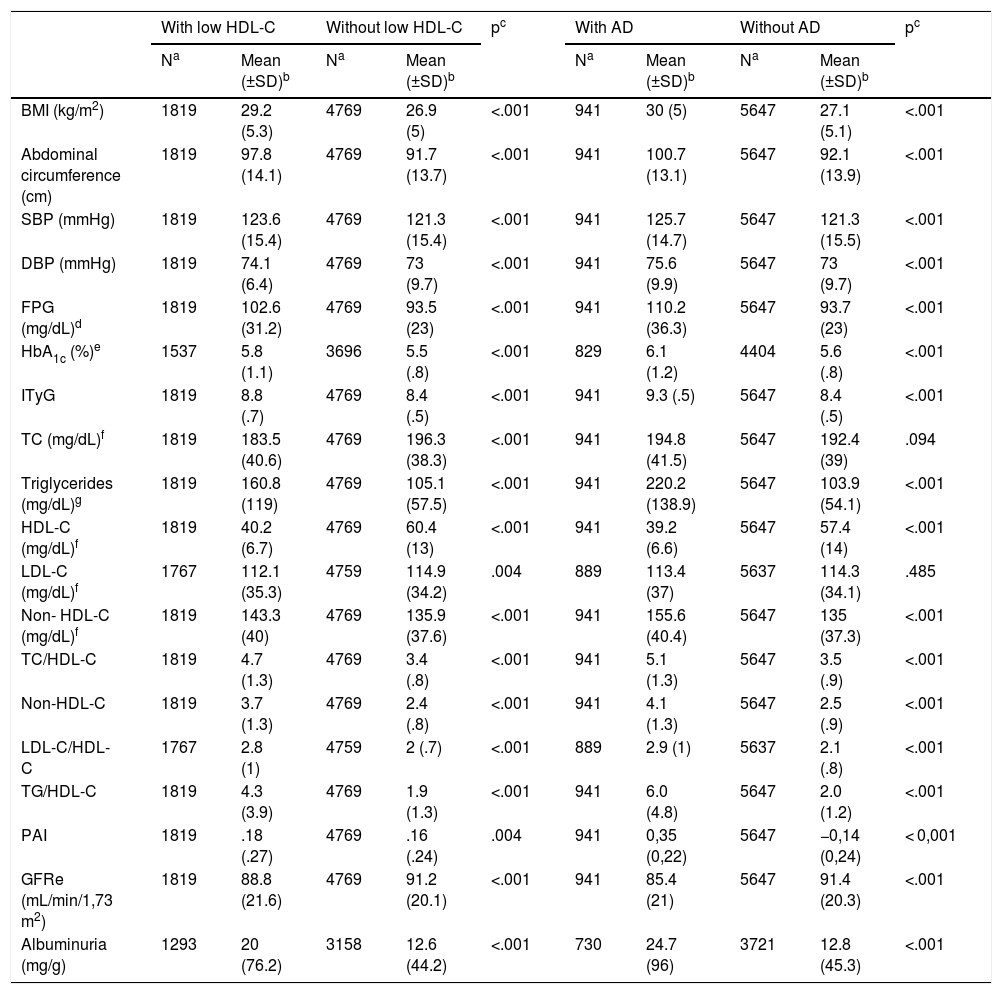
The clinical characteristics of the study population have been described previously in this journal.21 The means (±SD) of lipid parameter concentrations were total cholesterol (TC) 192.8 (±39.3) mg/dL; TG 120.5 (±83.2) mg/dL; HDL-C 54.8 (±14.7) mg/dL; LDL-C 114.2 (±34.5) mg/dL. The mean [±SD] HDL-C was significantly lower (p < .001) in males (49.2 [±12.6] mg/dL) than in females (59.2 [±14.7] mg/dL), and the mean [±SD] TG was significantly higher (p < .001) in males (135.7 [±100.6] mg/dL) than in females (108.6.2 [±63.8] mg/dL).
Description of the clinical characteristics of populations with and without low HDL-C and with and without AD are shown in Table 1. The median (IQR) TG concentrations in the populations with and without low HDL-C were 134 (96–194) mg/dL and 92 (68–127.5) mg/dL, respectively. The medians (IQR) of the concentrations of TG in the populations with and without AD were 191 (156–246.5) mg/dL and 93 (69–125) mg/dL, respectively.
Clinical characteristics of populations with and without low HDL-C, and with and without AD.
