Controlling cardiovascular risk factors (CV) is essential for patients with cardiovascular disease. The CV polypill contains aspirin 100mg, atorvastatin 20mg or 40mg, and ramipril 2.5mg, 5mg or 10mg in a fixed combination pill. The objective was to review the evidence on the secondary prevention of cardiovascular disease, to establish the eventual patient profiles suitable to consider the use of CV polypill with atorvastatin 40mg in secondary CV prevention (P40PS), and to define the priority situations most adequate for the use of P40PS. A bibliographic review was carried out, which was complemented with the clinical opinion of 19 specialists. During hospitalisation and discharge, P40PS is an option for patients admitted because of an atherothrombotic event, peripheral arterial disease, or other causes, and with the indication of the monocomponents. Its priority use is proposed in: prior intolerance to the highest dose of atorvastatin (80mg), age >75 years, low weight, stage 3 of chronic renal failure, hypothyroidism, drug interactions and Asian origin. Outside the hospital setting, the P40PS is a therapeutic alternative in patients with a need for secondary CV prevention and with indication to receive the monocomponents. The priority situations to receive the P40PS are: to be taking the three components separately, to require polypharmacy, lack of adherence or understanding of the treatment, and lack of control of CV risk factors. This work is the first with proposals for the use of P40PS and can facilitate the treatment of patients with cardiovascular disease in secondary prevention.
El control de los factores de riesgo cardiovascular (CV) es esencial en pacientes con enfermedad cardiovascular. La polipíldora CV contiene ácido acetilsalicílico 100mg, atorvastatina 20mg o 40mg y ramipril 2,5mg, 5mg o 10mg en combinación fija. El objetivo fue revisar las evidencias sobre la prevención secundaria de la enfermedad cardiovascular, establecer los posibles perfiles de pacientes donde usar la polipíldora CV con atorvastatina 40mg en prevención CV secundaria (P40PS) y definir las situaciones prioritarias de empleo de la P40PS. Se realizó una revisión bibliográfica, que se complementó con la opinión clínica de 19 especialistas. Durante la hospitalización y al alta, la P40PS es una opción en pacientes ingresados por un evento aterotrombótico de cualquier territorio, enfermedad arterial periférica u otras causas y con indicación de los monocomponentes. Se plantea su uso prioritario en: intolerancia previa a la dosis de atorvastatina 80mg, edad >75 años, bajo peso, insuficiencia renal crónica estadio 3, hipotiroidismo, interacciones farmacológicas y origen asiático. En el ámbito extrahospitalario, la P40PS es una alternativa terapéutica en los pacientes con necesidad de prevención CV secundaria con indicación para recibir los monocomponentes y las situaciones prioritarias son recibir los tres componentes por separado, requerir polimedicación, falta de adherencia o de comprensión del tratamiento, y falta de control de los factores de riesgo CV. Este trabajo es el primero con propuestas de uso de la P40PS y puede facilitar el tratamiento de los pacientes con enfermedad cardiovascular en prevención secundaria.
Diseases of the circulatory system are the leading cause of death in Spain. Coronary heart and cerebrovascular disease head the list.1 It is estimated that one third of patients who develop acute coronary syndrome (ACS) die before reaching hospital. Is it therefore very necessary to identify patients at high vascular risk in order to provide the most appropriate preventive measures.2
In view of the fact that in our environment atherosclerosis is one of the main causes of morbidity and mortality, current efforts are focused on prevention through the control of cardiovascular risk factors (CVRF),3 either in primary or secondary prevention. There are various different therapeutic strategies for controlling these risk factors. One of these is polypills, which include combinations of drugs for different CVRF in fixed doses. The cardiovascular (CV) polypill is the first treatment approved in Europe for the secondary prevention of CV disease (CVD). It contains three active ingredients at different doses: acetylsalicylic acid (ASA) (100mg), ramipril (2.5mg, 5mg or 10mg) and atorvastatin (20mg or 40mg).
