Se sabe que los hechos desagradables irrelevantes influyen negativamente en nuestra capacidad para mantener en la memoria de trabajo información no emocional aunque importante para la tarea. Paralelamente, la ansiedad sesga nuestras respuestas atencionales a aquellos estímulos potencialmente amenazadores para, de este modo, mejorar adaptativamente su detección y valoración. En este trabajo hemos investigado las diferencias entre voluntarios sanos y ansiosos mientras realizaban una tarea de memoria de trabajo en la que se presentaban como distractores imágenes neutras y desagradables. Los resultados muestran que la ansiedad estado puede aumentar el efecto de interferencia de los distractores neutros pero no de los desagradables. Se comentan dichos resultados en relación a estudios anteriores que concluyen que la ansiedad y el estrés agudo pueden disminuir el nivel de especificidad en el mecanismo de vigilancia que sirve para optimizar la detección y evaluación de las amenazas.
Unpleasant irrelevant events are known to negatively affect our capacity to maintain neutral but task-relevant information in working memory (WM). In parallel, anxiety biases our attentional responses to those stimuli that may be potentially threatening in order to adaptively enhance their detection and assessment. In this study, we investigated differences between healthy anxious and non-anxious volunteers while they performed a WM task in which neutral and unpleasant pictures were presented as distractors. Our results revealed that state anxiety could increase the interfering effect of neutral but not unpleasant distractors. These findings are discussed in regard to previous studies suggesting that anxiety and acute stress can decrease the level of specificity in the vigilance mechanism that serves to optimize the detection and evaluation of threats.
It is widely accepted that cognition can be influenced by emotion, probably due to the biologically relevant information (e.g., food or predators) contained in emotional stimuli (Anderson & Phelps, 2001; LeDoux, 1996; Ohman, Flykt, & Ludqvist, 2000). It has been proposed that such stimuli have the capacity to recruit cognitive resources (Lang, Bradley, & Cuthbert, 1998; Lang, Greenwald, Bradley, & Hamm, 1993), in particular attentional resources, so that we are able to quickly detect them and efficiently respond when facing information that is directly linked to our survival (Bradley et al., 2003; Lang, Bradley, Fitzsimmons, et al., 1998; Morris, Ohman, & Dolan, 1998; Sabatinelli, Bradley, Fitzsimmons, & Lang, 2005). In the memory domain, it has been demonstrated that emotional materials are better remembered than non-emotional ones (Canli, Zhao, Brewer, Gabrieli, & Cahill, 2000). However, a number of studies in the last years have showed that the preferential access of emotional stimuli to our cognitive system can impair our cognitive performance when they are potentially distracting. Thus, unpleasant events have been reported as more distracting than neutral events when participants are maintaining non-emotional information in working memory (Anticevic, Repovs, & Barch, 2010; Chuah et al., 2010; Denkova et al., 2010; Dolcos, Diaz-Granados, Wang, & McCarthy, 2008; Dolcos & McCarthy, 2006). This phenomenon has been interpreted as the consequence of the competition between linked-to-survival distractors and task-relevant information for cognitive resources in the context of interference-based forgetting theories (Berman, Jonides, & Lewis, 2009). In that case, the deep processing of emotional stimuli would consume a significant part of the available attentional capacity, leaving insufficient resources for the actual ongoing task (Ellis & Ashbrook, 1988).
Although the attention bias to emotional stimuli is considered adaptive in healthy people, a number of pathological and non-pathological mood states can affect these dynamics, modifying the expected cognitive performance. In particular, anxiety and acute stress are known to induce a state of hypervigilance in which the detection and evaluation of potential threats is boosted (de Kloet, Joëls, & Holsboer, 2005; van Marle, Hermans, Qin, & Fernández, 2009). This state of hypervigilance is considered adaptive as it increases our chances of successfully dealing with dangers in those situations in which our survival is compromised. However, this attentional bias in favor of potentially threatening stimuli is accompanied by impairments in selective attention (Henderson, Snyder, Gupta, & Banich, 2012; Tanji & Hoshi, 2008) and increased vulnerability to distraction (Aston-Jones & Cohen, 2005; Braunstein-Bercovitz, Dimentman-Ashkenazi, & Lubow, 2001; Skosnik, Chatterton, Swisher, & Park, 2000).
Previous studies have shown that stress can impair WM performance (Arnsten, 2009; Luethi, Meier, & Sandi, 2008; Lupien, Gillin, & Hauger, 1999; Oei, Everaerd, Elzinga, van Well, & Bermond, 2006; Ramos & Arnsten, 2007; Schoofs, Preuss, & Wolf, 2008) and this has been related to the release of glucocorticoids (GCs) and their negative effect on WM (Elzinga & Roelofs, 2005; Lupien et al., 1999; Oei et al., 2006; Wolf et al., 2001).