| With low HDL-C | Without low HDL-C | pc | With AD | Without AD | pc | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Na | Mean (±SD)b | Na | Mean (±SD)b | Na | Mean (±SD)b | Na | Mean (±SD)b | |||
| BMI (kg/m2) | 1819 | 29.2 (5.3) | 4769 | 26.9 (5) | <.001 | 941 | 30 (5) | 5647 | 27.1 (5.1) | <.001 |
| Abdominal circumference (cm) | 1819 | 97.8 (14.1) | 4769 | 91.7 (13.7) | <.001 | 941 | 100.7 (13.1) | 5647 | 92.1 (13.9) | <.001 |
| SBP (mmHg) | 1819 | 123.6 (15.4) | 4769 | 121.3 (15.4) | <.001 | 941 | 125.7 (14.7) | 5647 | 121.3 (15.5) | <.001 |
| DBP (mmHg) | 1819 | 74.1 (6.4) | 4769 | 73 (9.7) | <.001 | 941 | 75.6 (9.9) | 5647 | 73 (9.7) | <.001 |
| FPG (mg/dL)d | 1819 | 102.6 (31.2) | 4769 | 93.5 (23) | <.001 | 941 | 110.2 (36.3) | 5647 | 93.7 (23) | <.001 |
| HbA1c (%)e | 1537 | 5.8 (1.1) | 3696 | 5.5 (.8) | <.001 | 829 | 6.1 (1.2) | 4404 | 5.6 (.8) | <.001 |
| ITyG | 1819 | 8.8 (.7) | 4769 | 8.4 (.5) | <.001 | 941 | 9.3 (.5) | 5647 | 8.4 (.5) | <.001 |
| TC (mg/dL)f | 1819 | 183.5 (40.6) | 4769 | 196.3 (38.3) | <.001 | 941 | 194.8 (41.5) | 5647 | 192.4 (39) | .094 |
| Triglycerides (mg/dL)g | 1819 | 160.8 (119) | 4769 | 105.1 (57.5) | <.001 | 941 | 220.2 (138.9) | 5647 | 103.9 (54.1) | <.001 |
| HDL-C (mg/dL)f | 1819 | 40.2 (6.7) | 4769 | 60.4 (13) | <.001 | 941 | 39.2 (6.6) | 5647 | 57.4 (14) | <.001 |
| LDL-C (mg/dL)f | 1767 | 112.1 (35.3) | 4759 | 114.9 (34.2) | .004 | 889 | 113.4 (37) | 5637 | 114.3 (34.1) | .485 |
| Non- HDL-C (mg/dL)f | 1819 | 143.3 (40) | 4769 | 135.9 (37.6) | <.001 | 941 | 155.6 (40.4) | 5647 | 135 (37.3) | <.001 |
| TC/HDL-C | 1819 | 4.7 (1.3) | 4769 | 3.4 (.8) | <.001 | 941 | 5.1 (1.3) | 5647 | 3.5 (.9) | <.001 |
| Non-HDL-C | 1819 | 3.7 (1.3) | 4769 | 2.4 (.8) | <.001 | 941 | 4.1 (1.3) | 5647 | 2.5 (.9) | <.001 |
| LDL-C/HDL-C | 1767 | 2.8 (1) | 4759 | 2 (.7) | <.001 | 889 | 2.9 (1) | 5637 | 2.1 (.8) | <.001 |
| TG/HDL-C | 1819 | 4.3 (3.9) | 4769 | 1.9 (1.3) | <.001 | 941 | 6.0 (4.8) | 5647 | 2.0 (1.2) | <.001 |
| PAI | 1819 | .18 (.27) | 4769 | .16 (.24) | .004 | 941 | 0,35 (0,22) | 5647 | −0,14 (0,24) | < 0,001 |
| GFRe (mL/min/1,73 m2) | 1819 | 88.8 (21.6) | 4769 | 91.2 (20.1) | <.001 | 941 | 85.4 (21) | 5647 | 91.4 (20.3) | <.001 |
| Albuminuria (mg/g) | 1293 | 20 (76.2) | 3158 | 12.6 (44.2) | <.001 | 730 | 24.7 (96) | 3721 | 12.8 (45.3) | <.001 |
AD: Atherogenic dyslipidaemia; Body Mass Index (BMI); Diastolic blood pressure (DBP); GFRe: Estimated glomerular filtration rate (Chronic Kidney Disease Epidemiology Collaboration, CKD-EPI); Fasting plasma glucose (FPG); HbA1c: Glycosylated haemoglobin A1c (HbA1c); HDL-C: High-density lipoprotein-bound cholesterol; ITyG39: Triglyceride and glucose index; LDL-C: Low-density lipoprotein-bound cholesterol; Low HDL-C: High-density lipoprotein-bound cholesterol <40mg/dL (men) and <50mg/dL (women); Non-HDL-C: non-HDL-bound cholesterol; PAI40 plasma atherogenic index (log (TG/HDL-C)); Systolic blood pressure (SBP); TC: Total cholesterol; Triglyceride index (TG).
In the population with low HDL-C, the mean [±SD] HDL-C was significantly higher (p < .001) in females (43.2 [±5.7] mg/dL) than in males (36.8 [±5.9] mg/dL), and the mean [±SD] TG was significantly higher (p < .001) in males (182.2 [±143.3] mg/dL) than in females (142.3 [±88.9] mg/dL).
In the population with AD, the mean [±SD] HDL-C was significantly higher (p < .001) in females (42.3 [±6] mg/dL) than in males (36.6 [±6] mg/dL), and the mean [±SD] TG was significantly higher (p < .001) in males (233.2 [±163.3] mg/dL) than in females (204.6 [±100.2] mg/dL).
The crude prevalence rates of low HDL-C, HTG, and AD were 30.8% (CI: 29.7–31.9); 29.6% (CI: 28.4–30.7) and 14.3% (CI: 13.5–15.2), respectively. The difference in crude prevalence of low HDL-C between females (32.1% [CI: 30.6–33.6]) and males (29.1% [CI: 27.5–30.8]) was significant (p = .009). The difference in crude prevalence of AD between males (17.7% [CI: 16.3–19.1]) and females (11.7%11.7% [CI: 10.6–12.7]) was significant (p < .001).
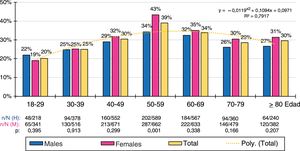
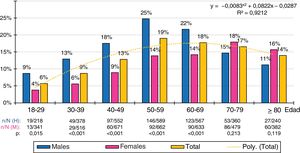
The age- and sex-adjusted prevalence rates for HTG were 27% (overall); 34.6% (males) and 21.4% (females); those for low HDL-C were 29.6% (overall), 28% (males) and 31% (females); and those for AD were 13.1% (overall); 16.4% (males) and 10.6% (females).
