Chronic pain is one of the primary reasons why people seek medical help. It produces functional limitation, affects mood, and impairs job performance. Many times it is difficult to manage and responds poorly to pharmacological therapies, posing a challenge for the multidisciplinary team in charge of treating this disorder. A number of approaches to relieve pain are the subject of growing research, including the application of agents such as botulinum toxin on painful points.
ObjectiveTo carry out a non-systematic narrative review of the scientific evidence available on the use of botulinum toxin for the treatment of chronic pain.
Materials and methodsA search was conducted in the PUBMED database, including meta-analyses, systematic reviews, Cochrane reviews, clinical trials, narrative reviews and case series, published between 1997 and 2013, in order to carry out a non-systematic narrative review.
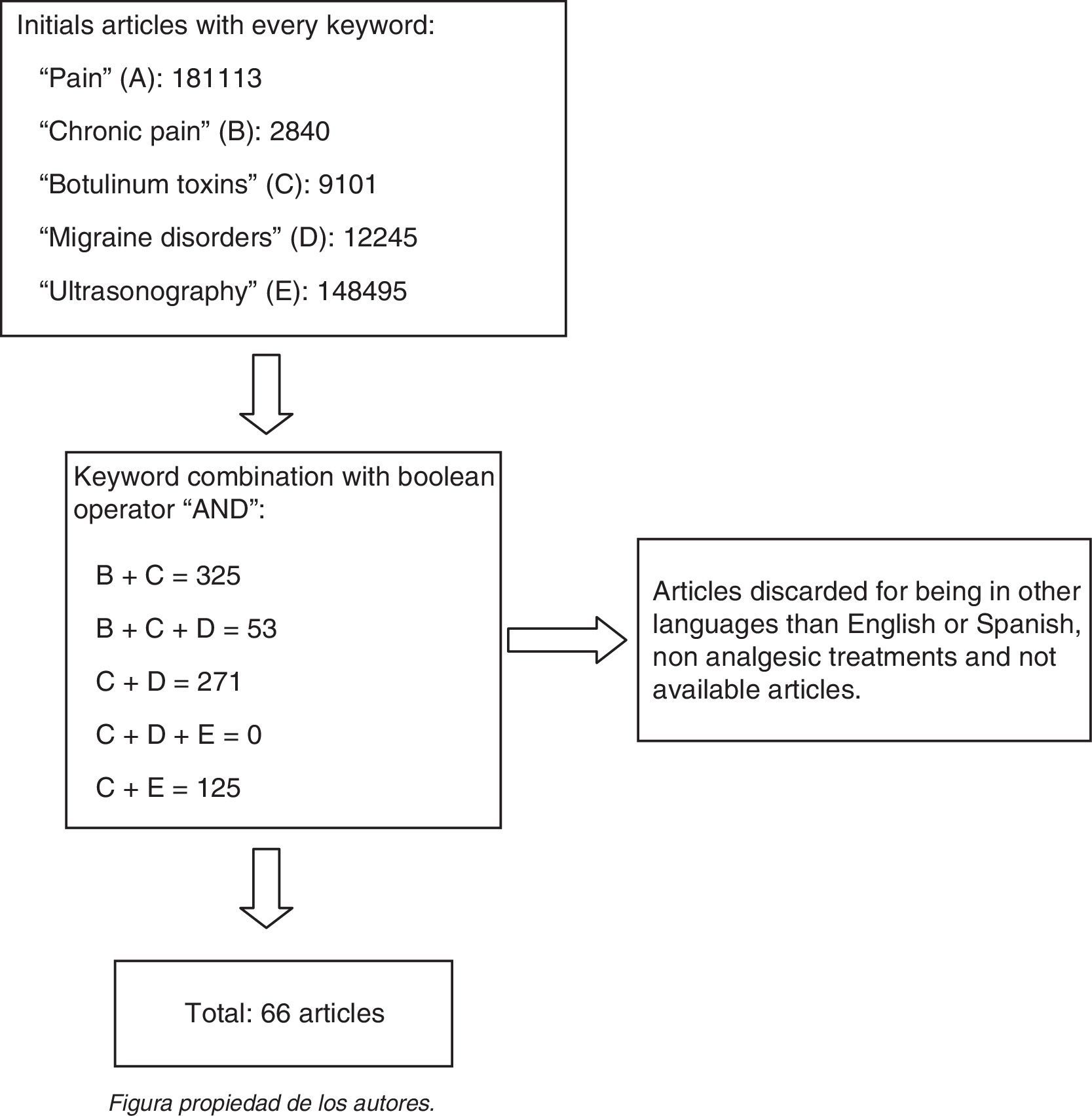
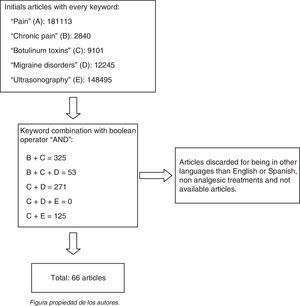
ResultsOverall, 66 articles were considered for an update on the proposed topic.