In the present study we aimed to investigate whether state anxiety in healthy young volunteers can affect the cognitive control of emotional and neutral distraction when maintaining non-emotional information in WM. If anxiety and acute stress are able to induce a state of hypervigilance, both neutral and unpleasant distractors should recruit more attentional resources in anxious participants than in non-anxious volunteers. Also, unpleasant distractors should be more interfering than neutral pictures in the anxious participants, since anxiety and acute stress produce an attentional bias towards the detection and evaluation of potential threats.
Method
Participants
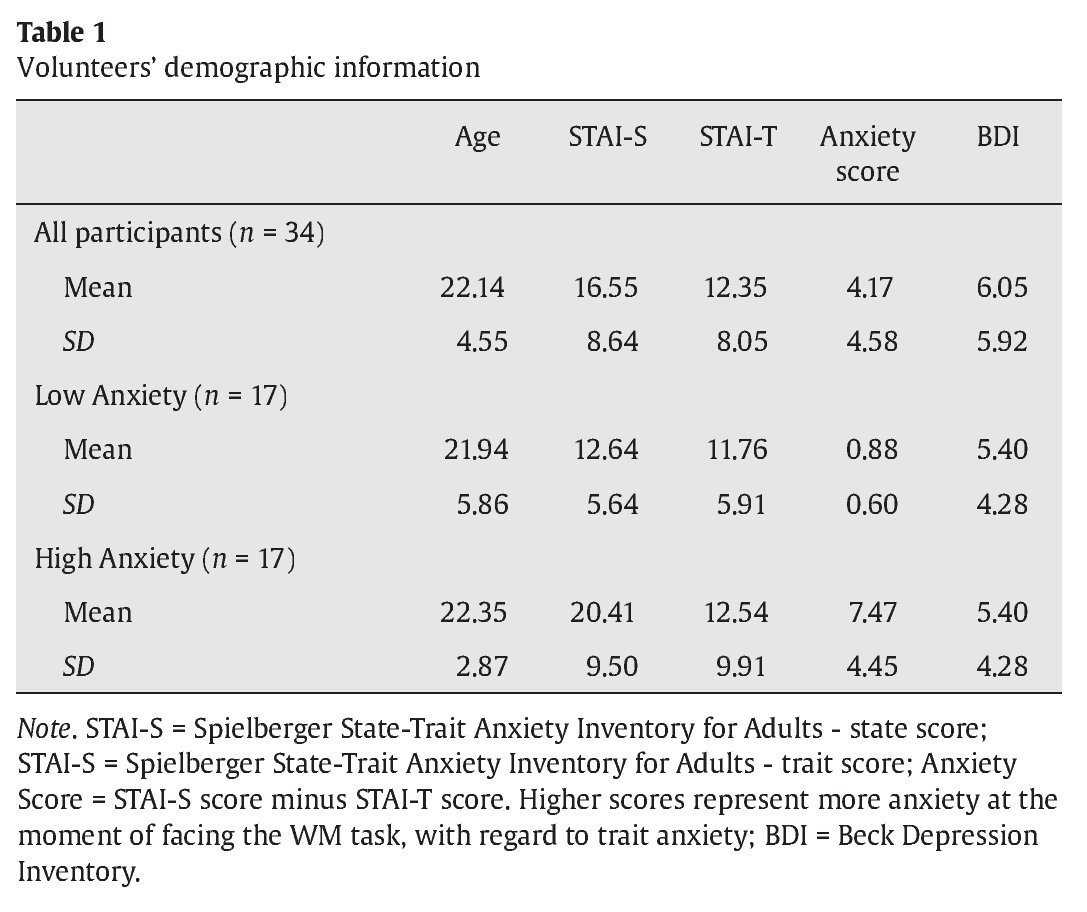
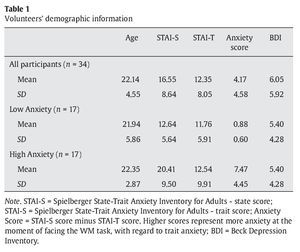
Thirty-four students from the Complutense University of Madrid and the Camilo José Cela University of Madrid (mean age 22.14 years and a range between 18 and 35 years) took part in the study. They had normal or corrected-to-normal vision. Eighteen participants were males (18-39 years old and a mean age of 22.16 years) and sixteen were females (18-33 years old and a mean age of 22.12 years). Before they performed the experimental task, they all completed the Spanish version of the Spielberger State-Trait Anxiety Inventory for Adults (Spielberger, Gorsuch, & Lushene, 2002) and the Beck Depression Inventory (Beck, Steer, & Brown, 2006) (see Table 1 for demographic information). Participants received course credits for their time.