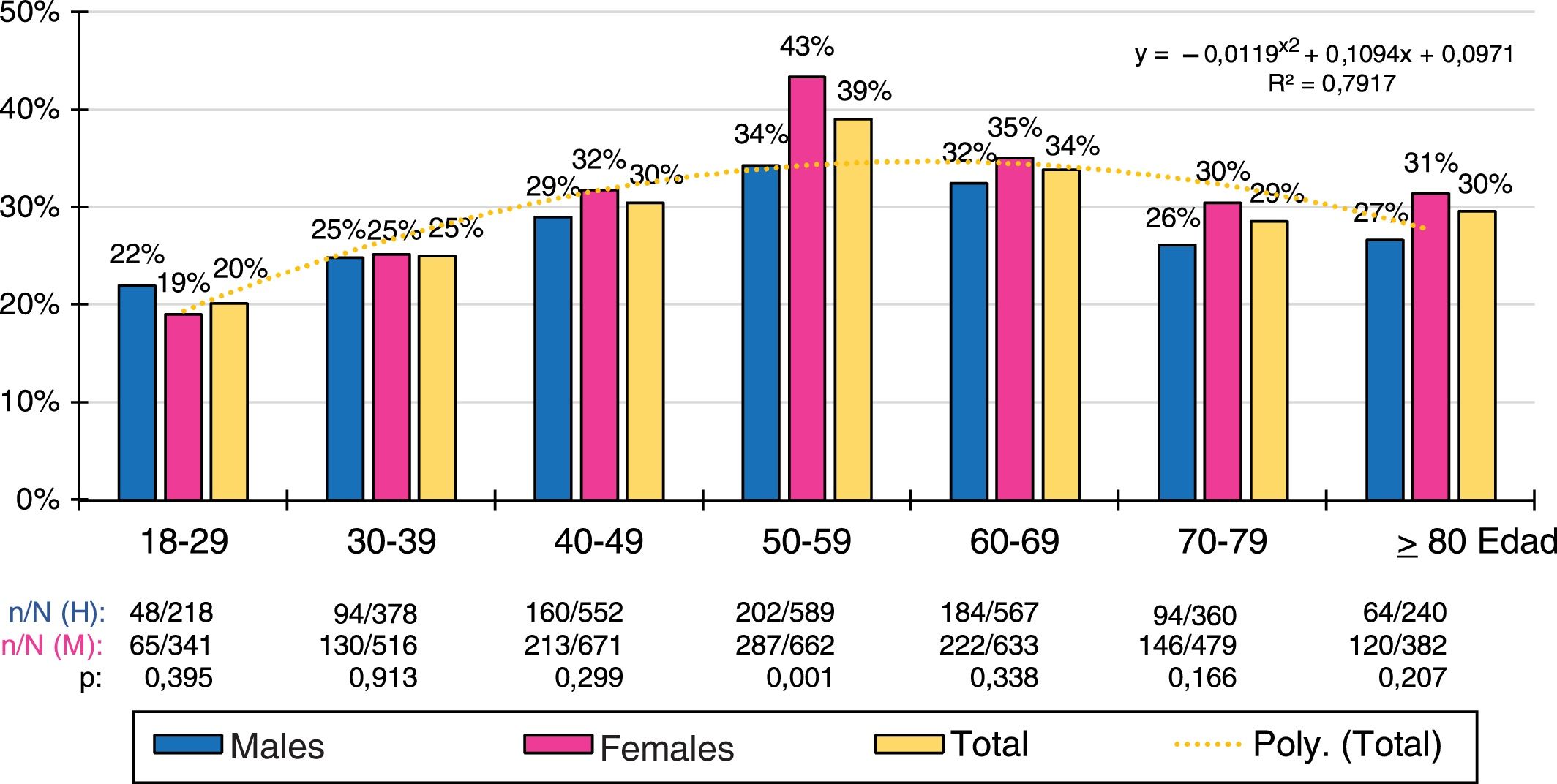
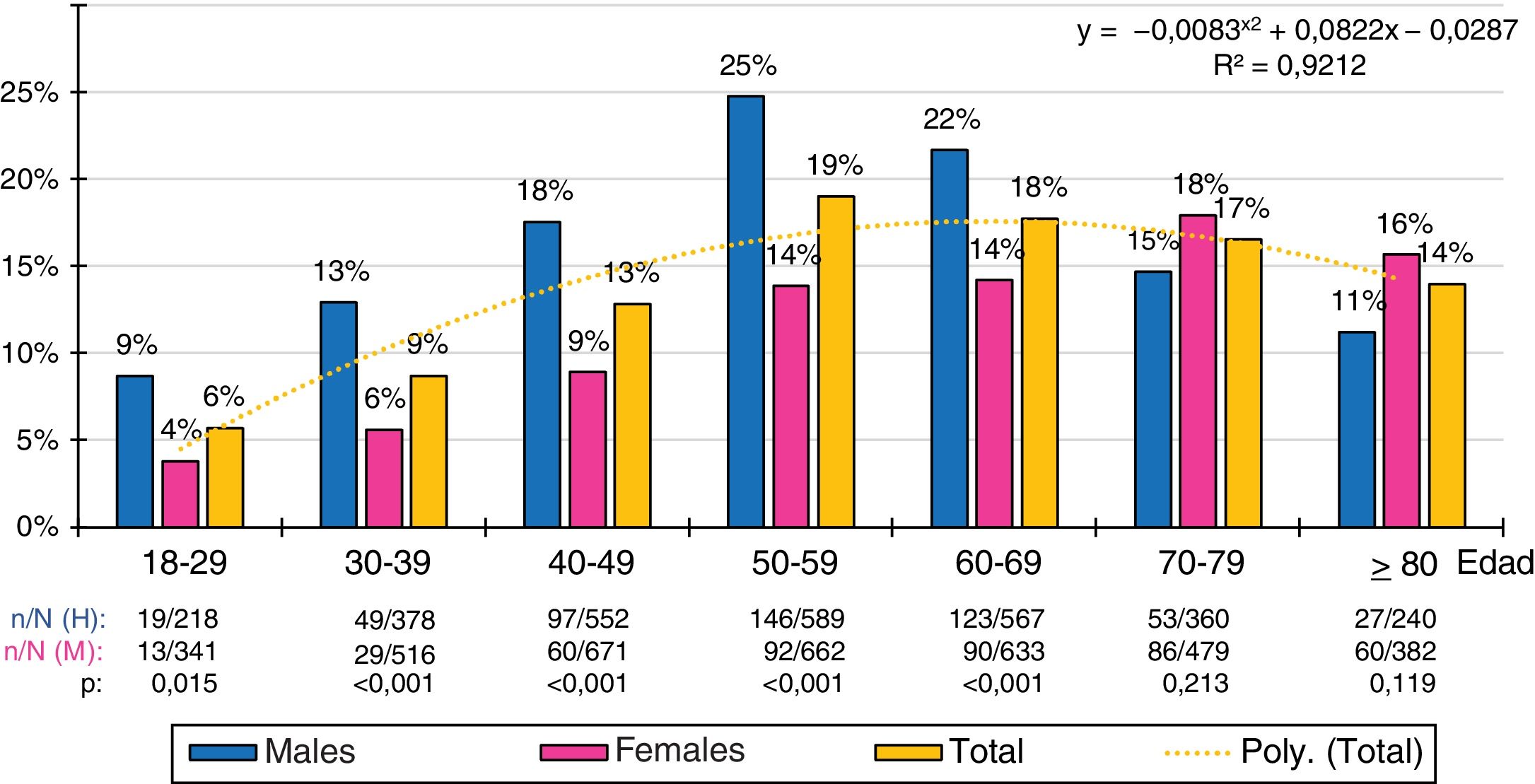
The ten-year age group distributions of the prevalence rates of low HDL-C and AD were adjusted (R2 = .79 and .92 respectively) to the following polynomial functions: y = −.0119x2 + .1094x + .0971; y = −.0083x2 + .0822x−.0287, respectively. The prevalence of low HDL-C was similar in males and females in all age groups, except in the 50 s, when there was a higher prevalence in the female population. The prevalence of AD increased with age, peaking in the 50 s in the male population and in the 70 s in the female population. The male population had higher prevalence rates of AD than the female population in all age groups until the 60 s, which later reversed with a higher prevalence of AD in the female population (Figs. 1 and 2).
Of the population with low HDL-C, 37.7% (CI: 35.4–39.9%) were on treatment with hypolipidaemic drugs, and 51.5% (CI: 48.1–54.9%) of the population with AD. The percentages of subjects categorized according