A 60-year-old male with familial combined hyperlipidemia, ischaemic heart disease and type 2 diabetes. Since childhood, intolerance to intense exercise. The patient was diagnosed of McArdle's disease after an episode of rhabdomyolysis associated with statins as treatment after a myocardial infarction. Since then, he had been treated with diet, fibrates and ezetimibe with good tolerance, despite this, LDL cholesterol (cLDL) remained >180mg/dl. He started to be treated with alirocumab 150mg/sc every 14 days, with excellent clinical response and a decrease in cLDL to 15mg/dl. Our case shows that PCSK9 inhibitors are effective and safe in patients with muscle diseases who have statin contraindication, and they are a good therapeutic tool for these patients.
Varón de 60 años con hiperlipidemia familiar combinada, cardiopatía isquémica y diabetes tipo 2. Desde la infancia, intolerancia al esfuerzo intenso. Se le diagnosticó enfermedad de McArdle a raíz de rabdomiólisis asociada a estatinas tras un infarto de miocardio. Desde entonces había seguido tratamiento con dieta, fibratos y ezetimiba con buena tolerancia, pero a pesar de ello las concentraciones de colesterol LDL (cLDL) eran >180mg/dl. Se asoció al tratamiento alirocumab 150mg subcutáneos cada 14 días, con excelente respuesta clínica y descenso de cLDL a 15mg/dl, manteniéndose estable desde entonces. Nuestro caso demuestra que los inhibidores de PCSK9 son eficaces y seguros en pacientes con enfermedades musculares que contraindican las estatinas y que son una alternativa terapéutica ideal para este tipo de pacientes.
McArdle's disease, also known as glycogenosis type V, glycogen storage disease type V or myophosphorylase deficiency, is a rare disease that causes muscle pain on minimal exertion.1,2 McArdle's disease is an autosomal recessive disease and affected patients present mutations in both alleles of the PYGM gene, which encodes myophosphorylase. To date, over 65 mutations of the PYGM gene have been identified to cause McArdle's disease.2,3 Myophosphorylase initiates the breakdown of glycogen in the muscles, as a result of a deficiency in this enzyme's activity. Those who suffer from the condition find it difficult to obtain energy from their glycogen stores.1,2 Consuming complex carbohydrates (vegetables, fruit, grains, bread, pasta and rice) before exercise and a total daily calorie intake of fat of 20% seems to improve tolerance.2 At present, there is no known definitive cure, and for patients diagnosed with dyslipidaemia who also have coronary artery disease, new treatments are proposed which may achieve therapeutic objectives in these high-risk patients. Consequently, anti-PCSK9 monoclonal antibodies may be used as a therapeutic alternative in these patients.
Material and methodsWe present the case of a 60-year-old male with a very high cardiovascular risk and a history of rhabdomyolysis from statins, who first attended our clinic at the Lipid Unit of the Hospital Universitario Miguel Servet in Zaragoza approximately 16 years ago. He was referred by his primary care physician for the investigation of his dyslipidaemia, with fasting total cholesterol levels of around 300mg/dl and triglycerides of 534mg/dl. The patient had been diagnosed with type 2 diabetes around 6 years prior.
Since childhood, he has reported myalgia and exercise intolerance. On several occasions, he presented elevated resting levels of creatine kinase (CK) and episodes of myoglobinuria. At 52 years of age he suffered a myocardial infarction. A few weeks later, he had symptoms of rhabdomyolysis with haematuria, intense myalgia and CK levels of >10,000IU/l, which appeared two weeks after he began treatment with atorvastatin 20mg/day.