Even though there is a wide range of treatments and strategies for controlling CVRF, some 50% of patients do not take the prescribed medication correctly.4 Over time, in both primary and secondary prevention, their neglect of the medication becomes worse, reducing the effectiveness of the treatments5–7 and increasing cardiovascular morbidity and mortality rates.8,9
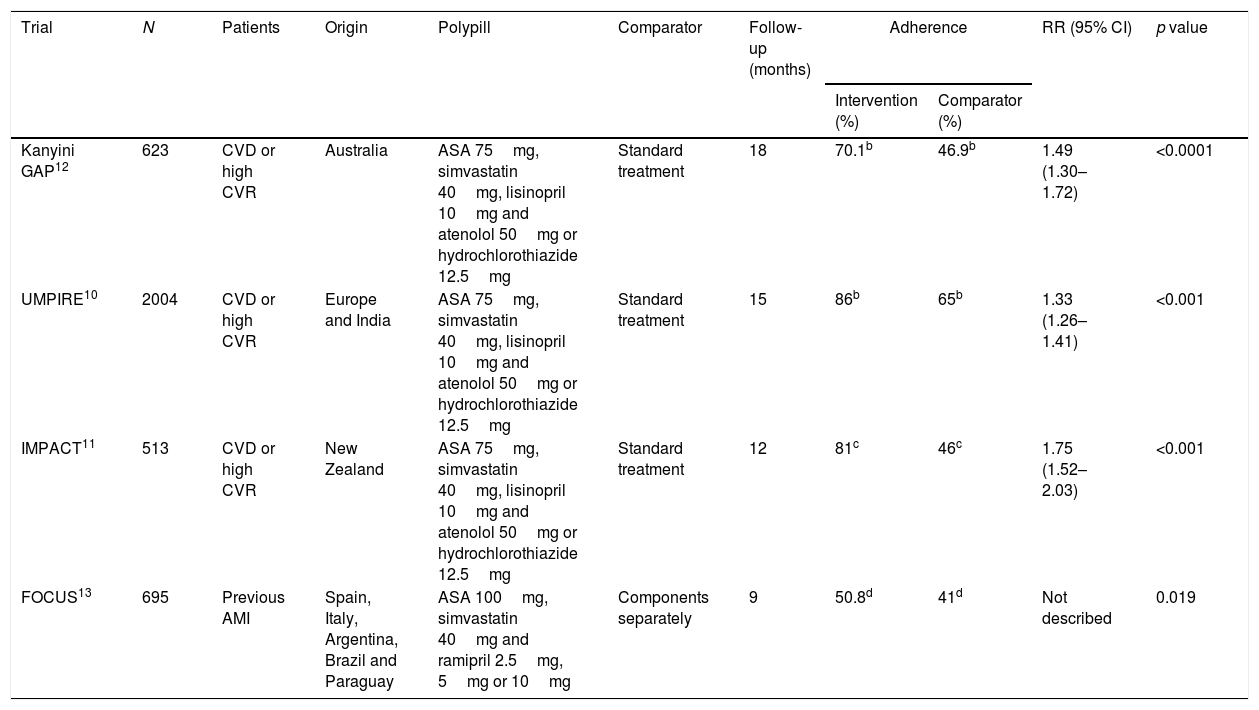
Different strategies have been developed in an effort to improve adherence and one of these is the CV polypill, which provides a combination of drugs at fixed doses in one tablet. These combinations have been shown to improve adherence in a number of different studies. The UMPIRE, IMPACT, Kanyini GAP and FOCUS studies analysed the outcomes in terms of adherence of different combinations of drugs at fixed doses versus usual treatments or the components separately. Adherence rates were higher in the intervention group in all the above studies10–13 (Table 1) and one even showed better results in other parameters such as blood pressure (BP) or LDL cholesterol (LDL-C).10 A recent Cochrane review evaluated the available evidence from 13 clinical trials with a total of 9059 participants, confirming the benefits of the use of fixed combination therapies in preventing the progression of CVD.14 Based on these results, the recent AMI-STEMI guidelines recommend the use of the polypill or combination treatments to improve adherence in patients in secondary prevention15 in line with the European guidelines for cardiovascular prevention.16 A consensus document was published on the clinical use of the CV polypill in secondary CV prevention which focused on the dose of atorvastatin 20mg, with the main benefits cited being related to the improvement in adherence.17,18 At that time, the 40mg dose was not marketed in Spain, but, once it became available, its possible implications in actual clinical practice had to be assessed.