to their CVR17 of the populations with low HDL-C and AD were respectively the following: low CVR: 19% (CI: 17.2%–20.9%) and 5.7% (CI: 4.2%–7.5%); moderate CVR: 22.1% (CI: 20.2%–24.0%) and 21.6% (CI: 18.8%–24.5%); high CVR: 19.4% (CI: 17.6%–21.3%) and 26.6% (CI: 23.6%–29.6%); remarkably high CVR: 39.5 (CI: 37.3%–41.8%) and 51.5% (CI: 48%–54.9%).
All the descriptive parameters (blood pressure, cardiometabolic, lipid and renal) were significantly higher in the population with low HDL-C than in the population without low HDL-C. Except for TC and LDL-C, all the descriptive parameters were significantly higher in the population with AD than in the population without AD (Table 1).
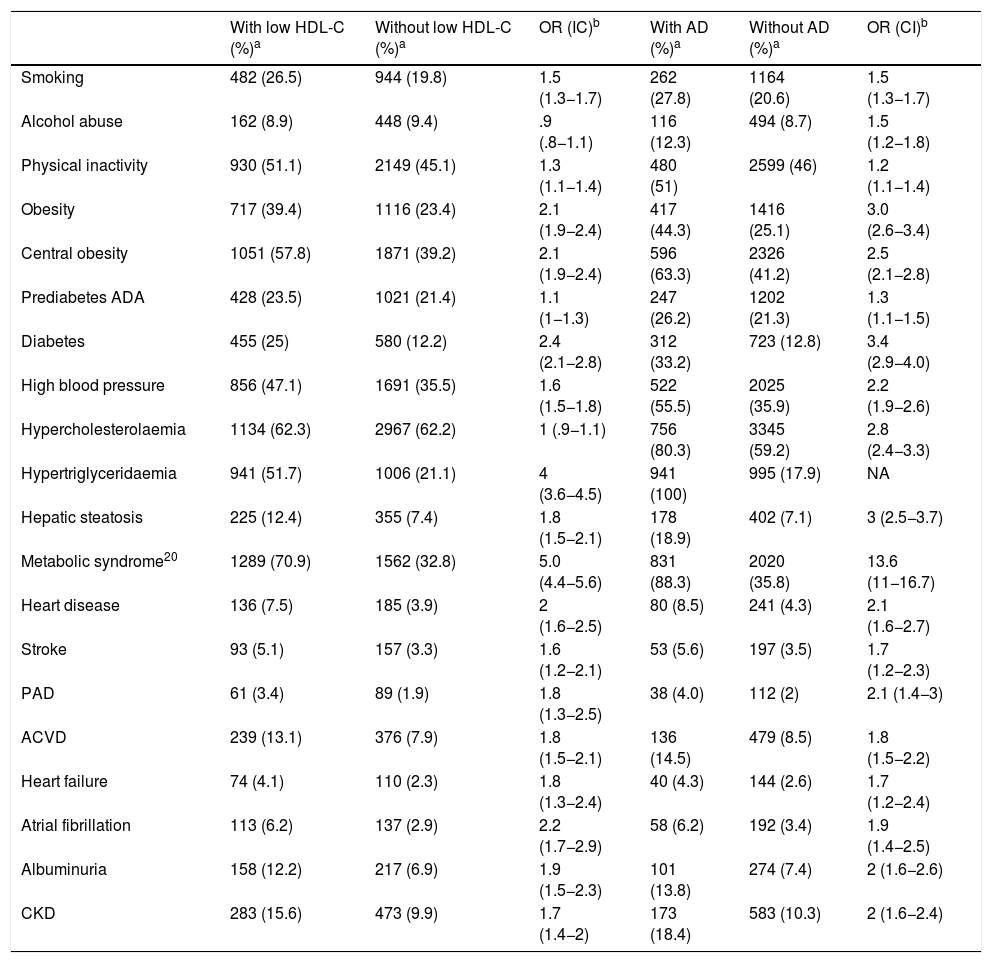
All cardiometabolic, cardiovascular and renal variables were statistically related to low HDL-C and AD, except for alcohol abuse and pre-diabetes, which were not related to low HDL-C (Table 2). The independent factors associated with low HDL-C and AD are shown in Tables 3 and 4, respectively.