ConclusionsBotulinum toxin has certain properties that might have a beneficial effect on chronic pain. However, there is not sufficient evidence supporting its use in the majority of indications in this group of patients. Additional studies are required to recommend its use. Ultrasound is considered a useful tool to guide its application.
El dolor crónico es una de las principales causas de consulta médica, produce limitación funcional, altera el estado del ánimo y disminuye el rendimiento laboral. En muchas ocasiones es de difícil manejo y pobre respuesta a terapias farmacológicas, generando un reto para el equipo interdisciplinario que trata esta enfermedad. Cada día se investigan más métodos enfocados a aliviar el dolor, entre ellos la aplicación de sustancias como la toxina botulínica en los puntos dolorosos.
ObjetivoRealizar una revisión narrativa no sistemática sobre la evidencia científica disponible acerca del uso de la toxina botulínica en el tratamiento del dolor crónico.
Métodos y materialesSe realizó una búsqueda en la base de datos PUBMED, que incluyó artículos de metaanálisis, revisiones sistemáticas, revisiones de Cochrane, ensayos clínicos, revisiones narrativas y series de casos, entre 1997 y 2013, para la realización de una revisión narrativa no sistemática.
ResultadosSe tuvieron en cuenta un total de 66 artículos para la realización de la actualización en el tema propuesto.
ConclusionesLa toxina botulínica presenta propiedades que posiblemente puedan tener algún beneficio en el área del dolor crónico. Sin embargo, no existe suficiente evidencia que soporte su uso en la mayoría de sus indicaciones en este grupo de pacientes. Se requieren más estudios para recomendar su uso. La guía ultrasonográfica es considerada una herramienta útil en su aplicación.
Chronic pain is one of the main reasons why patients seek help and are seen in the emergency services. It is an unpleasant experience that prevails throughout time, affecting quality of life and mood, and creating functional impairment and sleep disorders; it also destroys personal relations and is a cause of frequent work absenteeism. All of these are reflected in high costs for the healthcare system.
Because of all these reasons, relief of chronic pain is critical. There are many therapeutic options, always starting with conservative approaches such as local measures, simple analgesics, suppressing trigger factors, and changes in lifestyle. Analgesic potency is escalated depending on the individual response to those initial therapies.
In many patients, pain relief with drugs is impossible to achieve or there is a need to interrupt their use because of side effects. This creates the challenge for the multi-disciplinary team of finding other alternatives, including the use of botulinum toxin (BT). This use is the topic of this review, which seeks to provide recommendations based on the scientific evidence available at the present time.
Materials and methodsA search was conducted in the PUBMED database, including meta-analyses, systematic reviews, Cochrane reviews, clinical trials, narrative reviews and case series, published between 1997 and 2013, in order to carry out a non-systematic narrative review.
ResultsA total of 66 articles were taken into account to update the proposed theme (Fig. 1).
