To assess the impact of the coronavirus SARS-CoV-2 pandemic on the level of anxiety in low-risk pregnant women.
Material and methodEpidemiological, descriptive, prevalence study. A total of 74 patients who underwent low risk antenatal controls during the state of alarm because of COVID-19, were included. They filled in the Hamilton Anxiety Rating Scale and a specific document about the pandemic. Clinical histories and different variables of clinical interest were reviewed and compiled, respectively.
ResultsMean age was 34.05 years with average amenorrhoea of 28.17 weeks. A total of 77% of the sample presented symptoms and signs compatible with anxiety. Of these, 44.6% and 32.4% presented minor and major anxiety, respectively. Concern over the time of the birth and postpartum and fear of being at greater risk because of possible infection was present in 95.9% and 94.6% of the sample, respectively. A total of 93.2% of the sample was afraid of intrauterine virus transmission; 94.5% admitted fear over the neonatal consequences of infection.
ConclusionsThe pregnant women assessed had three times more anxiety during the COVID-19 pandemic. This incidence is independent of most study variables.
Evaluar el impacto de la pandemia del coronavirus SARS-CoV-2 en el nivel de ansiedad en mujeres embarazadas de bajo riesgo.
Material y métodoEstudio epidemiológico, descriptivo, de prevalencia. Se incluyeron un total de 74 pacientes que se sometieron a controles prenatales de bajo riesgo durante el estado de alarma por COVID-19. Completaron la escala de calificación de ansiedad de Hamilton y un documento específico sobre la pandemia. Se revisaron y recopilaron historias clínicas y diferentes variables de interés clínico, respectivamente.
ResultadosLa edad promedio fue de 34,05 años con amenorrea promedio de 28,17 semanas. El 77% de la muestra presentó síntomas y signos compatibles con la ansiedad. De estos, el 44,6 y el 32,4% presentaron ansiedad menor y mayor, respectivamente. La preocupación por el momento del parto y el puerperio y el temor de presentar mayor riesgo por una posible infección estuvieron presentes en el 95,9 y 94,6% de la muestra, respectivamente. El 93,2% de la muestra temía una posible transmisión del virus intrauterino; el 94,5% admitió tener miedo a las consecuencias neonatales tras una posible infección.
ConclusionesLas embarazadas evaluadas tenían tres veces más ansiedad durante la pandemia de COVID-19. Esta incidencia es independiente de la mayoría de las variables de estudio.
The current pandemic caused by coronavirus disease (COVID-19) – severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – has spread quickly across the globe and has led to a public health emergency. From December 31, 2019 to August, 2021, a total of 225,166,539 COVID-19 cases, including 4,636,120 deaths – according to data provided by the European Center for Disease Prevention and Control (ECDC),1 have been reported.
Pregnant women undergo immunologic and physiologic changes that could make them more susceptible to viral respiratory infections, including COVID-19. Therefore, they are a high-risk population during outbreaks of infectious disease. The impact of COVID-19 infection during pregnancy has been assessed and – although data are limited and confined to case series mainly during the third trimester – infection during pregnancy probably has a clinical presentation and severity that is similar to non-pregnant women. Moreover, some authors do not detect an association with poor maternal or perinatal results,2,3 although there is little current evidence, and this is under constant review. To date, there are only a few recorded cases of intrauterine infection caused by vertical transmission in women developing pneumonia because of COVID-19.4,5
Despite available evidence revealing reassuring maternal and fetal outcomes, the current state of the pandemic could lead to the onset of pathologic emotional states and a consequent reduction in the quality of life of pregnant women. The prevalence of maternal anxiety during a non-COVID-19 pregnancy is estimated to be 15%–23% of women according to the series consulted.6–9 Antenatal stress and anxiety have been related to the onset of both short and long term adverse maternal, fetal and neonatal events.10,11 Anxiety during pregnancy has, therefore, been strongly correlated to anxiety and depression during the postnatal period.12,13 Against this backdrop, low birth weight,14,15 premature birth16 and abnormal cognitive and behavioral neurologic development17,18 are among the negative outcomes reported at birth and during childhood.