Clinical trials on adherence with polypill.a
| Trial | N | Patients | Origin | Polypill | Comparator | Follow-up (months) | Adherence | RR (95% CI) | p value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention (%) | Comparator (%) | |||||||||
| Kanyini GAP12 | 623 | CVD or high CVR | Australia | ASA 75mg, simvastatin 40mg, lisinopril 10mg and atenolol 50mg or hydrochlorothiazide 12.5mg | Standard treatment | 18 | 70.1b | 46.9b | 1.49 (1.30–1.72) | <0.0001 |
| UMPIRE10 | 2004 | CVD or high CVR | Europe and India | ASA 75mg, simvastatin 40mg, lisinopril 10mg and atenolol 50mg or hydrochlorothiazide 12.5mg | Standard treatment | 15 | 86b | 65b | 1.33 (1.26–1.41) | <0.001 |
| IMPACT11 | 513 | CVD or high CVR | New Zealand | ASA 75mg, simvastatin 40mg, lisinopril 10mg and atenolol 50mg or hydrochlorothiazide 12.5mg | Standard treatment | 12 | 81c | 46c | 1.75 (1.52–2.03) | <0.001 |
| FOCUS13 | 695 | Previous AMI | Spain, Italy, Argentina, Brazil and Paraguay | ASA 100mg, simvastatin 40mg and ramipril 2.5mg, 5mg or 10mg | Components separately | 9 | 50.8d | 41d | Not described | 0.019 |
AMI: acute myocardial infarction; ASA: acetylsalicylic acid; CI: confidence interval; CVD: cardiovascular disease; CVR: cardiovascular risk; LDL-C: LDL cholesterol; N: number of patients; RR: relative risk; SBP: systolic blood pressure.
The aim of this study was to review the evidence on the therapeutic approach to secondary cardiovascular prevention, to establish the potential profiles of patients with whom the CV polypill with atorvastatin 40mg (P40PS) should be used in secondary CV prevention, taking into account when it is prescribed, and define the priority-use situations for the new therapeutic strategy.
Material and methodsA committee of experts was created to carry out this work, composed of seven specialists involved in the treatment of patients with cardiovascular risk (CVR) in secondary prevention (cardiology, internal medicine and clinical pharmacology). We drew up an outline of contents (subject index) covering the therapeutic approach to secondary prevention of CVD and the use of P40PS in this context. We then carried out a literature review, after which 19 recognised experienced experts in secondary CV prevention (cardiology, primary care, internal medicine and geriatrics) were interviewed and asked for their opinions on the subjects in the index. Based on the scientific evidence and the clinical experience and opinions obtained through the interviews, the expert committee created the document, which includes recommendations for the use of the P40PS and a proposed algorithm.
Results and discussionTherapeutic approach in secondary cardiovascular preventionAntithrombotic treatmentThe reference antithrombotic treatment in secondary CV prevention includes anti-platelet aggregation with ASA. The CV protective effects are obtained with low doses of ASA (75–100mg/d).19 Treatment with ASA reduces the risk of coronary or cerebral vascular events by around 20%. It does have an increased risk of gastrointestinal bleeding and haemorrhagic stroke, but significantly lower than the clinical benefit obtained. Low-dose ASA is therefore the most appropriate antithrombotic agent for a combination treatment.
Anti-hypertensive treatmentAdequate control of blood pressure is a key component of secondary CV prevention. There continues to be debate about the optimal levels for BP control in patients with a previous history of coronary heart disease or stroke. In general, it is accepted that it is reasonable to maintain the BP <140/90mmHg, although there are differences of opinion with respect to more ambitious targets in different subgroups of patients.20–22 There is consensus that the main objective is the control of BP, with no clear preference over the pharmacological group used to achieve that. However, different drugs may be preferable depending on the patient.
Blockade of the renin-angiotensin system is one of the first-line anti-hypertensive treatments. Angiotensin-converting enzyme (ACE) inhibitors are recommended in patients with left ventricular dysfunction, heart failure or diabetes.20,21 Ramipril is the ACE inhibitor best supported in the scientific literature, especially in the area of cardiovascular prevention.23,24 Angiotensin II receptor blockers (ARB) are indicated in patients with intolerance to ACE inhibitors. Among the different renin-angiotensin system families, the interviewees expressed their preference for the use of ACE inhibitors.