HistoryIn 1897, Van Ermengem identified BT as a cause of fatal food poisoning. In 1949, botulinum toxin type A (BTA) was shown to block neuromuscular junction transmission. In 1989, the Food and Drug Administration of the United States (FDA) approved the use of BTA in the management of strabismus, blepharospasm and hemifacial spasm in patients over 12 years of age. In 1992, otolaryngologist William Binder reported that the cosmetic application of BTA for the correction of facial lines produced significant improvement in individuals with migraine. In 2000, BT was approved by the FDA for cervical dystonia, and in 2002 it was approved for the temporal management of glabellar lines in patients less than 65 years of age.1
CharacteristicsBT is the fermentation product of the bacterium Clostridium botulinum. It is a Gram-positive anaerobic bacterium found in water and soil. The neurotoxins produced are proteins, and seven different serological types have been identified (A, B, C1, D, E, F and G). There is another protein apart from those mentioned previously (C2), but it is not considered a neurotoxin.2,3
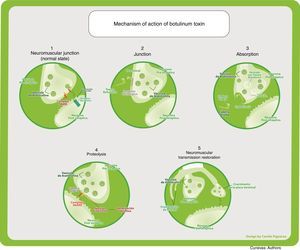
Deben suprimirse los nombres de marcas registradas: Botulinum toxin type A and B is comercially available. The best studied and most widely used of the two is BTA; however, toxin B has been studied in several conditions such as myofascial pain, migraine, tension headache and cervical dystonia, showing efficacy, duration of action and adverse effects similar to those of toxin A, when used in equivalent doses. They are molecular complexes ranging between 300 and 900kDa formed by a neurotoxin of 150kDa consisting of a light 50kDa chain and a heavy 100kDa chain that bind in a non-covalent way with other non-toxin proteins, with or without haemagglutinins that help stabilize and protect the neurotoxin from degradation. The different serotypes are activated by means of proteases, creating a greater therapeutic effect. Up to 95% of the type A neurotoxin is cleaved by endogenous proteases (Figs. 2 and 3).
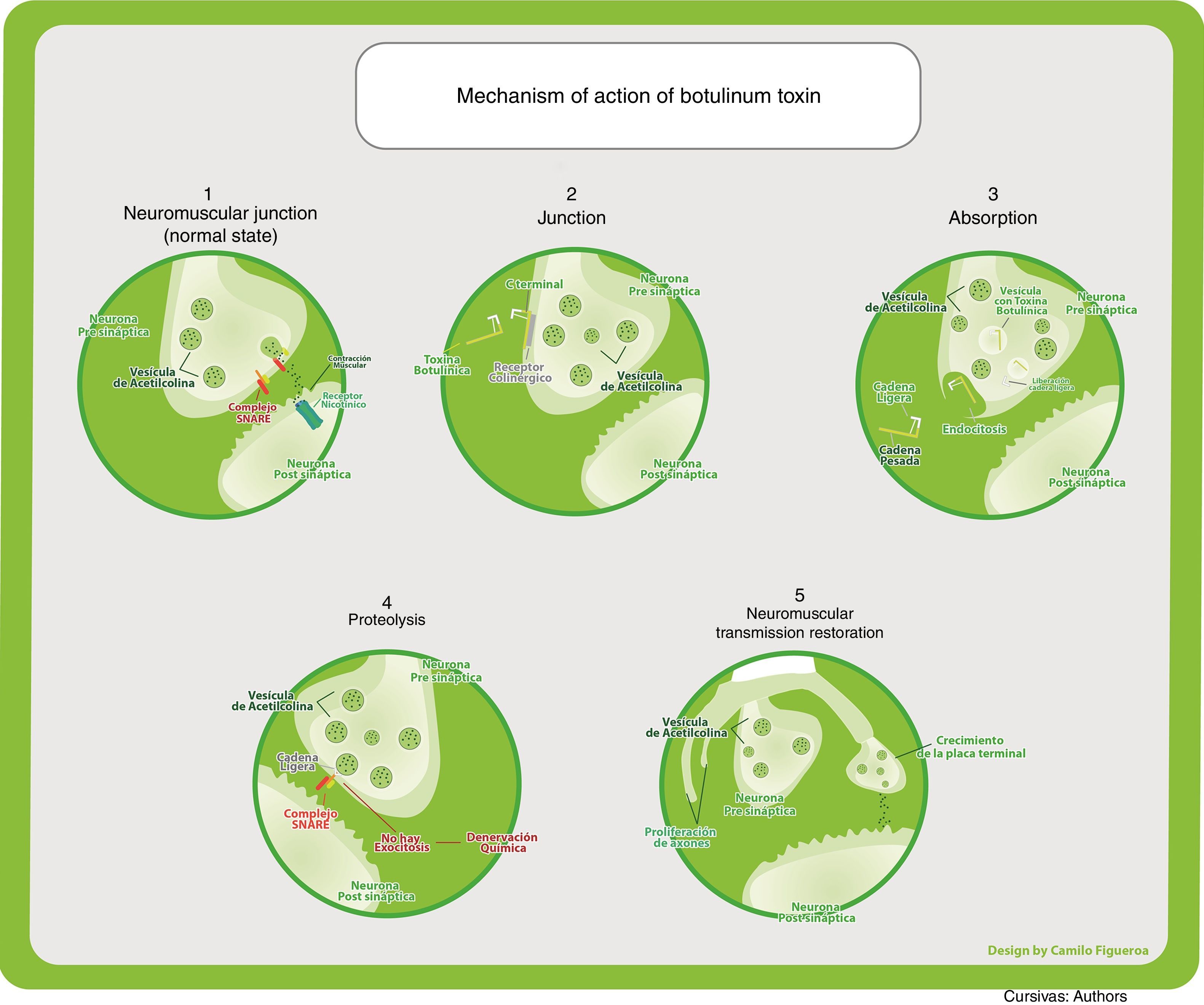
Mechanism of actionThe toxin is injected in the muscle belly where the motor plate, its main effector site, is located. Most of the therapeutic effects are secondary to the inhibition of acetylcholine release from nerve endings, including sympathetic and parasympathetic motor neurons. It produces a chemical denervation and the resulting muscle relaxation. As a reaction to this denervation, it gives rise to new dendritic branching towards the muscle cell, favouring the partial recovery of its effect, which translates into a 3–4 month period of therapeutic action.