Materials
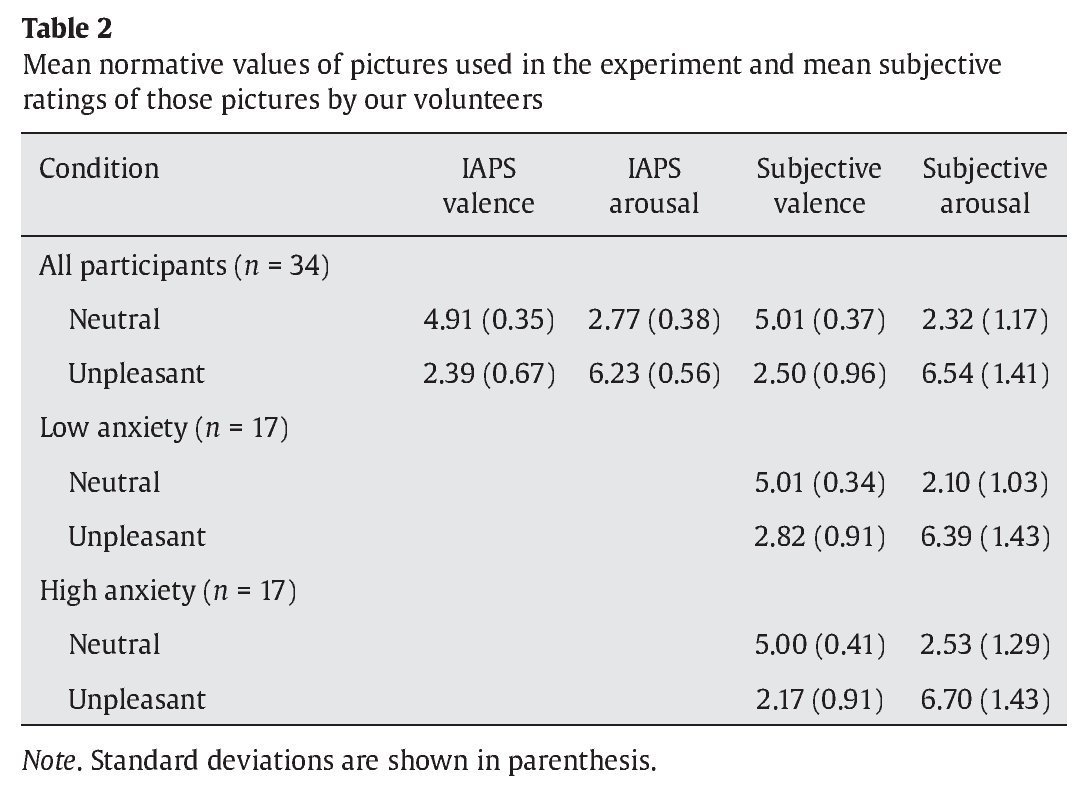
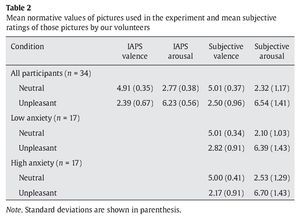
Items at encoding and recognition stages consisted of colored images of neutral faces. An oval mask was applied along the contours of the faces to remove ears and hair and avoid any potential non-face specific cues. A pair of faces was presented at the encoding stage while just one face was displayed at the recognition stage. Faces were counterbalanced across experimental conditions. For the interfering items presented at the maintenance period, 30 neutral and 30 unpleasant pictures were selected from the International Affective Picture System (IAPS) (Lang, Bradley, & Cuthbert, 2005) and matched in luminance, contrast, color, and figure-ground relationships (see Table 2 for mean normative values).
Procedure
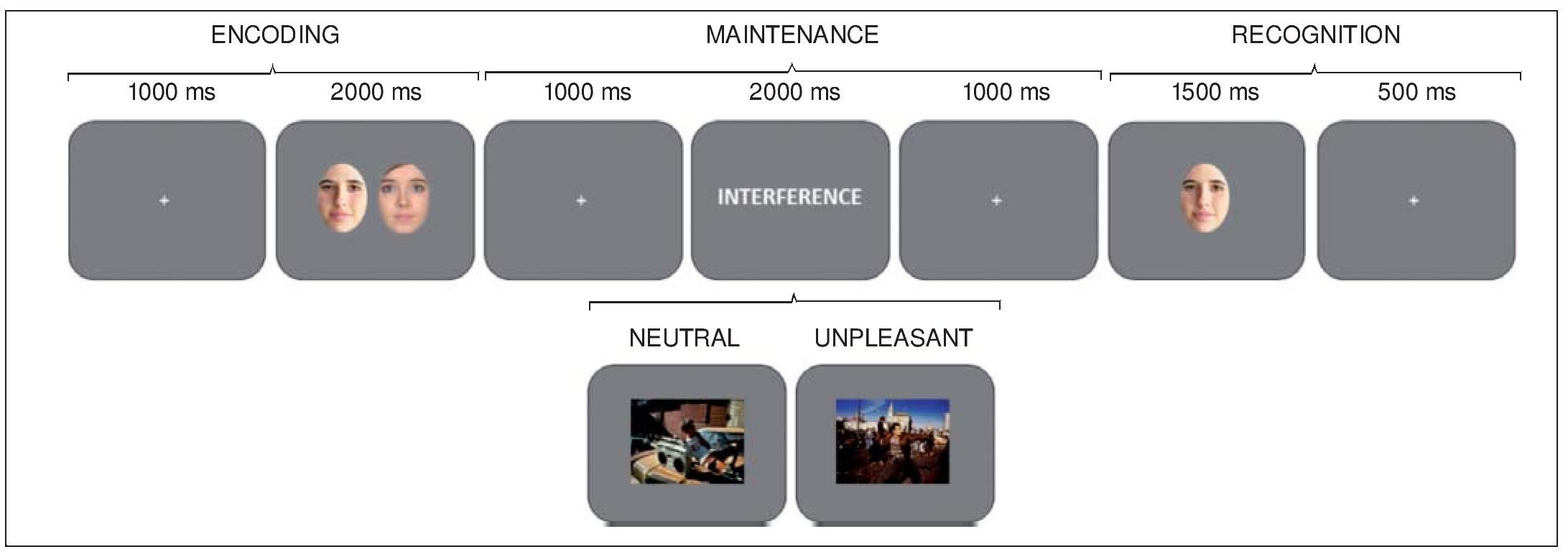
A delayed-recognition WM paradigm with two experimental conditions, neutral and unpleasant distraction, was used. Each condition comprised 30 trials. Each trial began with a 1,000-ms intertrial interval (ITI), followed by the presentation of a pair of faces for 2000 ms (encoding phase). After a 1000-ms blank screen, an interfering stimulus was displayed for 2000 ms, followed by another 1000-ms blank screen (maintenance phase). Next, just one face appeared on the screen for 1500 ms, followed by a 500-ms blank screen (recognition stage). Participants had to decide whether or not the face at the recognition stage has been one of the two previously encoded, by pressing one of two keys (Figure 1).