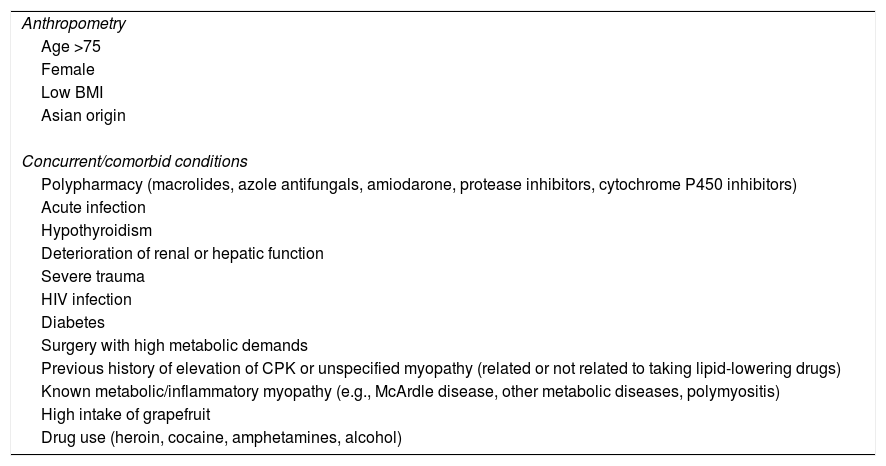
Lipid-lowering treatmentIntensive lipid-lowering therapy with statins is recommended in all cardiovascular prevention guidelines for patients who have suffered a cardiovascular event, regardless of the territory affected.16,25–28 In general, atorvastatin 40–80mg or rosuvastatin 20–40mg are considered high-intensity treatments. The initial versions of the CV polypill only provided a 20mg daily dose of atorvastatin, which made it insufficient for the majority of patients in secondary prevention. Additionally, a large number of patients have risk factors for statin toxicity (muscle toxicity), such as those shown in Table 2 (adapted from Stroes et al.).29 In these cases, using atorvastatin 40mg means we can combine the use of an intensive lipid-lowering treatment with a reduction in the risk of toxicity.
Risk factors for statin-induced muscle toxicity.
| Anthropometry |
| Age >75 |
| Female |
| Low BMI |
| Asian origin |
| Concurrent/comorbid conditions |
| Polypharmacy (macrolides, azole antifungals, amiodarone, protease inhibitors, cytochrome P450 inhibitors) |
| Acute infection |
| Hypothyroidism |
| Deterioration of renal or hepatic function |
| Severe trauma |
| HIV infection |
| Diabetes |
| Surgery with high metabolic demands |
| Previous history of elevation of CPK or unspecified myopathy (related or not related to taking lipid-lowering drugs) |
| Known metabolic/inflammatory myopathy (e.g., McArdle disease, other metabolic diseases, polymyositis) |
| High intake of grapefruit |
| Drug use (heroin, cocaine, amphetamines, alcohol) |
CPK: creatine phosphokinase; BMI: body mass index; HIV: human immunodeficiency virus.
Adapted from Stroes et al.29
In routine clinical practice, the general recommendation for the use of P40PS in secondary CV prevention is based on the patient having the indication for its three components and not having contraindication for any of the active substances. If there are any contraindications, they should be reassessed during the patient's follow-up, then the treatment can be prescribed if they disappear. Beyond this general recommendation, the experts established profiles of candidate patients and situations where P40PS use would be recommended.
Profiles of candidate patients for P40PS according to when prescribed (hospital admission and discharge or outpatient setting)One of the questions raised was in which patient profiles and at what point the indication for P40PS should be assessed after an acute ischaemic event. These points were defined as “during hospitalisation” or “at discharge” or “in an outpatient setting”, meeting the conditions for the recommended situations described below.
During hospitalisation and at hospital discharge, the experts considered that P40PS could be a treatment option in the following profiles:
- •
Patients admitted for an atherothrombotic event such as ACS, peripheral arterial disease or stroke with indication to receive the three individual components (ASA, ramipril and atorvastatin), and
- •
Patients admitted for other reasons with indication for the individual components.