An increase in premature birth rates, low birth weight and higher child mortality within the scope of other traumatic events, such as the terrorist attacks in New York and Madrid, has also been recorded. The negative effect on reproductive outcomes reported is related to probable posttraumatic stress undergone by the pregnant women exposed to these events.19–21
Therefore, having set out the significant consequences of antenatal anxiety on maternal and child health, the aim of our study will be to determine the impact of the current COVID-19 pandemic on maternal anxiety of the low risk pregnant woman who has not been diagnosed with COVID-19.
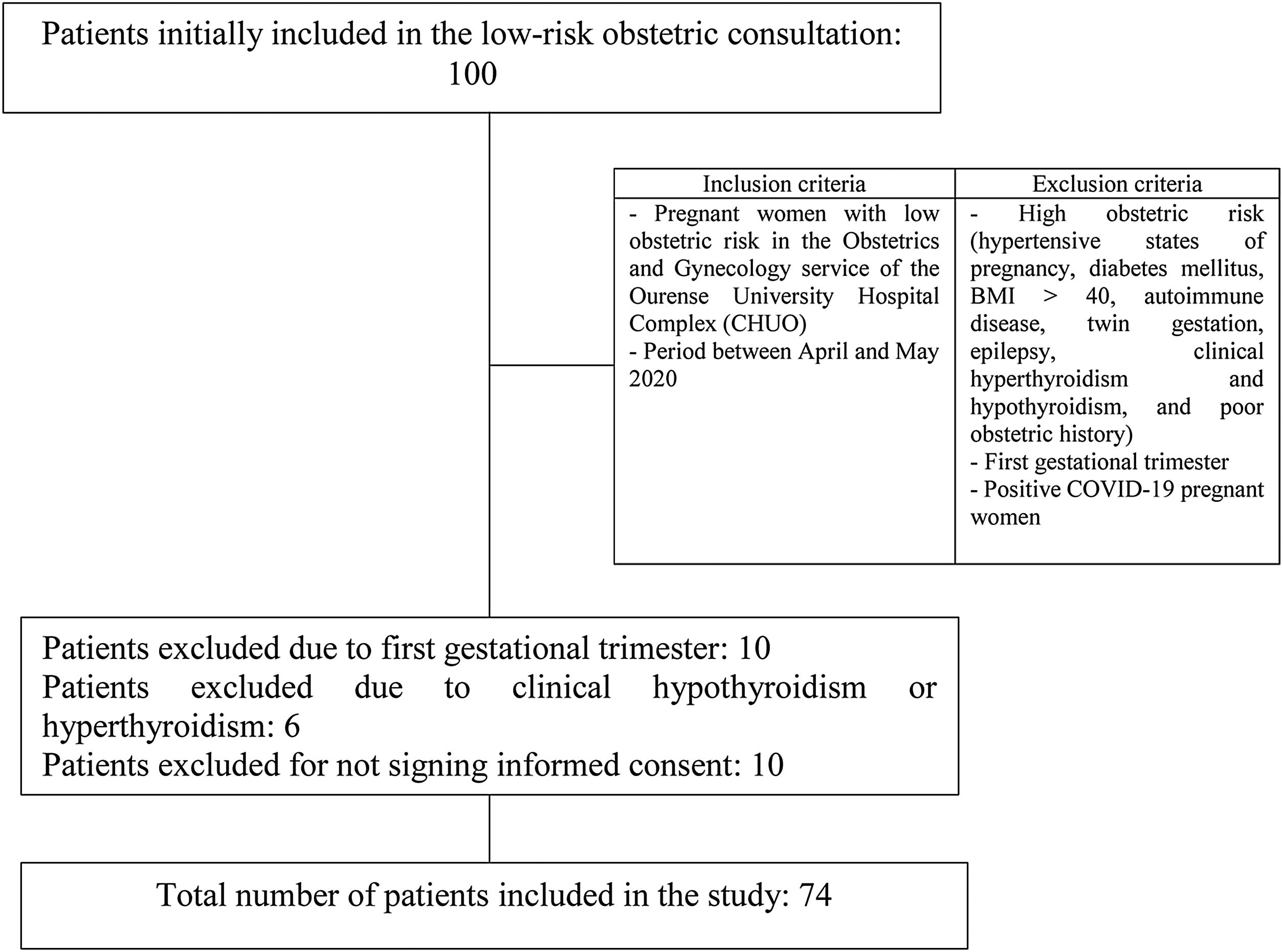
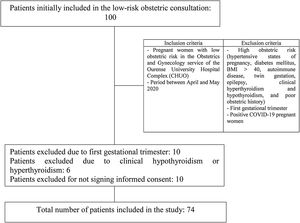
Materials and methodsAn epidemiological, descriptive, prevalence study was performed. A total of 74 patients was included. The inclusion criteria were those pregnant women who underwent low obstetric risk, antenatal, gynecologic controls in the department of Obstetrics and Gynecology of Ourense Teaching Hospital Complex during the healthcare state of alarm because of coronavirus SARS-CoV-2 between April and May 2020. Pregnant women at high obstetric risk (hypertensive states of pregnancy, diabetes mellitus, BMI>40, autoimmune disease, twin gestation, epilepsy, clinical hypothyroidism and hyperthyroidism, and poor obstetric history), pregnant women in the first trimester, and those diagnosed with COVID-19 were excluded. Therefore, the sampling method was non-probabilistic for convenience. The selection process is shown in Fig. 1.
For study purposes patients filled in two short questionnaires: first, the Hamilton Anxiety Rating Scale (HARS), a document comprising 14 items and the gold standard for the study of anxiety, validated in Spanish22–24 and, second, a questionnaire comprised of 9 specific questions on the current state of the COVID-19 pandemic, with the aim of relating the scores obtained on the previous form with the current pandemic. To assess whether or not anxiety is present the proposal made by Bech was used; by way of guidance, this sets out the following ranges: 0–5 points (no anxiety), 6–14 (less anxiety), 15 or more (more anxiety).25 With the aim of obtaining the study parameters the electronic clinical histories of patients included were also reviewed. These variables are shown in Table 1.