Figure 1. Diagram of the delayed-recognition WM paradigm Note. Neutral and unpleasant distractors were pseudorandomly presented during the maintenance stage. Volunteers were trained to learn and maintain the pair of faces into WM, look at the distracter, and then decide whether the face at the recognition stage is one of the two previously encoded or not, by pressing one of two keys.
Before the experiment, all the volunteers underwent four training trials in order to ensure that they completely understood the task. To avoid inducing long-lasting mood states, the order of trials were constrained so that no more than three trials of the same condition were consecutively presented. Once the WM paradigm was completed, all the pictures used as interference were presented to the participants and they were asked to rate them regarding emotional valence and arousal, using the Self-Assessment Manikin (SAM) self-report scale (Lang, 1980). Participants were allowed to see each picture as long as they wanted, and the order of presentation of the pictures was also constrained in the same way, but in a different sequence, than for the WM task.
Median Split Design
Once the thirty-four participants completed the experimental procedure, we used their individual scores at the STAI scale to extract a measurement of how anxious their felt when facing the WM task. To do this, we subtracted the state score from the trait score for every single participant. Thus, low scores represent low anxiety with regard to basal anxiety levels, while high scores represent high anxiety, when facing the cognitive task, in comparison to state anxiety levels. Then, we split the whole group of participants in two subgroups, based on the median of the 34 volunteers in this anxiety score. Participants whose anxiety scores were below the median were included in the low anxiety group (mean anxiety score 0.88 in a range between 0 and 2) while volunteers with anxiety scores over the median were included in the high anxiety group (mean anxiety score 7.47 in a range between 3 and 18) (see Table 1 for means and standard deviations in each group).
Results
Di fferences between Groups in Anxiety and Depression
Groups did not differ in age (U = 101.50, Z-score = -1.49, p > .1, r = -.25), STAI-S (U = 137.50, Z-score = 0.24, p = .08, r = -.04), and BDI scores (U = 130.50, Z-score = -0.48, p > .1, r = -.08). Volunteers in the high anxiety group showed higher STAI-T scores than participants in the low anxiety group (U = 57.50, Z-score = -3.01, p < .005, r = -.51). As expected, anxiety scores in the high anxiety group were higher than in the low anxiety group (U = 0.00, Z-score = -5.07, p < .005, r = -.87)
Accuracy
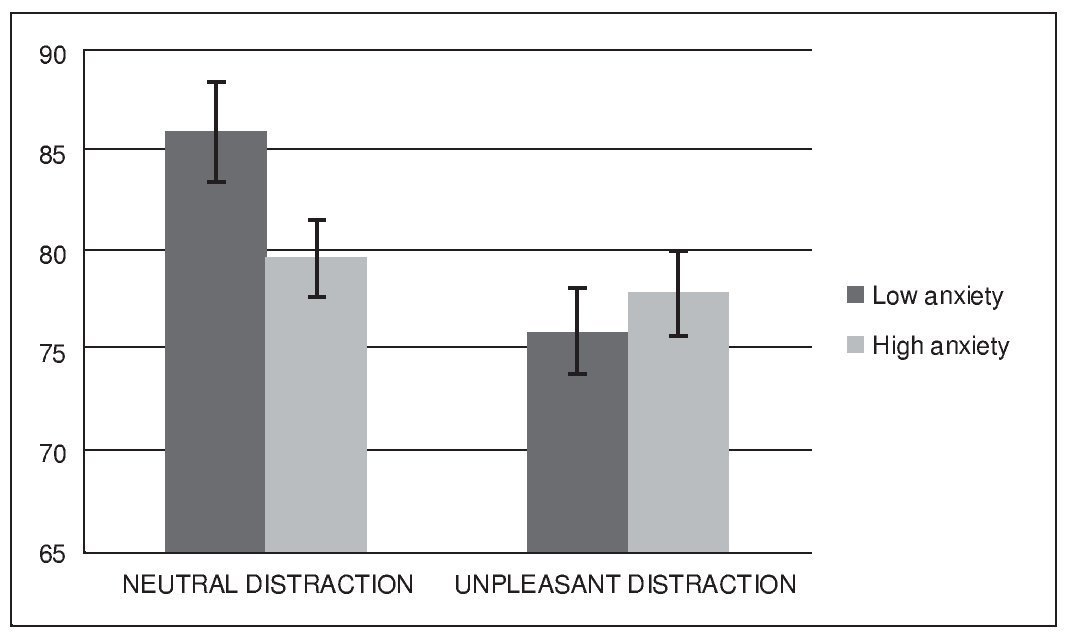
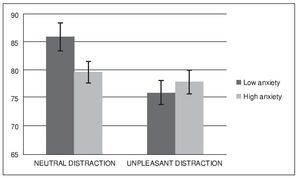
Figure 2 shows the mean accuracy (hits and correct rejections) for each condition and group. A repeated-measures analysis of variance (ANOVA) revealed a significant main effect of condition, F(1, 32) = 12.02, p < .005, eta squared = .27, and a significant effect of condition x group interaction F(1, 32) = 5.97, p < .05, eta squared = .15. Analysis did not reveal a significant main effect of group, F(1, 32) = 0.79, p > .1, eta squared = .02. Post-hoc comparisons using the Bonferroni correction revealed a lower performance during unpleasant distraction compared to neutral distraction (p < .005). They also showed that this difference in performance between condition was present in the low anxiety group (p < .05) but not in the high anxiety group (p > .05).
Figure 2. Mean accuracy (expressed as percent correct) in the WM task