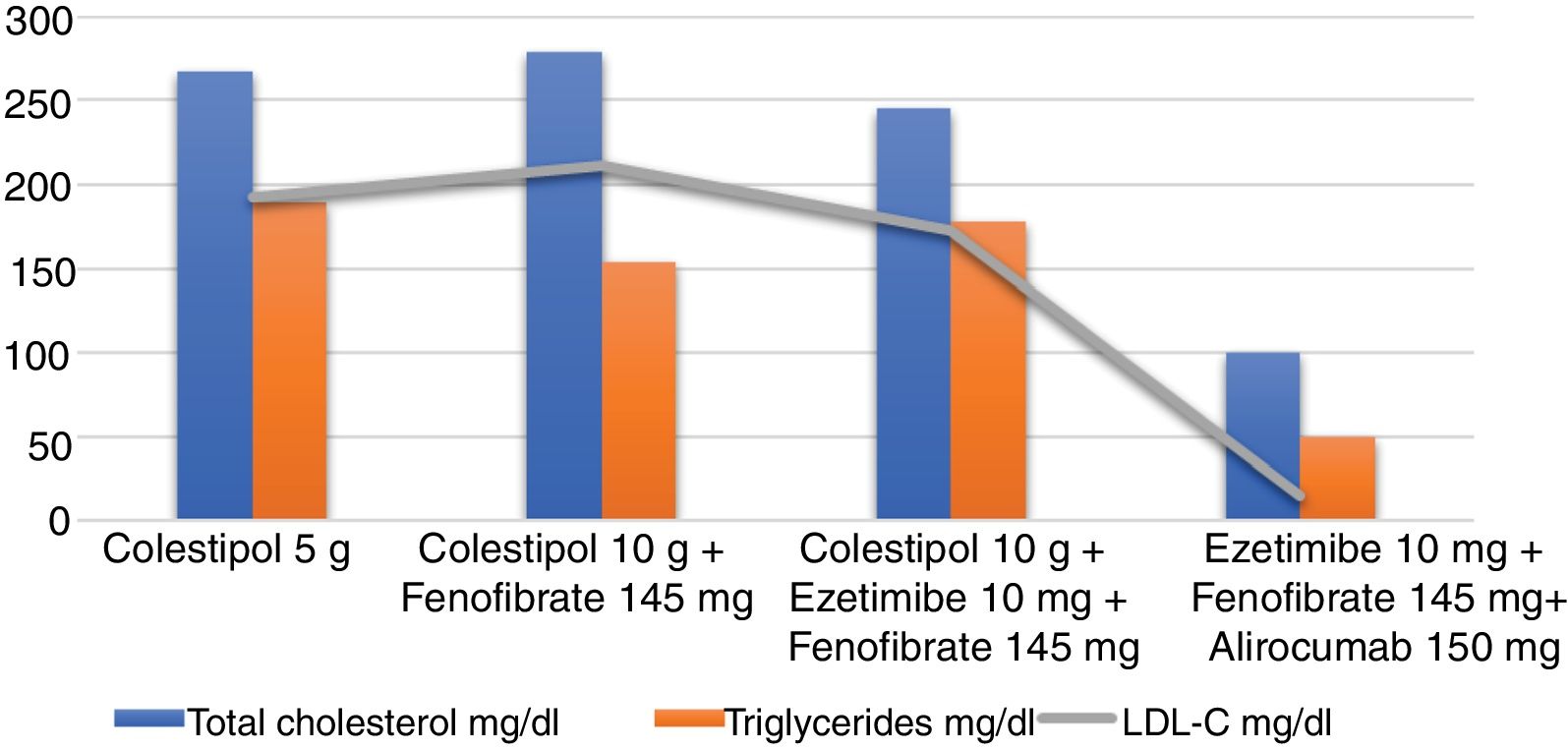
When the patient first attended our clinic, he presented with severe asthenia which had been ongoing for several months. His physical examination was normal and he weighed 81.7kg, with a body mass index (BMI) of 27.2kg/m2. He had no corneal arcus or xanthomas. He did, however, exhibit telangiectases on the cheeks. He had been following a strict diet low in saturated fat and high in complex carbohydrates, and undergoing pharmacological treatment with colestipol 5g per day for four years. Nevertheless, his lipid profile revealed the following: total cholesterol 267mg/dl, triglycerides 189mg/dl, HDL cholesterol (HDL-C) 36mg/dl, LDL cholesterol (LDL-C) 193.2mg/dl, apolipoprotein B (apoB) 186mg/dl. His liver enzymes were also raised: GGT 82U/l, GPT 82U/l, GOT 53U/l, and he also presented elevated CK levels of 4505U/l and lactate dehydrogenase (LDH) of 523U/l. His other biochemical parameters, including thyroid hormones, glucose and creatinine, were within the normal ranges. In light of suspected primary muscle disease, a muscle biopsy confirmed the diagnosis of McArdle's disease or glycogen storage disease type V.
It was recommended that he begin treatment with fenofibrate 145mg per day and his colestipol dose was doubled to 10g per day. He was also told to limit his physical activity. The nutritionist reinforced the importance of following a diet high in complex carbohydrates, limiting the intake of saturated fats, simple sugars and alcohol, and consuming oily fish at least once a week as well as nuts (at least three units) every day. At the next visit a year later, the patient had lost 5kg, but still felt tired and suffered from muscle pain. His CK levels were still raised, at 3000U/l, while his LDL-C was measured at 212mg/dl and glycosylated haemoglobin (HbA1c) 6.3%. At this stage, treatment with ezetimibe 10mg was also introduced. The patient was periodically reviewed by our unit, with no substantial changes in the physical examination or lipid profile.