In the outpatient setting, P40PS is considered a therapeutic option in patients with a need for secondary CV prevention with indication to receive the individual components.
Despite the fact that drug treatments provide great benefit to patients with CV diseases, a large percentage of patients do not adhere to the optimal medical treatment, even in the early stages after hospital discharge. There is no doubt that any early intervention with the patient in the initial phases of hospitalisation post-ACS, or even at discharge, will have a greater impact in terms of them incorporating the treatment into their daily life. P40PS is therefore a good therapeutic option to increase the likelihood of adherence and compliance with drug treatment, as long as the patient meets the recommended objectives in secondary prevention.
After hospital discharge following a CV event, some patients have not been prescribed either P40PS or its components separately. Most experts suggest that they would titrate the components individually before prescribing P40PS. However, the CV polypill includes six different dose combinations of ramipril (three) and atorvastatin (two), which facilitates the titration using a single tablet. Moreover, P40PS offers an advantage over previous formulations, as the 40mg dose of atorvastatin extends the spectrum of patients in whom dyslipidaemia can be more adequately controlled.
In order to correctly identify the profiles of patients who are candidates for P40PS, we need to have resources or support tools, such as a P40PS protocol, inclusion of these patients in the clinical practice guidelines, specific information for patients, and the running of case report sessions in the healthcare centres.
Recommended situations for considering the use of P40PS according to patient profilesIn the case of patients admitted for an acute ischaemic event, P40PS may be particularly useful when they have toxicity risk factors that advise avoiding the 80mg dose of atorvastatin. The recommendation to start P40PS during hospitalisation or at discharge is prioritised, always taking into account the individual characteristics of the patient, in the following situations:
- •
Previous intolerance of atorvastatin at 80mg,
- •
Aged over 75,
- •
Underweight: BMI <20kg/m2,
- •
Stage 3 chronic renal failure: GFR <60ml/min/m2,
- •
Hypothyroidism,
- •
Drug–drug interactions: amiodarone and verapamil, and
- •
Asian origin.
The simplification of the treatment regimen at discharge can contribute to better treatment adherence10–14,30 and possibly to a reduction in cardiovascular complications.31,32 The use of P40PS can, in turn, facilitate the transition from hospital to primary care follow-up and continuity of the prescribed treatment.
In patients in secondary prevention admitted to hospital for other reasons, it would be a good idea to review the indications for P40PS and assess the possibility of prescribing it, particularly in order to simplify complex treatment regimens and so facilitate adherence.
The following situations are those outside the hospital setting in which it is more appropriate to consider prescribing P40PS:
- •
When the patient is taking the three components separately,
- •
When the patient requires polypharmacy,
- •
When there is a lack of adherence or difficulty understanding the treatment regimen, and
- •
When there is lack of control of the CVRF.
Both the European33 and American25 guidelines, and the experts interviewed, consider 40 and 80mg doses of atorvastatin to be high-intensity doses. The recommendation is to check for a high CVRF index before prescribing a statin treatment at maximum dose. The dosage of 40mg may be a reasonable compromise for patients at high risk of statin toxicity, while maintaining high-intensity treatment.29
Patients who are in secondary prevention after ACS usually have follow-up and monitoring post-hospital discharge with the cardiology specialist, followed by cardiac rehabilitation and then primary care. To a lesser extent, they may be followed up by general or vascular risk clinics in internal medicine and geriatrics. The indication for the use of P40PS may therefore initially come from a cardiology assessment. However, any doctor carrying out follow-up of patients with a vascular risk may consider the indication. This is especially important when the patient's comorbid conditions, usually treated by non-cardiologists, mean that simplification or readjustment of the patient's overall treatment regimen is advisable.