Associated comorbidity with and without low HDL-C, and associated with and without AD.
| With low HDL-C (%)a | Without low HDL-C (%)a | OR (IC)b | With AD (%)a | Without AD (%)a | OR (CI)b | |
|---|---|---|---|---|---|---|
| Smoking | 482 (26.5) | 944 (19.8) | 1.5 (1.3−1.7) | 262 (27.8) | 1164 (20.6) | 1.5 (1.3−1.7) |
| Alcohol abuse | 162 (8.9) | 448 (9.4) | .9 (.8−1.1) | 116 (12.3) | 494 (8.7) | 1.5 (1.2−1.8) |
| Physical inactivity | 930 (51.1) | 2149 (45.1) | 1.3 (1.1−1.4) | 480 (51) | 2599 (46) | 1.2 (1.1−1.4) |
| Obesity | 717 (39.4) | 1116 (23.4) | 2.1 (1.9−2.4) | 417 (44.3) | 1416 (25.1) | 3.0 (2.6−3.4) |
| Central obesity | 1051 (57.8) | 1871 (39.2) | 2.1 (1.9−2.4) | 596 (63.3) | 2326 (41.2) | 2.5 (2.1−2.8) |
| Prediabetes ADA | 428 (23.5) | 1021 (21.4) | 1.1 (1−1.3) | 247 (26.2) | 1202 (21.3) | 1.3 (1.1−1.5) |
| Diabetes | 455 (25) | 580 (12.2) | 2.4 (2.1−2.8) | 312 (33.2) | 723 (12.8) | 3.4 (2.9−4.0) |
| High blood pressure | 856 (47.1) | 1691 (35.5) | 1.6 (1.5−1.8) | 522 (55.5) | 2025 (35.9) | 2.2 (1.9−2.6) |
| Hypercholesterolaemia | 1134 (62.3) | 2967 (62.2) | 1 (.9−1.1) | 756 (80.3) | 3345 (59.2) | 2.8 (2.4−3.3) |
| Hypertriglyceridaemia | 941 (51.7) | 1006 (21.1) | 4 (3.6−4.5) | 941 (100) | 995 (17.9) | NA |
| Hepatic steatosis | 225 (12.4) | 355 (7.4) | 1.8 (1.5−2.1) | 178 (18.9) | 402 (7.1) | 3 (2.5−3.7) |
| Metabolic syndrome20 | 1289 (70.9) | 1562 (32.8) | 5.0 (4.4−5.6) | 831 (88.3) | 2020 (35.8) | 13.6 (11−16.7) |
| Heart disease | 136 (7.5) | 185 (3.9) | 2 (1.6−2.5) | 80 (8.5) | 241 (4.3) | 2.1 (1.6−2.7) |
| Stroke | 93 (5.1) | 157 (3.3) | 1.6 (1.2−2.1) | 53 (5.6) | 197 (3.5) | 1.7 (1.2−2.3) |
| PAD | 61 (3.4) | 89 (1.9) | 1.8 (1.3−2.5) | 38 (4.0) | 112 (2) | 2.1 (1.4−3) |
| ACVD | 239 (13.1) | 376 (7.9) | 1.8 (1.5−2.1) | 136 (14.5) | 479 (8.5) | 1.8 (1.5−2.2) |
| Heart failure | 74 (4.1) | 110 (2.3) | 1.8 (1.3−2.4) | 40 (4.3) | 144 (2.6) | 1.7 (1.2−2.4) |
| Atrial fibrillation | 113 (6.2) | 137 (2.9) | 2.2 (1.7−2.9) | 58 (6.2) | 192 (3.4) | 1.9 (1.4−2.5) |
| Albuminuria | 158 (12.2) | 217 (6.9) | 1.9 (1.5−2.3) | 101 (13.8) | 274 (7.4) | 2 (1.6−2.6) |
| CKD | 283 (15.6) | 473 (9.9) | 1.7 (1.4−2) | 173 (18.4) | 583 (10.3) | 2 (1.6−2.4) |
ACVD: Atherosclerotic cardiovascular disease; AD: Atherogenic dyslipidaemia; ADA Prediabetes (American Diabetes Association): fasting plasma glucose (FPG) 100–125 mg/dL, or HbA1c 5.7%–6.4%: Albuminuria: albumin-creatinine ratio >30 mg/g; Alcohol abuse: habitual alcohol consumption >210 g/week (males) and >140 g/week (females); BMI: Body Mass Index; Central obesity: abdominal circumference ≥102 cm (males); ≥88 cm (females); CKD: Chronic kidney disease; Diabetes: FPG ≥126 mg/dL or HbA1c ≥6.5% or diagnosis recorded in clinical history; HbA1c: Glycosylated haemoglobin A1c; Hypercholesterolaemia: Total cholesterol ≥200 mg/dL or diagnosis in clinical history; Hypertriglyceridaemia: Triglycerides ≥150 mg/dL or diagnosis in clinical history; Low HDL-C: Cholesterol bound to high density lipoproteins <40 mg/dL (males) and <50 mg/dL (females); NA: Not applicable; Obesity: BMI ≥30 kg/m2; PAD: Peripheral artery disease; Physical inactivity: Physical activity <150 min/week; Smoking: Consumption of any amount of cigarettes or tobacco over the last month.