Under normal circumstances, the acetylcholine-containing vesicles are located in the nerve endings. They bind to the cell membranes through SNARE protein complexes (N-ethylmaleimide-sensitive factor attachment protein receptor), which include VAMP (vesicle-associated membrane protein) where type B exercises its main action, and SNAP-25 (synaptosome-associated protein of 25kDa) where type A has its action. They then release their content into the synaptic cleft through exocytosis. Acetylcholine crosses the synaptic space where it binds to nicotinic receptors found in muscle cells, producing muscle contraction.
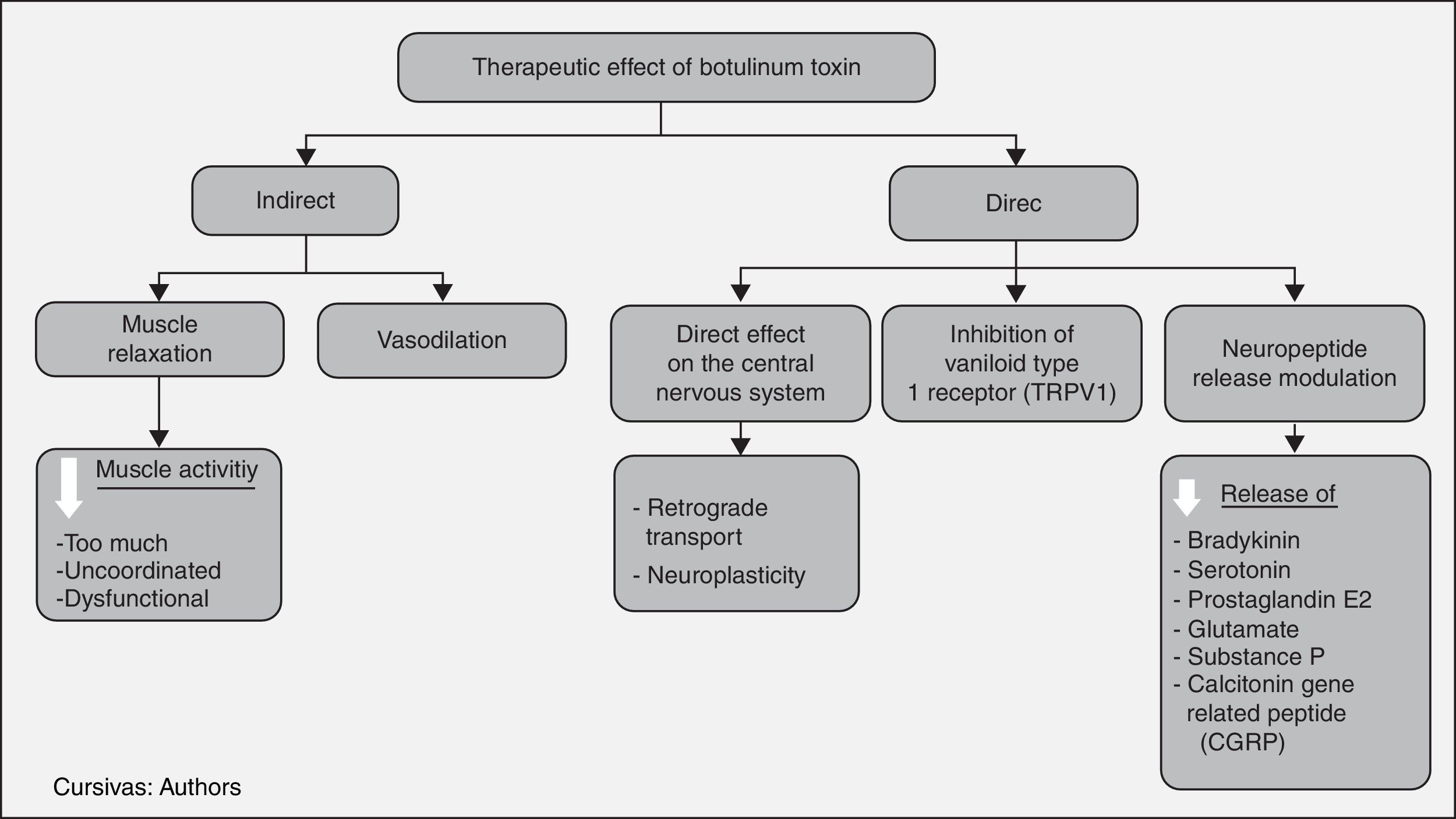
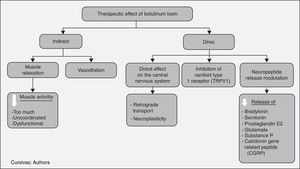
It has been proposed that BT reduces pain directly by producing molecular changes in nociceptive fibre function, and indirectly by reducing excess dysfunctional muscle activity. Others propose that it acts in three different ways: producing muscle blockade, modulating the release of different neuropeptides involved in pain genesis, and improving circulation, when ischemia is considered a cause of pain.4
The most studied is the indirect mechanism, which involves three steps:
- -
Binding: Through the C terminal region, the heavy chain binds irreversibly to presynaptic cholinergic receptors, which contain gangliosides as part of their structure, specific for the different neurotoxins. Low and high affinity binding sites have been identified, leading to the dual-receptor hypothesis: when the ganglioside, being the low-affinity part, binds the neurotoxin, it induces a conformational change in the toxin, allowing recognition by the protein receptor. This binding is independent of nerve activity.
- -
Internalization: It happens through toxin endocytosis. It is receptor mediated and gives rise to the formation of vesicles that carry the neurotoxin inside. This process is partially dependent on nerve stimulation.
- -