Subjective Emotional Ratings
As expected, subjective valence ratings differed as a function of affective category in the whole group of participants, with unpleasant pictures rated as more unpleasant than neutral ones (Z-score = 5.06, p < .001, r = .61). Arousal ratings also varied as a function of affective category unpleasant pictures rated as more arousing than neutral pictures (Z-score = 5.08, p < .001, r = -.61). Differences in valence appeared in the same direction in the low anxiety group (Z-score = 3.62, p < .001, r = .62) and in the high anxiety group (Z-score = 3.57, p < .001, r = .61). Differences in arousal also appeared in the same direction in the low anxiety group (Z-score = 3.62, p < .001, r= .62) and in the high anxiety group (Z-score = 3.62, p < .001, r = .622). Although there were no differences between the low anxiety and the high anxiety group in valence (U =131.50, Z-score = -0.44, p > .1, r = -.07) and arousal (U = 116.50, Z-score = -0.96, p > .1, r = -.16) for neutral pictures, participants in the high anxiety group rate unpleasant distractors as more unpleasant than volunteers in the low anxiety group did (U = 74.50, Z-score = -2.41, p < .05, r = -.41). There were no differences between groups in their arousal ratings for unpleasant distractors (U = 130.50, Z-score = -.48, p > .1, r = -.08).
Discussion
The present study aimed to investigate the effect of acute anxiety on the cognitive control of emotional and neutral distraction while maintaining non-emotional information in WM in healthy young volunteers. Using a median split procedure we compared participants that showed low levels of state anxiety at the beginning of the WM task to those who experienced a higher level of anxiety in such situation. Overall, unpleasant distractors produced higher levels of distraction during the WM task, leading to a worsening of performance at the recognition stage of the task. This result is consistent with previous literature in the field, and provides further evidence regarding the detrimental influence of negatively-valence distractors in the on-line maintenance of non-emotional information in WM (Anticevic et al., 2010; Chuah et al., 2010; Denkova et al., 2010; Dolcos et al., 2008; Dolcos & McCarthy, 2006). This effect has been interpreted in regard to the concept of motivated attention (Bradley et al., 2003), as the consequence of the preferential attentional capture of information linked to survival, so that emotional but irrelevant events become powerful interferences that compete with task-relevant information for cognitive resources. Contrary to our initial hypothesis, results from our analysis did not show significant overall differences between groups. However, they did reveal a significant effect of the interaction between group and condition, in a way that high anxiety participant's performance after neutral distraction was worse than it was in the low anxiety group. In parallel, both groups did not differ in successful performance after unpleasant distraction. In other words, while low anxiety participants were more distracted by unpleasant events than by neutral stimuli, as previously reported in the literature (Anticevic et al., 2010; Chuah et al., 2010; Denkova et al., 2010; Dolcos et al., 2008; Dolcos & McCarthy, 2006), high anxiety volunteers experienced the same level of distraction after neutral than after unpleasant information. Therefore, participants with high levels of anxiety when facing the WM task experienced a reduction in their capacity to control neutral interference, when compared to non-anxiety participants.