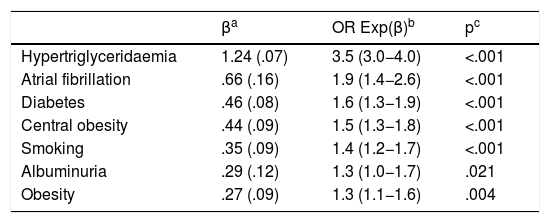
Independent factors associated with low HDL-C.
| βa | OR Exp(β)b | pc | |
|---|---|---|---|
| Hypertriglyceridaemia | 1.24 (.07) | 3.5 (3.0−4.0) | <.001 |
| Atrial fibrillation | .66 (.16) | 1.9 (1.4−2.6) | <.001 |
| Diabetes | .46 (.08) | 1.6 (1.3−1.9) | <.001 |
| Central obesity | .44 (.09) | 1.5 (1.3−1.8) | <.001 |
| Smoking | .35 (.09) | 1.4 (1.2−1.7) | <.001 |
| Albuminuria | .29 (.12) | 1.3 (1.0−1.7) | .021 |
| Obesity | .27 (.09) | 1.3 (1.1−1.6) | .004 |
Albuminuria: Albumin-creatinine ratio >30 mg/g; Atrial fibrillation: Diagnosis in clinical history; BMI: Body Mass Index; Central obesity: abdominal circumference ≥102 cm (males); ≥88 cm (females); Diabetes: Fasting plasma glucose ≥126 mg/dL or HbA1c ≥6.5% or diagnosis recorded in clinical history; HbA1c: Glycosylated haemoglobin A1c; Hypertriglyceridaemia: Triglycerides≥150mg/dL or diagnosis in clinical history; Low HDL-C: Cholesterol bound to high density lipoproteins < 40mg/dL (males) and <50 mg/dL (females); Obesity: BMI ≥30 kg/m2; Smoking: Consumption of any amount of cigarettes or tobacco over the last month.
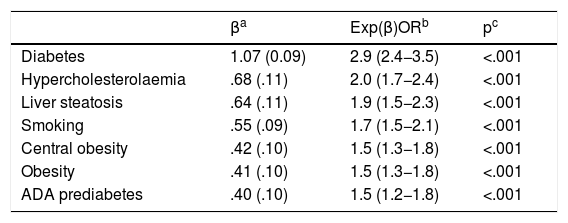
Independent factors associated with atherogenic dyslipidaemia.
| βa | Exp(β)ORb | pc | |
|---|---|---|---|
| Diabetes | 1.07 (0.09) | 2.9 (2.4−3.5) | <.001 |
| Hypercholesterolaemia | .68 (.11) | 2.0 (1.7−2.4) | <.001 |
| Liver steatosis | .64 (.11) | 1.9 (1.5−2.3) | <.001 |
| Smoking | .55 (.09) | 1.7 (1.5−2.1) | <.001 |
| Central obesity | .42 (.10) | 1.5 (1.3−1.8) | <.001 |
| Obesity | .41 (.10) | 1.5 (1.3−1.8) | <.001 |
| ADA prediabetes | .40 (.10) | 1.5 (1.2−1.8) | <.001 |
ADA prediabetes (American Diabetes Association): Fasting plasma glucose 100–125 mg/dL, or HbA1c 5.7%–6.4%; Central obesity: Abdominal circumference ≥102 cm (males); ≥88 cm (females); Diabetes: Fasting blood glucose ≥126 mg/dL or HbA1c ≥6.5% or diagnosis recorded in clinical history; HbA1c: Glycosylated haemoglobin A1c; Hypercholesterolaemia: Total cholesterol ≥200 mg/dL or diagnosis in clinical history; Liver steatosis: Diagnosis in clinical history; Obesity: Body mass index ≥30 kg/m2; Smoking: Consumption of any amount of cigarettes or tobacco over the last month.
The present study updates the information on mean HDL-C values and prevalence rates of low HDL-C and AD in the adult population, offering results that are intermediate between those published by other international and national studies. The cardiometabolic and renal characteristics of the population with low HDL-C were significantly higher than those of the population without low HDL-C, the differences being more pronounced when compared between populations with and without AD (Table 1). The mean HDL-C in the SIMETAP-AD study was similar to those in the United States22 (53 mg/dL), France23 (57 mg/dL) and Germany24 (56 mg/dL), and lower than those in China25 (50 mg/dL) and India26 (49 mg/dL). The average HDL-C was similar to those of the DARIOS27 (54 mg/dL) and ENRICA28 (53 mg/dL) studies conducted in Spain. The PREDIMERC29 study, conducted in Madrid, showed a higher mean than the present study in females (64 mg/dL) and similar in males (50 mg/dL).