Vesicles have an acid pH, allowing changes in the protein structure to facilitate passage through the lipid layers where ion channels will form. These are needed for the translocation of the endosomal light chain into the neuronal cytoplasm.
- -
Neuromuscular blockade, also called proteolysis: It happens with the translocation of the light chain, characterized by a catalytic activity. Through the action of zinc-dependent endopeptidases, there is cleavage of the different SNARE proteins according to the specificity of each type of toxin. In this way, through different molecular mechanisms, they prevent vesicle-receptor coupling and fusion, inhibiting exocytosis of acetylcholine into the synaptic space.5
In this way, the cholinergic transmission of alpha and gamma motor neurons is inhibited, producing a muscle relaxing effect that, in turn, reduces blood vessel compression, releases nerve compression and reduces nociceptive receptor activation. These changes create analgesia, considering that pain in dystonia, contracture and spasm results mainly from the compressive focal ischemia of muscle blood vessels. This creates a hypersensitivity state in peripheral nociceptive receptors (group IV fibres) to biochemical changes such as bradykinin, pH reduction and ATP release due to the damage to the muscle cell membrane. In the past it was believed that pain was triggered by lactic acid due to muscle fatigue, but this concept is now revised. Moreover, the pain receptor activation threshold is lowered, leading to pain from muscle activity. On the other hand, low intensity contractions, although they do not compress the vessels, cause pain as excess ATP is released through anaerobic glycolysis.6
It has been found that the toxin might be useful in other conditions where pain is not related to muscle contraction and where muscle relaxation is not a prerequisite for analgesia, as is the case in migraine. This happens through a direct mechanism of action.