It has been previously reported that acute stress and anxiety can impair WM performance (Arnsten, 2009; Luethi et al., 2008; Lupien et al., 1999; Oei et al., 2006; Ramos & Arnsten, 2007; Schoofs et al., 2008), probably through the associate GCs release (Elzinga & Roelofs, 2005; Lupien et al., 1999; Oei et al., 2006; Wolf et al., 2001). Indeed, psychological stress can reduced WM-related activity over the dorsolateral prefrontal cortex (dlPFC) (Qin, Hermans, van Marle, Luo, & Fernández, 2009), a part of the prefrontal cortex that has been consistently related to WM function (D'Esposito, Postle, & Rypma, 2000; Nee et al., 2013; Smith & Jonides, 1999).
Although acute stress and anxiety are known to reallocate attentional processes in favor of threatening stimuli (de Kloet et al., 2005; van Marle et al., 2009), our main finding revealed that in healthy volunteers high levels of anxiety can make neutral events as distracting as unpleasant events, even when the former do not represent any potential threat. However, it has been also reported that stress also decreases the level of specificity in the vigilance mechanism that serves to optimize the detection and evaluation of threats (van Marle et al., 2009). Following this rationale, it is conceivable that participants with high anxiety scores responded to both neutral and unpleasant distractors in a similar manner, as if both were equally threatening. Interestingly, subjective ratings of emotional valence and arousal for neutral distractors did not differ between the low anxiety and the high anxiety groups. Since these subjective assessments took place after completing the WM task, we suggest that such decreased specificity might only affect the most automatic attentional capture and not the entire appraisal processing, at least in no-pathological individuals.
However, one might have expected to observe enhanced emotional distraction effects in participants with highest levels of anxiety. By contrast, our groups did not differ in their ability to resist unpleasant distractors in WM. However, some studies have reported similar results, and they have also demonstrated that stress-related GCs release can even be beneficial in coping with unpleasant distraction (Oei et al., 2011; Oei, Tollenaar, Spinhoven, & Elzinga, 2009; Putman, Hermans, Koppeschaar, van Schijndel, & van Honk, 2007).
Finally, it is important to highlight that these differences between anxiety groups in the cognitive control of both neutral and unpleasant distraction in WM were mainly related to state anxiety rather than to trait anxiety and depressive moods, since both groups did not differ in STAI-T and BDI scores, but were different in STAI-S score. Trait anxiety has been associated with enhanced attentional capture by threatening images (Mathews & MacLeod, 1994) and with increased capture by irrelevant information without clear emotional value (Moser, Becker, & Moran, 2012). Besides, the hypervigilance theory (Eysenck, 1992) proposes that people with high levels of trait anxiety also showed an attentional bias toward potentially threatening events. If we have used the pure state anxiety score from the STAI, one might be concerned about the possibility that individuals with highest scores in this scale also showed high levels of anxiety in the trait scale of the STAI. In that case, there would have been a confound between the specific contribution of state and trait anxiety levels to the WM effects. Nevertheless, our procedure contributed to control the effect of trait anxiety and suggest that differences between groups in the WM task were mainly related to state anxiety.
In summary, our results provide further evidence in favor of the detrimental effect of unpleasant distractors on the maintenance of non-emotional information in WM (Anticevic et al., 2010; Chuah et al., 2010; Denkova et al., 2010; Dolcos et al., 2008; Dolcos & McCarthy, 2006). Also, they suggest that healthy anxious individuals might experience an increased vulnerability to non-threatening irrelevant stimuli in such a way that performance during the actual relevant task might be compromised, while potentially threatening distractors did not affect them more than they do in non-anxious individuals.
Conflict of Interest
The authors of this article declare no conflict of interest.
Manuscrito recibido: 08/01/2014
Revisión recibida: 13/05/2014
Aceptado: 22/05/2014
Doi: http://dx.doi.org/10.1016/j.clysa.2014.10.002
*Correspondence concerning this article should be addressed to
Javier García-Pacios.
Department of Psychology.
Camilo José Cela University, Madrid.
C/ Castillo de Alarcón, 49, Urb.
Villafranca del Castillo.