The prevalence of low HDL-C in the SIMETAP-AD study was similar to the United States22 (30%), higher than China25 (22%), and lower than India26 (43%). The prevalence was higher than those of the DARIOS27 (20% [males]; 28% [females]), ENRICA28 (26%), and PREDIMERC29 (18% [males]; 9% [females]) studies. These lower prevalence rates than the SIMETAP-AD study, could be because the ENRICA28 study excluded patients with TG >400 mg/dL, the PREDIMERC29 study considered low HDL-C at a stricter concentration (<46 mg/dL) in females, and the DARIOS27 study selected a limited population between 35 and 74 years of age. In the present study, the prevalence of AD in adult subjects <35 years and >70 years was not negligible (8% and 16% respectively), and therefore it could be recommended to include these 2 age groups to assess the entire adult population more accurately.
Prevalence rates of low HDL-C and AD are higher in patients with ACVD. In the CLYDIA30 study, the prevalence of low HDL-C was 34.4% in patients with ACVD, higher than in the present study conducted in the general population. In heart patients, the LIPICERES31 study showed a prevalence of low HDL-C of 32% in males and 45% in females, and in the PRESENAP32 study, the prevalence of low HDL-C was 26%. The prevalence rates of AD in the LIPICERES31 and PRESENAP32 studies were 11.2% and 13% respectively, similar to that of the SIMETAP-AD study, considering the age ranges of these studies.
Likewise, the prevalence rates of low HDL-C and AD tend to be higher in patients with DM or with greater CVR. In the EDICONDIS-ULISEA33 study, which included patients assessed in SEA Lipid Units for dyslipidaemia, 36.1% had low HDL-C and 17.9% had AD. In a health survey conducted in Europe34 that included patients with DM or MS (93% with hypolipidaemic drugs), the prevalence of low HDL-C was 34%. In an analysis of the DYSIS35 study conducted in patients treated with statins, the prevalence of low HDL-C was 23% and the prevalence of AD was 13.1%, the same as the present study. The Di@bet.es36 study showed a prevalence of low HDL-C, 28%, lower than the SIMETAP-DM37 study, which showed a prevalence of 41% in patients with DM. Finally, in the population with AD, HDL-C concentrations in the present study were similar to the SEA Registry38 in patients with HTG (41 mg/dL [global]; 39 mg/dL [males]; 46 mg/dL [females]).
Metabolic involvement in populations with low HDL-C or AD is important. In addition to the obvious association with MS,20 DM was the comorbidity with the strongest association with AD and had a strong association with low HDL-C. In addition, obesity and increased abdominal girth were also independent factors associated with both entities. Furthermore, it has been suggested that the TG and glucose index (Ln [TG × FPG/2]) is a good marker of risk for DM.39 TG and glucose indices were also high in the low HDL-C and AD populations (8.8 and 9.3 respectively), suggesting that these entities may be good markers of insulin resistance.
Cardiovascular involvement in populations with low HDL-C or AD was also important as high blood pressure, ACVD (HD, stroke, and peripheral arterial disease), heart failure and atrial fibrillation were associated with low HDL-C and AD. CKD and albuminuria also appeared as factors associated with low HDL-C and AD, with the ORs being slightly higher in AD, although only albuminuria stood out as an independent factor associated with low HDL-C (Tables 2–4).
TG concentrations modulate the concentration of HDL particles towards denser and smaller subclasses (HDL3), producing a net result of decreased HDL-C. In the population with low HDL-C in the present study, the close relationship between HDL-C and TG in both the univariate (OR: 4) and the multivariate (OR: 3.5) analysis stands out, despite being evaluated in a population with low HDL-C, whose mean and median TG concentrations were 161 and 134 mg/dL, respectively (Tables 1–3).
The increase in TC was not associated with low HDL-C, however, it was strongly associated with AD, which could indicate that partial assessment of TC may underestimate the lipid phenotype and that a comprehensive assessment of the whole lipid profile, including HDL-C and TG concentrations, is important. The plasma atherogenic index (log [TG/HDL-C]) is a predictor of risk of atherosclerosis, where values between −.3 and .11 are associated with low CVR, between .1 and .21 with moderate CVR and >.21 with high CVR.40 In patients with HTG, the Castelli-I41 (CT/HDL-C) and Castelli-I41 (LDL-C/HDL-C) indices have been considered more sensitive and specific CD risk indices than CT, and associated with higher CD risk when they are >4 and >3, respectively.42 In patients in whom LDL-C cannot be calculated due to TG >400 mg/dL, the atherogenic coefficient (non-HDL-C/HDL-C) reflects the atherogenic potential of particles containing apolipoprotein B2. In the present study, all these rates were high in the populations with low HDL-C or AD, indicating that their atherogenic risks were higher than the populations without low HDL-C and without AD. This higher atherogenic risk together with a high prevalence of ACVD, DM, obesity, hypertension, and MS in the AD population could explain 73% of this population having a high or remarkably high CVR, and therefore it is advisable to detect AD in the lipid profile and consider it an important atherogenic risk marker.
A limitation of the present study was possible under-diagnosis by excluding as per protocol terminal, institutionalized, or cognitively impaired patients. Another limitation was that the investigators had to collect the information on the most recent parameters from the analyses carried out over the last year, and therefore the cross-sectional observation could have been influenced by the diseases the patients were suffering at that time and by their treatments. It should also be noted that the age and sex variables were not used in the multivariate analysis because they were strongly associated with CVRF, cardiovascular and cardiometabolic diseases, to highlight the other variables that could be associated with low HDL-C or AD. On the other hand, it is plausible that the prevalence rates of low HDL-C and AD could be somewhat higher if the populations with low HDL-C or AD were not under the influence of hypolipidaemic therapy (38% and 52% respectively), a circumstance that cannot be avoided ethically in this observational study. Another limitation was the inability of a cross-sectional study to determine causality.