In animal and in vitro studies, it has been found to produce desensitization and changes in the response pattern of the peripheral group III and group IV nociceptive fibres, indirectly reducing central sensitization. These fibres are activated by sensitizing substances, and the toxin is involved by reducing the release of bradykinins, serotonin, potassium, prostaglandin E2, glutamate, substance P, and calcitonin gene-related peptide (CGRP). In a subsequent phase, this reduces dorsal horn neuronal activity, which participates in the transmission of the painful stimulus to the central nervous system, with a concomitant reduction of neuropeptides in dorsal ganglion neurons.7
Things are complex at an autonomic level, given that both nicotinic and muscarinic receptors are active in this system. Moreover, neurotransmitters different from acetylcholine also participate, including enkephalins, neurotensin, somatostatin and substance P. The mechanism of action at this level involves in different ways, the most important being the suppression of neurogenic inflammation, with the involvement of vascular effects, cytokines and neuropeptides such as substance P which, when released, produces vasodilation and contributes to plasma extravasation. As mentioned previously, all of these changes favour the accumulation of agents that sensitize peripheral nerves. There is a change in blood flow patterns due to the effect of autonomic neurons in the vascular smooth muscle. Blood flow is related to inflammation and ischemic pain, and it may be involved in nociceptor sensitization. The toxin is also believed to alter pain perception or response to pain, through a direct effect on the central nervous system.8
BT also induces neuroplastic changes in afferent somatosensory processing at multiple levels of the neural axis, due to events that alter peripheral sensitization. Different mechanisms have been proposed, including excitatory and inhibitory spinal activity patterns, such as up-regulation of NMDA-mediated afferent signals as well as other non-NMDA activated pathways. It was demonstrated that when labelled botulinum toxin was injected in the sciatic nerve, it left part of the radioactive material in the spinal cord, suggesting the possibility of retrograde transport. However, evidence showed that only remnants and not the intact toxin were transported. To this date, there is no conclusive evidence of this retrograde transport in peripheral neurons.
Finally, vanilloid type 1 receptors (TRPV1) have been found to increase when the dorsal root ganglion is stimulated in vitro, contributing to the development and maintenance of an inflammatory state, with the involvement also of botulinum toxin type A.
Craniofacial painBT has been used in patients with bruxism,9 temporomandibular joint pain,10 trigeminal neuralgia,11 occipital neuralgia,12 tension headache, and chronic migraine, with conflicting results. Several randomized clinical trials in patients with orofacial pain or temporomandibular joint dysfunction have reported that the use of BTA does not offer any benefit in terms of pain relief when compared to placebo over a follow-up period of up to 24 weeks.13,14
In episodic headache and chronic migraine, there are hundreds of studies with conflicting results. Recently, a meta-analysis was published of randomized clinical trials comparing BTA with placebo or other interventions in patients with tension headache and patients with chronic migraine performed between 1966 and March 2012, reporting that there is little benefit from BTA compared to placebo in reducing the number of days of the month with headache in patients with chronic migraine (−2.06 days per month), and that there is no difference in the number of ictus days per month or in tension headache. No benefit was also reported when compared with valproic acid, topiramate and amitriptyline, with the added disadvantage that patients receiving BTA had a higher incidence of palpebral ptosis, taught skin, paresthesias, neck stiffness, muscle weakness and cervical pain.15 Other meta-analyses comparing BTA versus placebo also reported no benefit in reducing ictus in patients with chronic migraine.16
The PREEMPT 1 and 2 studies, sponsored by Allergan, the owner of the Botox brand, are studies of involving 1384 patients randomized to receive BTA (n=688) or placebo (n=696). The studies found a statistically significant difference in favour of the use of BTA in patients with chronic migraine in terms of reducing the number of days with headache per month (−8.4 vs. −6.6; P<.001), with a mean of prior days with headache per month of 19.9 and 19.8, respectively. No difference was found in the number of ictal days in the month or the need for rescue medication in acute pain. Additionally, more adverse events were reported in the BTA group (62.4 vs. 51.7%).17
Myofascial painMyofascial pain syndrome is a disorder caused by persistent acute or chronic muscle contraction, characterized by the identification of “trigger points” or fibrous bands that, when stimulated or pressed, transfer radiated pain to the distribution area of the affected muscle. The pathophysiology of this condition is unclear, but it appears to include several complex interactions of countless pathogenic mechanisms such as ischemia induced by muscle spasm, hyperactivity of the neuromuscular spindle or the motor plate, and central or peripheral sensitization. Therapies used to date for its management include oral medication, use of local anaesthetics and/or steroids in the painful area, dry puncture, and different cycles of physical therapy, most of them with limited benefits and the presence of some side effects.