ResultsSince the patient was far off his LDL-C targets, which are below 70mg/dl due to him being very high risk, and given the possibility of prescribing proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, in early 2017 the patient began treatment with alirocumab 150mg administered subcutaneously every 14 days, while continuing on ezetimibe 10mg per day and fenofibrate 145mg per day. Colestipol was voluntarily withdrawn due to digestive discomfort. He presented no clinical changes and remained angina-free, leading a normal life and tolerating light exercise on a daily basis. His CK levels have stayed between 1212 and 6000U/l, his liver enzymes have remained normal and there has been a marked decrease in his LDL-C, with levels of 15mg/dl. His latest blood work revealed the following: total cholesterol 100mg/dl, triglycerides 175mg/dl, HDL-C 50mg/dl, LDL-C 15mg/dl, apoB 41.4mg/dl, HbA1c 6.6%. Enzymes: GGT 28U/l, GPT 51U/l, GOT 47U/l, CK 1212U/l, LDH 222U/l. He is currently on the same treatment as stated above (Fig. 1).
DiscussionThis case report depicts a patient with McArdle's disease whose LDL-C levels improved on PCSK9 inhibitors, with excellent clinical tolerance.
McArdle's disease is a hereditary autosomal recessive disease caused by a deficiency in myophosphorylase, the enzyme in charge of skeletal muscle glycogen breakdown.4 It is characterised by asthenia, muscle weakness, cramping, myalgia and exercise intolerance, as well as high resting CK levels and episodes of myoglobinuria, particularly after exertion.1,3
This is the first case described in the literature with a favourable evolution of dyslipidaemia in the context of this disease. It also shows that PCSK9 inhibitors may be used as a treatment in these patients. Statins tend to aggravate muscular symptoms in patients with myopathies. It is therefore recommended that they be avoided or, where applicable, that low doses be used and strict follow-up performed.1,5 Our patient had episodes of myalgia and malaise when he performed exercise, which worsened while receiving statin therapy. He even developed an episode of severe rhabdomyolysis. However, on treatment with PCSK9 inhibitors, his lipid and enzyme profiles improved favourably, with no effects on his muscles. In spite of the very low LDL-C levels achieved in the patient, his doses of lipid-lowering agents were maintained in light of the excellent tolerance results and the clinical benefit of low LDL-C levels with PCSK9 inhibitors.6
The LDL receptor concentration on the surface of the liver is controlled by the PCSK9 protein.7 This protein reduces the uptake of LDL particles, which leads to an increase in plasma LDL-C levels. Monoclonal antibodies act by inhibiting PCSK9 binding to the LDL receptor. They have shown dose-dependent reductions of LDL-C (44–65%), apoB (48–59%) and lipoprotein(a) (27–50%), with no significant adverse effects, including in patients who are intolerant to statins.8 In the Odyssey Alternative study, 314 statin-intolerant patients were randomised to receive alirocumab 75mg every two weeks, ezetimibe 10mg/day or atorvastatin 20mg/day. If the LDL-C targets were not achieved, the alirocumab dose was increased to 150mg every two weeks. Muscular side effects were less prevalent with alirocumab than with atorvastatin.9
These findings suggest that PCSK9 inhibitors could be a promising alternative to reduce LDL-C in patients with contraindications for statins.10
ConclusionsMcArdle's disease is a myopathy caused by myophosphorylase deficiency. Affected patients experience acute muscle crises after intense exercise. There is currently no treatment, but a diet high in complex carbohydrates and limiting intense exercise are recommended. Statin therapy is contraindicated, so in the presence of high vascular risk and raised LDL-C levels, PCSK9 inhibitors represent a therapeutic alternative, as shown in our case.
Conflicts of interestFernando Civeira received financial contributions from Amgen and Sanofi for conferences and advisory committees. Victoria Marco-Benedí and Estíbaliz Jarauta received travel bursaries from Sanofi to attend congresses.
This case was granted a prize by the Spanish Society of Atherosclerosis (Sociedad Española de Arteriosclerosis, SEA) at the SEA National Congress held in Girona in June 2018.
Please cite this article as: Marco-Benedí V, Jarauta E, Pérez-Calahorra S, Bea AM, Civeira F. Tratamiento de un varón con enfermedad de McArdle y muy alto riesgo cardiovascular con inhibidores de PCSK9. Clín Investig Arterioscler. 2019;31:89–92.