28692 Madrid, Spain.
E-mail: jgpacios@ucjc.edu
References
Anderson, A. K., & Phelps, E. A. (2001). Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature, 411, 305-9. doi: 10.1038/35077083
Anticevic, A., Repovs, G., & Barch, D. M. (2010). Resisting emotional interference: brain regions facilitating working memory performance during negative distraction. Cognitive, Affective & Behavioral Neuroscience, 10, 159-73. doi: 10.3758/CABN.10.2.159
Arnsten, A. F. T. (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews. Neuroscience, 10, 410-22. doi:10.1038/ nrn2648
Aston-Jones, G., & Cohen, J. D. (2005). An integrative theory of locus coeruleusnorepinephrine function: adaptive gain and optimal performance. Annual Review of Neuroscience, 28, 403-450. doi: 10.1146/annurev.neuro.28.061604.135709
Beck, A. T., Steer, R. A., & Brown, G. K. (2006). BDI-II, Inventario de Depresión de Beck - II. Barcelona: Paidós. Retrieved from http://www.pearsonpsychcorp.es/ Producto/54/bdi-ii-inventario-de-depresion-de-beck---ii
Berman, M. G., Jonides, J., & Lewis, R. L. (2009). In search of decay in verbal short-term memory. Journal of Experimental Psychology. Learning, Memory, and Cognition, 35, 317-33. doi: 10.1037/a0014873
Bradley, M. M., Sabatinelli, D., Lang, P. J., Fitzsimmons, J. R., King, W., & Desai, P. (2003). Activation of the visual cortex in motivated attention. Behavioral Neuroscience, 117, 369-80. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12708533
Braunstein-Bercovitz, H., Dimentman-Ashkenazi, I., & Lubow, R. E. (2001). Stress affects the selection of relevant from irrelevant stimuli. Emotion, 1, 182-192. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12899196
Canli, T., Zhao, Z., Brewer, J., Gabrieli, J. D., & Cahill, L. (2000). Event-related activation in the human amygdala associates with later memory for individual emotional experience. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 20(19), RC99. Retrieved from http://www.ncbi.nlm.nih.gov/ pubmed/11000199
Chuah, L. Y. M., Dolcos, F., Chen, A. K., Zheng, H., Parimal, S., & Chee, M. W. L. (2010). Sleep deprivation and interference by emotional distracters. Sleep, 33, 1305-1313. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=29414 16&tool=pmcentrez&rendertype=abstract
D'Esposito, M., Postle, B. R., & Rypma, B. (2000). Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Experimental Brain Research, 133, 3-11. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10933205
De Kloet, E. R., Joëls, M., & Holsboer, F. (2005). Stress and the brain: from adaptation to disease. Nature Reviews. Neuroscience, 6, 463-475. doi: 10.1038/nrn1683
Denkova, E., Wong, G., Dolcos, S., Sung, K., Wang, L., Coupland, N., & Dolcos, F. (2010). The impact of anxiety-inducing distraction on cognitive performance: a combined brain imaging and personality investigation. PloS One, 5(11), e14150. doi: 10.1371/ journal.pone.0014150
Dolcos, F., Diaz-Granados, P., Wang, L., & McCarthy, G. (2008). Opposing influences of emotional and non-emotional distracters upon sustained prefrontal cortex activity during a delayed-response working memory task. Neuropsychologia, 46, 326-35. doi: 10.1016/j.neuropsychologia.2007.07.010
Dolcos, F., & McCarthy, G. (2006). Brain systems mediating cognitive interference by emotional distraction. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 26, 2072-2079. doi: 10.1523/JNEUROSCI.5042-05.2006
Ellis, H. C., & Ashbrook, P. W. (1988). Resource allocation model of the effects of depressed mood states on memory. In K. F. J. Forgas (Ed.), Affect, cognition and social behavior (pp. 25-43). Toronto: C. J. Hogrefe.
Elzinga, B. M., & Roelofs, K. (2005). Cortisol-induced impairments of working memory require acute sympathetic activation. Behavioral Neuroscience, 119, 98-103. doi: 10.1037/0735-7044.119.1.98
Eysenck, M. W. (1992). Anxiety: The Cognitive Perspective. Hove, UK: Psychology Press. Retrieved from http://books.google.co.uk/books/about/Anxiety.html?id=uUu1aFkq_ h0C&pgis=1
Henderson, R. K., Snyder, H. R., Gupta, T., & Banich, M. T. (2012). When does stress help or harm? The effects of stress controllability and subjective stress response on stroop performance. Frontiers in Psychology, 3, 179. doi: 10.3389/fpsyg.2012.00179
Lang, P. J. (1980). Behavioral treatment and bio-behavioral assessment: computer applications. In J. B. Sidowski, J. H. Johnson, & T. A. Williams (Eds.), Technology in Mental Health Care Delivery Systems (pp. 119- 137).
Norwood, NJ: Ablex Publishing. Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (1998). Emotion, motivation, and anxiety: brain mechanisms and psychophysiology. Biological Psychiatry, 44, 1248-1263. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9861468
Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (2005). International Affective Picture System (IAPS). Afective ratings of pictures and instruction manual (Technical Report A-6). Gainesville, FL.
Lang, P. J., Bradley, M. M., Fitzsimmons, J. R., Cuthbert, B. N., Scott, J. D., Moulder, B., & Nangia, V. (1998). Emotional arousal and activation of the visual cortex: An fMRI analysis. Psychophysiology, 35, 199-210. Retrieved from http://journals.cambridge. org/abstract_S0048577298970809
Lang, P. J., Greenwald, M. K., Bradley, M. M., & Hamm, A. O. (1993). Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology, 30, 261-73. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8497555
LeDoux, J. E. (1996). The emotional brain: The mysterious underpinnings of emotional life. New York, NY: Simon & Schuster.