BT has anti-nociceptive and muscle relaxant properties that have led it to be used successfully since 1968 in chronic pain found in several disorders due to focal muscle hyperactivity, such as cervical dystonia. It has been proposed that BT could be very useful in the treatment of the myofascial pain syndrome by breaking the painful vicious circle.18 However, some studies have demonstrated that the sole use of BT is not enough to relieve pain and that, in addition to active treatment, physical therapy is fundamental.19
In 2011, the Cochrane collaboration published a review of the literature on BT application in subacute and chronic cervical pain. They analysed nine studies with 503 patients and concluded that, based on the evidence available to date, it is not possible to demonstrate a benefit for BT when compared with placebo, normal saline solution, common analgesics or physical therapy in these types of patients, including those suffering from cervicogenic headache.20
Another review of the literature was published in 2012 (Cochrane collaboration), this time with the objective of determining the efficacy and safety of BT in the treatment of myofascial syndrome, excluding head and neck muscles. Based on four studies (233 patients), the authors concluded that the evidence is non-conclusive to support treatment with BT, considering that the studies included were of good methodological quality but included a small number of patients, and were very heterogeneous in terms of the evaluation measures and the type of endpoint analysed.21 In another systematic review, five clinical trials were compared, and only one provided favourable evidence for the use of BT in myofascial pain syndrome, while the other four trials did not show significant differences for the different variables when compared to placebo.22 In another review, the use of BT in myofascial pain was evaluated in nine clinical trials and only two trials provided favourable evidence, while the rest failed to show any differences when compared to placebo. Similar to the previous review, the heterogeneity of the studies makes it difficult to arrive at a clear conclusion.4
In a systematic review and meta-analysis including 12 trials relating to the use of BT for the treatment of myofascial pain, only three studies showed positive pain relief results compared to placebo or other treatments. In the subgroup analysis, the use of BT in doses over 25IU showed significant pain reduction, while lower doses did not show clinical benefit.23
Neuropathic painThe analgesic potential of BT has been explored recently in the treatment of neuropathic pain because of its mechanism of action that involves not only blocking the release of acetylcholine in the neuromuscular plate, but also inhibiting the release of substance P, CGRP, glutamate, and vanilloid receptor TRPV1 expression, which are all involved to a significant extent in maintaining hyperalgesia. Preclinical studies support the analgesic qualities of BT when used as pre-treatment in formalin-induced neuropathic pain models in rats and capsaicin-induced neuropathic pain in humans.24,25
In the clinical realm, multiple studies have been conducted, mainly case series or case reports, using BT in the treatment of localized neuropathic pain (post-herpetic neuralgia, trigeminal neuralgia, chronic facial pain, post-operative neck pain, and carpal tunnel syndrome).26–32
The relevant studies include those conducted by Yuan33 and Ranoux et al.34 (case series) with 18 and 29 patients, respectively. They studied the use of BT in the treatment of localized neuropathic pain (diabetic foot, and trauma-related allodynia, post-operative pain or post-herpetic neuralgia). After subcutaneous BT injection (5 and 4U) in different points around the affected site, they found that more than 4% of patients in the two studies experienced more than 50% pain relief compared to baseline, or a reduction of 3 points in the analogue visual scale (AVS). The benefit was obtained from the first week of application and was maintained up to a maximum of 12 or 14 weeks, respectively, without relevant side effects in either of the two studies, consistent with results found by other authors.