Study variables.
| Pregnant woman variables | |
| Age | BMI |
| Number of prior pregnancies | Preconception |
| Number of prior deliveries | Pregestational hypothyroidism |
| Number of prior miscarriages | |
| Gestational variables | |
| Gain in gestational weight | Fetal weight percentile on ultrasound 3rd trimester |
| Result of chromosomal disorder screening | Trimester of pregnancy |
The pregnant women included in the study gave their written informed consent. The study was approved by the institutional ethics committee.
A descriptive analysis where qualitative variables were expressed as frequency and percentage, was initially performed. Continuous variables were expressed as mean±standard deviation, median and minimum–maximum.
Non-parametric tests were performed to determine the potential association between the study variables (Chi-squared, Kruskal–Wallis, Mann–Whitney U tests). Correlations were studied to detect a relationship or interaction between the different variables.
Differences with P<0.05 were considered statistically significant for all analyses. Analyses were performed using the software IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY, USA: IBM Corp.
ResultsDescriptive analysis of the sampleA total of 74 pregnant women were included in the study. Mean age at the time of the intervention was 34.05 years (minimum age: 19; maximum age: 46). Mean gestational age was 28.17 weeks (minimum gestational age:15.28; maximum gestational age: 41.42). Most subjects did not present previous deliveries, miscarriages or c-sections (63.5%, 68.9% and 91.9%, respectively). A total of 52.7% of patients were in the third trimester of pregnancy and 47.3% remained in the second trimester. Subjects in the first trimester of pregnancy were not included in the study. Prior history of anxiety was only presented by 4.1% of the sample. Furthermore, only 13.5% of pregnant women were diagnosed with a thyroid disorder in form the subclinical hypothyroidism. A total of 23%, 66.2% and 10.8% had excessive weight gain, normal weight or remained under weight, respectively. Most pregnant women (83.8%) had a low risk result of chromosomal disorder screening compared to 6.8% and 9.5% classified as intermediate and high-risk, respectively. The general characteristics are shown in Table 2.