The main strengths of the present study were the population-based randomized selection of a large sample that included the entire adult age range from 18 to 102 years, the description of the prevalence rates in all adult ten-year age groups, the presentation with age- and sex-adjusted rates, and the evaluation of the possible associations between these entities and CVRF cardiometabolic diseases, CKD, and ACVD.
SEA recommends promoting and disseminating existing knowledge of AD and associated risk, and implementing measures for its correct identification, treatment, and control.43 More epidemiological studies are needed to analyse the prevalence of low HDL-C and AD throughout the population, for better planning of intervention policies for cardiovascular prevention, to optimise the available health resources, and improve medical care and quality of life for AD patients. In this sense, it is hoped that this study will contribute towards the epidemiological knowledge of AD and assessing the importance of the risk associated with ACVD that AD entails.
ConclusionsThere is great variability in the studies conducted on the prevalence of low HDL-C or AD, depending on the comorbidities and the mean age of the populations studied. The present study shows medium HDL-C concentrations and medium prevalence rates of low HDL-C and AD compared to other studies.
The prevalence of these entities is high, as almost one third of the adult population had low HDL-C, and half of them met criteria for AD.
Cardiometabolic variables were associated with low HDL-C and AD, highlighting HTG as the main factor associated with low HDL-C, and DM as the main factor associated with AD.
The frequent association of these entities with CVRF, DM and ACVD means these patients must be identified rapidly to implement treatment and monitor for risk factors and cardiovascular disease as soon as possible.
FundingFunding of the SIMETAP study (grant code: 05/2010RS) RS_AP10/8 was approved in accordance with Order 472/2010, of 16 September, of the Regional Ministry of Health, approving the regulatory bases and the call for grants for 2010 from the "Pedro Laín Entralgo" Agency for Training, Research and Health Studies of the Community of Madrid, to conduct research projects in the field of health outcomes in primary care.
Research ethics committeeResearch Commission of Assistant Planning and Quality Management.
Primary Care Management. Health Service of the Community of Madrid (SERMAS).
Conflict of interestsThe authors have no conflict of interest to declare.
We would like to thank the doctors who participated in the SIMETAP Study Research Group: Abad Schilling C, Adrián Sanz M, Aguilera Reija P, Alcaraz Bethencourt A, Alonso Roca R, Álvarez Benedicto R, Arranz Martínez E, Arribas Álvaro P, Baltuille Aller MC, Barrios Rueda E, Benito Alonso E, Berbil Bautista ML, Blanco Canseco JM, Caballero Ramírez N, Cabello Igual P, Cabrera Vélez R, Calderín Morales MP, Capitán Caldas M, Casaseca Calvo TF, Cique Herráinz JA, Ciria de Pablo C, Chao Escuer P, Dávila Blázquez G, de la Peña Antón N, de Prado Prieto L, del Villar Redondo MJ, Delgado Rodríguez S, Díez Pérez MC, Durán Tejada MR, Escamilla Guijarro N, Escrivá Ferrairó RA, Fernández Vicente T, Fernández-Pacheco Vila D, Frías Vargas MJ, García Álvarez JC, García Fernández ME, García Alcañiz MP, García Granado MD, García Pliego RA, García Redondo MR, García Villasur MP, Gómez Díaz E, Gómez Fernández O, González Escobar P, González-Posada Delgado JA, Gutiérrez Sánchez I, Hernández Beltrán MI, Hernández de Luna MC, Hernández López RM, Hidalgo Calleja Y, Holgado Catalán MS, Hombrados Gonzalo MP, Hueso Quesada R, Ibarra Sánchez AM, Iglesias Quintana JR, Íscar Valenzuela I, Iturmendi Martínez N, Javierre Miranda AP, López Uriarte B, Lorenzo Borda MS, Luna Ramírez S, Macho del Barrio AI, Magán Tapia P, Marañón Henrich N, Mariño Suárez JE, Martín Calle MC, Martín Fernández AI, Martínez Cid de Rivera E, Martínez Irazusta J, Migueláñez Valero A, Minguela Puras ME, Montero Costa A, Mora Casado C, Morales Cobos LE, Morales Chico MR, Moreno Fernández JC, Moreno Muñoz MS, Palacios Martínez D, Pascual Val T, Pérez Fernández M, Pérez Muñoz R, Plata Barajas MT, Pleite Raposo R, Prieto Marcos M, Quintana Gómez JL, Redondo de Pedro S, Redondo Sánchez M, Reguillo Díaz J, Remón Pérez B, Revilla Pascual E, Rey López AM, Ribot Catalá C, Rico Pérez MR, Rivera Teijido M, Rodríguez Cabanillas R, Rodríguez de Cossío A, Rodríguez De Mingo E, Rodríguez Rodríguez AO, Rosillo González A, Rubio Villar M, Ruiz Díaz L, Ruiz García A, Sánchez Calso A, Sánchez Herráiz M, Sánchez Ramos MC, Sanchidrián Fernández PL, Sandín de Vega E, Sanz Pozo B, Sanz Velasco C, Sarriá Sánchez MT, Simonaggio Stancampiano P, Tello Meco I, Vargas-Machuca Cabañero C, Velazco Zumarrán JL, Vieira Pascual MC, Zafra Urango C, Zamora Gómez MM, Zarzuelo Martín N.
Please cite this article as: Ruiz-García A, Arranz-Martínez E, García-Fernández ME, Cabrera-Vélez R, García-Pliego RA, Morales-Cobose LE, et al. Factores cardiometabólicos asociados y prevalencia de concentraciones bajas de colesterol HDL y de dislipidemia aterogénica. Estudio SIMETAP-DA. Clin Investig Arterioscler. 2021;33:19–29.