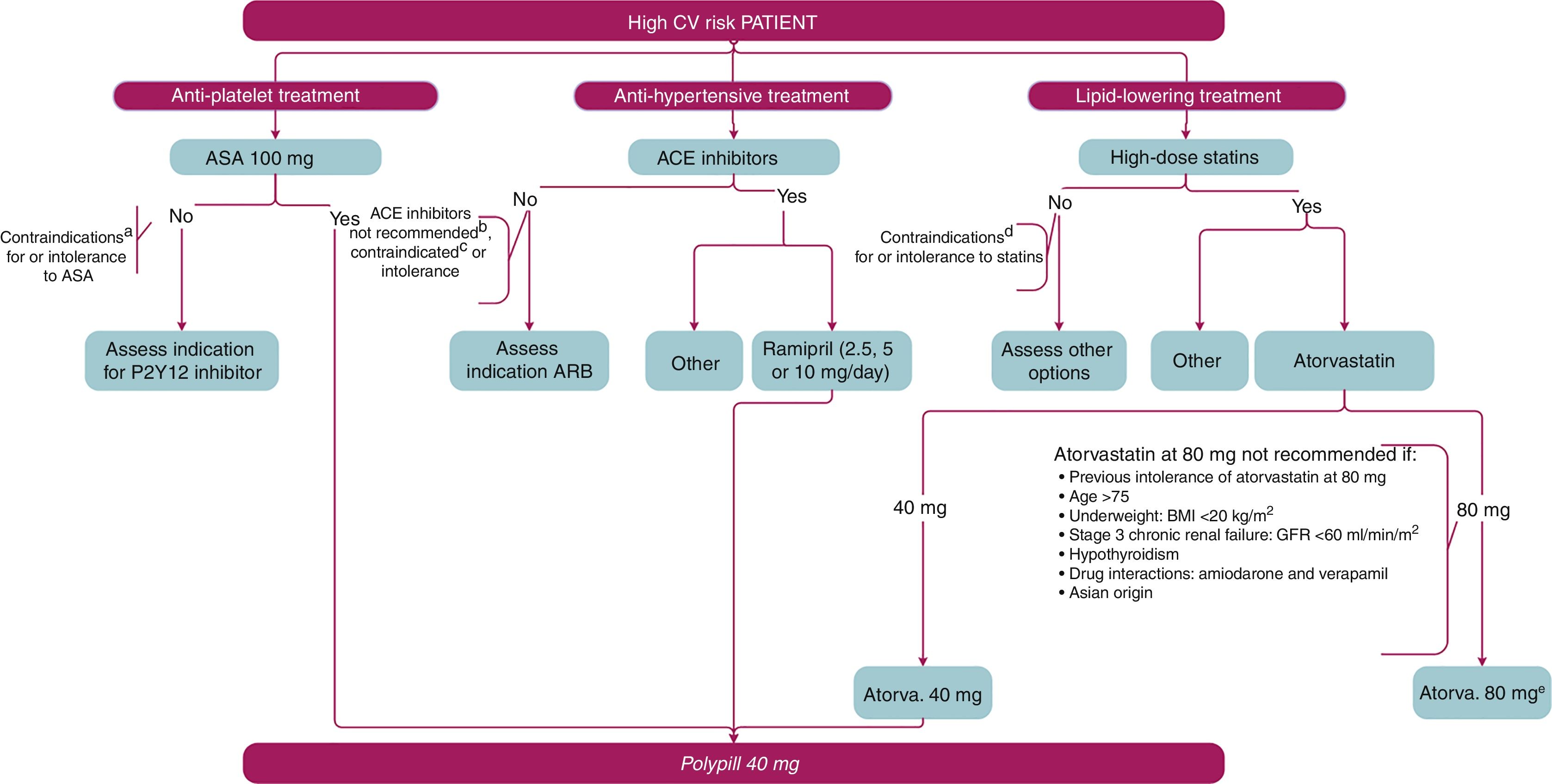
Algorithm for prescribing P40PSBased on the evidence on use of the different components of P40PS and the proposal for patient profiles and clinical situations where its use would be recommended, the group of experts created a prescribing algorithm, shown in Fig. 1. With a patient who has a high CVRF index, the advisability of prescribing an antiplatelet agent, an antihypertensive and a lipid-lowering treatment should be assessed. The first therapeutic option for antiplatelet treatment is ASA 100mg. In the event that administering ASA is not possible due to contraindication or intolerance, other alternatives will be recommended. Among the anti-hypertensive drugs available, the first choice would be ACE inhibitors. If these are not recommended, are contraindicated or there are problems with intolerance, ARB should be considered. In patients who can be prescribed an ACE inhibitor, the active substance considered to be the most suitable, including ramipril, should be selected. For lipid control, it is advisable to use statins at high doses, unless they are contraindicated or there are problems with intolerance. Of the available active substances, the use of atorvastatin 40 or 80mg should be assessed. Therefore, patients who are going to be treated or are already being treated with ASA 100mg, ramipril (2.5mg, 5mg or 10mg) and atorvastatin 40mg will be suitable candidates for P40PS. The algorithm includes the circumstances that contraindicate the use of ASA, ACE inhibitors or statins and would therefore mean the patients are not candidates for the polypill. Also included are the conditions in which the use of atorvastatin 80mg is unadvisable, thereby giving pride of place to the use of the P40PS.