Luethi, M., Meier, B., & Sandi, C. (2008). Stress effects on working memory, explicit memory, and implicit memory for neutral and emotional stimuli in healthy men. Frontiers in Behavioral Neuroscience, 2, 5. doi: 10.3389/neuro.08.005.2008
Lupien, S. J., Gillin, C. J., & Hauger, R. L. (1999). Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: a dose-response study in humans. Behavioral Neuroscience, 113, 420-430. Retrieved from http://www.ncbi. nlm.nih.gov/pubmed/10443770
Mathews, A., & MacLeod, C. (1994). Cognitive approaches to emotion and emotional disorders. Annual Review of Psychology, 45, 25-50. doi: 10.1146/annurev. ps.45.020194.000325
Morris, J. S., Ohman, A., & Dolan, R. J. (1998). Conscious and unconscious emotional learning in the human amygdala. Nature, 393, 467-470. doi: 10.1038/30976
Moser, J. S., Becker, M. W., & Moran, T. P. (2012). Enhanced attentional capture in trait anxiety. Emotion, 12, 213-216. doi: 10.1037/a0026156
Nee, D. E., Brown, J. W., Askren, M. K., Berman, M. G., Demiralp, E., Krawitz, A., & Jonides, J. (2013). A meta-analysis of executive components of working memory. Cerebral Cortex (New York, N.Y. : 1991), 23, 264-282. doi: 10.1093/cercor/bhs007
Oei, N. Y. L., Everaerd, W. T. A. M., Elzinga, B. M., van Well, S., & Bermond, B. (2006). Psychosocial stress impairs working memory at high loads: an association with cortisol levels and memory retrieval. Stress, 9, 133-141. doi: 10.1080/10253890600965773
Oei, N. Y. L., Tollenaar, M. S., Spinhoven, P., & Elzinga, B. M. (2009). Hydrocortisone reduces emotional distracter interference in working memory. Psychoneuroendocrinology, 34, 1284-1293. doi: 10.1016/j.psyneuen.2009.03.015
Oei, N. Y. L., Veer, I. M., Wolf, O. T., Spinhoven, P., Rombouts, S. A. R. B., & Elzinga, B. M. (2011). Stress shifts brain activation towards ventral "affective" areas during emotional distraction. Social Cognitive and Affective Neuroscience, 7, 403-412. doi: 10.1093/scan/nsr024
Ohman, A., Flykt, A., & Ludqvist, D. (2000). Unconscious emotion: Evolutionary perspectives, psychophysiological data and neuropsychological mechanisms. In R. D. R. Lane, L. Nade, & G. L. Ahern (Eds.), Cognitive neuroscience of emotion (pp. 296-327). New York, NY: Oxford University Press.
Putman, P., Hermans, E. J., Koppeschaar, H., van Schijndel, A., & van Honk, J. (2007). A single administration of cortisol acutely reduces preconscious attention for fear in anxious young men. Psychoneuroendocrinology, 32, 793-802. doi:1 0.1016/j. psyneuen.2007.05.009
Qin, S., Hermans, E. J., van Marle, H. J. F., Luo, J., & Fernández, G. (2009). Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biological Psychiatry, 66, 25-32. doi: 10.1016/j.biopsych.2009.03.006
Ramos, B. P., & Arnsten, A. F. T. (2007). Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacology & Therapeutics, 113, 523-536. doi: 10.1016/j. pharmthera.2006.11.006
Sabatinelli, D., Bradley, M. M., Fitzsimmons, J. R., & Lang, P. J. (2005). Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. NeuroImage, 24, 1265-1270. doi: 10.1016/j.neuroimage.2004.12.015
Schoofs, D., Preuss, D., & Wolf, O. T. (2008). Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology, 33, 643-653. doi: 10.1016/j.psyneuen.2008.02.004
Skosnik, P. D., Chatterton, R. T., Swisher, T., & Park, S. (2000). Modulation of attentional inhibition by norepinephrine and cortisol after psychological stress. International Journal of Psychophysiology, 36, 59-68. Retrieved from http://www.ncbi.nlm.nih. gov/pubmed/10700623
Smith, E. E., & Jonides, J. (1999). Storage and executive processes in the frontal lobes. Science, 283, 1657-1661. Retrieved from http://www.ncbi.nlm.nih.gov/ pubmed/10073923
Spielberger, C. D., Gorsuch, R. L., & Lushene, R. E. (2002). Cuestionario de Ansiedad Estado-Rasgo (STAI) : Manual. Madrid: TEA.
Tanji, J., & Hoshi, E. (2008). Role of the lateral prefrontal cortex in executive behavioral control. Physiological Reviews, 88, 37-57. doi: 10.1152/physrev.00014.2007
Van Marle, H. J. F., Hermans, E. J., Qin, S., & Fernández, G. (2009). From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biological Psychiatry, 66, 649-655. doi: 10.1016/j.biopsych.2009.05.014
Wolf, O. T., Convit, A., McHugh, P. F., Kandil, E., Thorn, E. L., De Santi, S., ... de Leon, M. J. (2001). Cortisol differentially affects memory in young and elderly men. Behavioral Neuroscience, 115, 1002-1011. Retrieved from http://www.ncbi.nlm.nih. gov/pubmed/11584913