Phantom limb pain syndromeAs with many of the uses of BT, case reports are the most prevalent in the literature, with varying results.35,36 One clinical trial compared BT application with lidocaine plus steroid and found reduced stump pain in both groups, with longer duration of this effect in patients who received BT, but with no improvement of phantom limb pain in either of the groups.37
Peripheral ischemic diseaseIntra-digital BTA injection at a dose of 10–100 units has been studied since 2004 when a pilot study conducted in two patients revealed improved clinical symptoms and blood flow. Consequently, it has been considered a promising therapy in the treatment of peripheral ischemia refractory to pharmacological or even surgical management of patients diagnosed with Raynaud's syndrome, with both primary and systemic sclerosis.38 Other studies have been conducted since then, including one case series with 11 patients, of which 9 reported diminished frequency and severity of vasospasm episodes,39 as well as a case report showing a noticeable reduction in pain at rest, immediately after the injection.40 A retrospective study reported that 16 of 19 patients (84%) experienced marked reduction of pain at rest, and all of them went on to heal their digit ulcers within 60 days.41 However, although BTA has been shown to confer benefits in patients with digit ischemia, different studies recommend performing additional research before it can be included as part of the therapeutic options.42–44
Joint painIntra-articular use of BT has also been described. A study of 36 patients with chronic shoulder pain due to osteoarthritis and rheumatoid arthritis in which 43 joints were infiltrated with 100U of BTA plus lidocaine was compared to saline solution plus lidocaine showed a reduction of 2.4 points on the VAS during the first month.45
Likewise, in 43 patients with severe knee pain, 100U of BTA plus lidocaine compared to saline solution plus lidocaine were compared, showing a reduction of 4.2 units on the McGill joint pain scale at three months, but with no improvement at one month.46
ComplicationsAlthough electromyography has shown that neuromuscular junctions in the entire body may be affected with BTA injections, generalized paralysis is very rare. BTA lacks systemic effects, which makes it a safe medication.47 Although rare, there are reports of skin irritation and anaphylactic reactions triggered by its use.48,49
Depending on the region where it is injected and the indication, it may produce different adverse effects50:
- •
Strabismus: ptosis, subconjunctival haemorrhage and transient vertical deviation of the eyeball.
- •
Blepharospasm: ptosis, diplopia, hemifacial paralysis and epiphora.
- •
Hemifacial spasm: the most common adverse effect is ptosis and transient facial palsy.
- •
Cervical dystonia: dysphagia, cervical weakness, cervical pain, shoulder and hand pain.
Of the patients treated with BTA, 3–10% develop resistance.
In a recently published meta-analysis of clinical trials in patients with headache,51 the main adverse effects reported were blepharoptosis (RR, 9.5; 95% CI, 4.7–18.9), muscle paralysis (RR, 8.9; 95% CI, 2.5–30.9), neck pain (RR, 4.7; 95% CI, 3.2–6.9), neck stiffness (RR, 3.2; 95% CI, 1.9–5.6), paresthesias (RR, 3.3; 95% CI, 1.3–7.9), and skin numbness (RR, 3.6; 95% CI, 1.6–8.3).
Other reported adverse effects were flu-like discomfort,52 fever and shivering.53,54
Ultrasound guidance for BT infiltrationThere is worldwide consensus regarding the fact that the safest way of administering BT to the different tissues is to avoid blind injections. Several reports have been published showing the efficacy and safety of ultrasound use in different conditions such as in patients with sialorrhea,55–59 thoracic outlet syndrome,60 external bladder sphincter dyssynergia61 and cerebral palsy-associated spasticity.62,63
It is also recommended when performing percutaneous lavage, dry puncture, electrocauterization, infiltrations of steroids and local anaesthetics, and prolotherapy.64,65
ConclusionsChronic pain continues to have a high prevalence in healthcare institutions, often requiring the involvement of a multi-disciplinary team and the use of multiple therapeutic tools. The pharmacokinetic and pharmacodynamic characteristics of BT make it a promising agent for the treatment of some conditions causing chronic pain. However, the evaluation of current evidence shows that an important clinical benefit of BT cannot be demonstrated for the time being in craniofacial and myofascial pain because of the impossibility to estimate adjusted impact measures supporting absolute recommendation, as a result of the heterogeneity of the articles.
In joint paint, ischemic disease, painful phantom limb syndrome, and neuropathic pain, the evidence is limited to case reports, with equally heterogeneous results.
Given the above, it is important to undertake additional research studies with patients affected by diseases that meet clear diagnostic criteria in order to allow comparability, using adequate protocols for the use of BT that assess optimal endpoints with adequate follow-up, in order to derive more accurate conclusions.
Finally, the use of ultrasound is essential in situations requiring injections into any tissue of the body.
FundingThis review was funded with the authors’ own resources and with the advice of Universidad CES (Medellín – Colombia).
Conflicts of interestThe authors declare no conflicts of interest.
We thank Camilo Figueroa and Diego Salazar Uribe for their help with the figures.
Please cite this article as: Rivera Día RC, Lotero MAA, Suarez MVA, Saldarriaga SE, Martínez MG. Toxina botulínica para tratamiento del dolor crónico. Rev Colomb Anestesiol. 2014;42:205–213.