Algorithm for prescribing P40PS. This algorithm shows the assessment process for a patient with high cardiovascular risk, and the therapeutic alternatives which include the different individual components of the CV polypill in secondary prevention and which lead to it being prescribed.
ACE inhibitors: angiotensin-converting enzyme inhibitors; ARB: angiotensin II receptor blockers; ASA: acetylsalicylic acid; BMI: body mass index; CV: cardiovascular; GFR: glomerular filtration rate. a ASA contraindications: hypersensitivity, high bleeding risk, active gastroduodenal ulcer. b They do not follow the recommendations for the use of ACE inhibitors in secondary prevention included in the text. c ACE inhibitor contraindications: hypersensitivity, bilateral stenosis of renal arteries; history of angioedema; second/third trimester of pregnancy or lactation; precautions for use: dual RAAS blockade is not recommended by the combined use of ACE inhibitors, angiotensin II receptor blockers or aliskiren. d Statin contraindications: hypersensitivity, active liver disease or persistent and unexplained elevations of serum transaminases (>3 times upper limit of normal); myopathy; pregnancy and lactation. e The 80mg dose of atorvastatin is not recommended: previous intolerance to atorvastatin 80mg, age >75, low body weight: BMI <20kg/m2, stage 3 chronic renal failure: GFR <60ml/min/m2, hypothyroidism, drug–drug interactions: sacubitril/valsartan, amiodarone and verapamil; Asian origin.
The aim of this document was to assess the most appropriate circumstances for considering the use of the CV polypill, establishing the profiles of patients who might obtain greater potential benefit in different clinical contexts. To achieve this, we reviewed the scientific evidence and the opinion of experts in CVD in our environment. The attached algorithm summarises these concepts in a simplified manner to facilitate decision making for medical professionals who care for patients with CVD in secondary prevention.
Conflicts of interestThe authors declare that they received personal fees from Ferrer during the study.
The authors wish to thank the experts who were interviewed: José Antonio Alarcón (San Sebastián), Alfredo Bardají (Tarragona), Elena Bello (Madrid), Carlos Brotons (Barcelona), Alberto Cordero (Alicante), Regina Dalmau (Madrid), Rosa Fernández (Jaén), Iluminada García (Madrid), Juan José Gómez (Málaga), Pilar Mazón (Santiago Compostela, La Coruña), Nuria Muñoz-Rivas (Madrid), Juan Carlos Obaya (Alcobendas, Madrid), Domingo Orozco-Beltrana (Alicante), Pablo Pérez-Martínez (Córdoba), José Polo (Casar de Cáceres, Cáceres), Miguel Ángel Prieto-Díaz (Oviedo), Leocadio Rodríguez (Getafe, Madrid), Alessandro Sionis (Barcelona) and Fernando Worner (Lérida). We would also like to express our gratitude for the technical support provided by the GOC Networking consultants: Antoni Torres, Liliana Ramalho and Sonia Pisa, for their methodological support in the development of the project, and Veronica Albert, for her support with the writing of the manuscript. Lastly, we thank Laboratorios Ferrer for their support, allowing us to develop our work independently, with no interference in the scientific discussions.
Please cite this article as: Marzal D, Rodríguez Padial L, Arnáiz J-A, Castro A, Cosín J, Lekuona I, et al. Uso de la polipíldora cardiovascular 40mg en prevención cardiovascular secundaria. Clín Investig Arterioscler. 2018;30:240–247.