General characteristics.
| Study variables | Mean | Standard deviation | Minimum | Maximum |
|---|---|---|---|---|
| Age | 34.05 | 5.76 | 19 | 40 |
| Gestational age | 28.17 | 7.55 | 15.28 | 41.42 |
| Frequency | Valid percentage (%) | |||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| Deliveries | 27 | 47 | 36.5 | 63.5 |
| Miscarriages | 23 | 51 | 31.1 | 68.9 |
| C-sections | 6 | 68 | 8.1 | 91.9 |
| Prior anxiety | 3 | 71 | 4.1 | 95.9 |
| Thyroid abnormality | 10 | 64 | 13.5 | 86.5 |
| Weight gain (kg) | Frequency | Valid percentage (%) |
|---|---|---|
| No gain | 8 | 10.8 |
| Normal | 49 | 66.2 |
| Excessive | 17 | 23.0 |
| Chromosomal disorder screening | Frequency | Valid percentage (%) |
|---|---|---|
| Low risk | 62 | 83.7 |
| Intermediate risk | 5 | 6.8 |
| High-risk | 7 | 9.5 |
| HARS | Frequency | Valid percentage (%) |
|---|---|---|
| No anxiety | 17 | 23.0 |
| Less anxiety | 33 | 44.6 |
| More anxiety | 24 | 32.4 |
| Total | 74 | 100.0 |
According to results obtained approximately 8 in 10 patients (77%) reported presenting symptoms and signs compatible with anxiety. Of these, 44.6% and 32.4% presented minor and major anxiety, respectively.
Most pregnant women (95.9%) said they were concerned about the time of the birth and postpartum after starting the confinement because of COVID-19. Moreover, a total of 94.6% confessed worry in regard to the possibility of being more at risk because of pregnancy in the event of a possible infection. A total of 93.2% of the sample revealed unease in the event of possible intrauterine transmission of the virus to the baby. And 94.5% confessed being scared about the consequences the baby could present at birth after a possible maternal infection. Moreover, 9 out of every 10 pregnant women (91.4%) were worried about contagiousness during the birth or subsequent stay on the obstetrics ward. To a lesser extent, half of the patients (50.7%) were concerned about the possibility of less healthcare personnel being present at the time of the birth. Conversely, most of the sample (62.9%) had no doubts over starting to breastfeed. Only 37.1% were wary of starting to breastfeed during the current pandemic. These results are shown in Table 3.
COVID-19 questionnaire.
| Questions | Frequency | Valid percentage | |||
|---|---|---|---|---|---|
| Yes | No | NA | Yes | No | |
| Given that you are pregnant are you worried about being more at risk in the event of a possible Covid-19 infection? | 70 | 4 | 0 | 94.6 | 5.4 |
| Are you worried about intrauterine Covid-19 transmission to your baby? | 68 | 5 | 1 | 93.2 | 6.8 |
| Have you thought about the consequences your baby could present at birth? | 69 | 4 | 1 | 94.5 | 5.5 |
| Are you more worried about the time of the birth and postpartum after starting the healthcare state of alarm because of Covid-19? | 70 | 3 | 1 | 95.9 | 4.1 |
| Are you scared about your child's Covid-19 contagion during the birth or subsequent stay on the obstetric ward? | 64 | 6 | 4 | 91.4 | 8.6 |
| Are you scared about less healthcare personnel being available at the time of the birth, in case of need? | 35 | 34 | 5 | 50.7 | 49.3 |
| Do you have doubts over the suitability of starting to breastfeed given the Covid-19 pandemic? | 26 | 44 | 4 | 37.1 | 62.9 |
| Do you repeatedly think about the previous questions? | 39 | 34 | 1 | 53.4 | 46.6 |
| Do you believe these thoughts affect your quality of life? | 31 | 41 | 2 | 43.1 | 56.9 |
NA: no answer.
No statistically significant differences were observed between most of the different study variables and their relationship with maternal anxiety indices. However, a statistically significant association was observed between those patients who had not given birth previously and more concern over the time of the birth and postpartum after the healthcare state of alarm began (Exact Fisher test: P<0.05). It was also observed that those patients with diagnostic anxiety scores thought repeatedly about the above issues, which affected their quality of life (Chi-squared test=7.960, P<0.05).
DiscussionThe data obtained in the present study seem to demonstrate an increase in maternal anxiety in the context of the SARS-COV-2 pandemic when the results obtained are compared with those previously published by other authors. Recent publications conclude similar results and also report increased levels of antenatal anxiety.26–30
The prevalence of antenatal anxiety in our sample tripled that reported in scientific literature (15%–21%6–9). This figure is notable among those disseminated by different works. The study by Mappa et al.26 reported half the number of women who attained abnormal anxiety levels; specifically 38.2% of the sample as opposed to 77% obtained in our study. Along the same lines, the work by Wu et al.27 reported 29.6% of pregnant women with symptoms and anxiety and depression. Such discrepancies might be accounted for by the use of different instruments to measure maternal anxiety or be due to the confinement in Spain – among the strictest in the world – which might increase the perception of level of severity perceived by the population.
This increase can be observed in low obstetric risk pregnant women regardless of specific features studied (age, BMI, existence of preconception anxiety or thyroid pathology). Nor have significant differences been observed in regard to factors such as the number of prior gestations, births and miscarriages, trimester of pregnancy, increase in gestational weight or result of chromosomal disorder screening. Similar results were obtained by Taubman et al.28 when they reported an increase in gestational anxiety regardless of the various sociodemographic features presented. Mappa et al.26 did not reveal differences either according to the variables studied except for the high educational level associated with higher percentages of perinatal anxiety. However, the work by Wu el al.27 reported a higher risk of presenting symptoms of anxiety and depression in those patients giving birth for the first time with a low gestational BMI, aged under 35, employed full time and with an average income.
The most important concerns reported by pregnant women in our study were in more than 9 out of 10 cases: fear of being at risk patients in the event of a possible infection, fear of intrauterine viral transmission, neonatal consequences in the event of a possible maternal infection and infection during the birth or subsequent stay on the obstetric ward. To a lesser extent, up to half the women admitted they were scared about the possibility of less healthcare personnel available at the time of the birth. Other studies have reported similar maternal concerns although it is notable that these were present in lower percentages. Therefore, Taubman et al.28 reported higher anxiety levels in regard to maternal exposure in public places and transport (87.5% and 70%, respectively); to a lower extent fear of infection of other family members or fetal health, followed by attending gynecologic consultations and maternal infection at the time of the birth. Lower figures were revealed by Mappa et al.26 given that 65% of the sample were afraid of a possible restriction in fetal growth; and to a lesser degree, premature birth and the possibility of fetal structural abnormalities.
The most important limitations of our study are the small sample size and subjects belonging to just one center which does not enable generalization of the data. Furthermore, as this is a prevalence study it is not possible to set out a causal relationship with the onset of possible future maternal and fetal consequences. However, to the best of our knowledge this work is the first to be performed in Spain and to date one of the few to be published in other settings.
To conclude, our work reveals a marked increase in maternal anxiety after onset of the COVID-19 pandemic. This increase was observed regardless of the majority of variables studied; both sociodemographic factors and specific features of the pregnancy.
Ethics approvalThis study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Galician drug research ethics committee (CEIm-G) (28-05-2020).
Authors’ contributionsAll authors contributed to the conception and design of the study and performed material preparation, data collection, and analysis.
Ethical responsibilitiesProtection of people and animalsThe authors declare that the procedures followed were in accordance with the ethical standards of the responsible human experimentation committee and in accordance with the World Medical Association and the Declaration of Helsinki.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects referred to in the article. This document is in the possession of the corresponding author.
FundingNo grants or other types of support were used to draw up this article.
Conflicts of interestThe authors declare no competing interests.