Breathing affects cardiac output on a cycle-by-cycle basis, through changes in pressure and intrathoracic volume of key components in cardiovascular function Hence, heart–lung interactions affect cardiovascular functioning and the heart's ability to adapt. The heart–lung interaction is an area of applied physiology broadly studied but in Colombia, this is the first trial ever done with a thoracic bioreactance monitor. The hemodynamic profiles of 38 patients scheduled for myocardial revascularization were measured at the Fundación Clínica Abood Shaio using thoracic bioreactance monitor, which provided ventricular volume and cardiac output measurements. The ventilator volumes and pressures with which corrections were made to interpret the effects of mechanical ventilation were recorded.
La respiración afecta el rendimiento cardiaco, ciclo a ciclo, a través de los cambios en la presión y el volumen intratorácicos sobre las determinantes de la función cardiovascular. De esta manera, las interacciones corazón-pulmón afectan el funcionamiento cardiovascular y la capacidad del corazón para adaptarse. La interacción corazón-pulmón es un área de la fisiología aplicada ampliamente estudiada, pero en Colombia es el primer estudio que se realiza con un monitor de biorreactancia torácica. Se midieron los perfiles hemodinámicos de 38 pacientes programados para cirugía de revascularización miocárdica en la Fundación Clínica Abood Shaio utilizando un monitor de biorreactancia torácica, con el cual se obtuvieron medidas de volúmenes ventriculares y gasto cardiaco. Se registraron los volúmenes y presiones ventilatorias con las que se hicieron correlaciones para interpretar los efectos de la ventilación mecánica.
Spontaneous natural breathing that involves the integrity of the respiratory system begins with inspiration through the action of muscles that expand the chest volume, generating a negative pressure in the inter-pleural space that also allows for an increased pulmonary volume and airflow into the alveolar zone. Simultaneously, negative pressure trough a pressure gradient facilitates the inflow of blood to the chest and the heart for a natural venous return. During this phase, an enhanced right ventricle filling promotes a higher right systolic volume and blood flow through the pulmonary artery that increases the capillary refill to facilitate hematosis. During expiration, the mechanical inversion of forces produces divergent effects; but, according to Frank Starling's phenomenon, the left ventricle will receive more blood with enhanced stroke volume at this stage.
Pulmonary or cardiac disease that affects the blood flow dynamics or the gas exchange demands the use of methods and systems that temporarily support a compromised function. Usually these are based on external pulmonary insufflation using the Positive Pressure principle. Mechanical ventilation is one of the most commonly used and important aids for the comprehensive management of the critical patient. Intrathoracic positive pressures have beneficial effects on the respiratory function that are directly related with gas exchange but, on the other hand, lead to adverse effects on the lungs and the heart that have to adapt their function to the pressure forces and vectors.
Positive intrapulmonary pressures during mechanical ventilation bring about changes in the heart–lung interaction that may alter directly or indirectly the regulation of the cardiorespiratory system. The intensity of the effects depends on several factors: the severity of the involvement of the lung parenchyma, the integrity of the intrapleural space, the strength of positive pressures, the volemic status, and heart failure. However, the effects of mechanical ventilation (MV) over the composition of blood gasses will depend on the underlying pulmonary disease and the peripheral perfusion.
During the last few years, clinical research has been focusing on non-invasive monitoring methods because of the adverse events resulting from invasive devices in the ICUs. These new devices allow for a continuous evaluation of the hemodynamic variables, of any acute changes in the respiratory cycle and immediate response to treatment, hence making some headway toward achieving the management objectives.
In order to analyze the changes in the heart–lung interaction, a research protocol was designed to take measurements in patients with ischemic heart disease scheduled for myocardial revascularization surgery. First, because mechanical ventilation may then be initiated in a programmed manner, and second, because the hemodynamic changes associated with mechanical ventilation could be potentially evaluated early.
Materials and methodsThis research protocol was approved by the Ethics Committee of the School of Medicine of the National University of Colombia and by the Ethics Committee of the Fundación Clínica Abood Shaio, in keeping with the Declaration of Helsinki.
This was a pre-experimental trial with a pre-test and post-test single group design.1 The study took place during the second and third quarter of 2011, at the Fundación Clínica Shaio. Three time periods were used:
- 1.
Informed consent and baseline pre-anesthetic evaluation.
- 2.
Induction of anesthesia – the same anesthetic induction protocol was followed in every patient: Midazolam 0.1mg/kg, Fentanyl 4mcg/kg, Cisatracurium 0.1mg/kg and Sevofluorane initial dose 4% and 2% maintenance dose.
- 3.
Mechanical ventilation – the following parameters were applied: volume controlled ventilation, RR 12–14rpm, PEEP 5cmH2O, CV 8ml/kg approximately, FIO2 100%.
The trial included both male and female patients with the following characteristics:
- -
Documented coronary disease.
- -
Patients scheduled for myocardial revascularization surgery.
- -
Patients at the Fundación Clínica Shaio.
Patients with the following pathologies were excluded:
- -
Severe chronic pulmonary disease.
- -
Moderate to severe pulmonary hypertension.
- -
Aortic and/or mitral valve insufficiency.
- -
Aortic aneurysm.
- -
Counterpulsation balloon.
- -
Ejection fraction <30%.
Non-Invasive Cardiac Output Monitor, based on frequency change analysis and current signal oscillation occurring when the current traverses the thoracic cavity; this is in contrast with Bioimpedance that only measures signal amplitude changes. This device uses a high frequency of 75kHz applied to the thorax through trigger and signal reception electrodes.
These signals are processed individually and combined before digital processing. Frequency and phase changes are equivalent and are representative of the reactance in the circuit. Based on the algorrhythms, any changes in Bioimpedance and reactance of the current injected and the current received, reflect changes in aortic blood flow. There is therefore a close relationship between the exchange rate in blood flow and the exchange rate of reactance (ΔX/Δtmax) as expressed in the following equation (Fig. 1).2
Statistical analysisComparison of measurements. (ANOVA test, Multiple ranges). The statistical program used was: Statgraphics centurion XVI version 16.1.11.
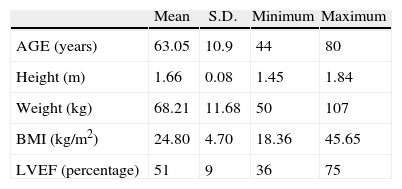
ResultsThe patients were from the Fundación Abood Shaio in Bogotá, and included 192 heart surgeries, 75 of which were myocardial revascularization; 38 patients met the inclusion criteria. 33 males and 5 women with a mean age of 63 years (Table 1).
Demographic distribution.
| Mean | S.D. | Minimum | Maximum | |
| AGE (years) | 63.05 | 10.9 | 44 | 80 |
| Height (m) | 1.66 | 0.08 | 1.45 | 1.84 |
| Weight (kg) | 68.21 | 11.68 | 50 | 107 |
| BMI (kg/m2) | 24.80 | 4.70 | 18.36 | 45.65 |
| LVEF (percentage) | 51 | 9 | 36 | 75 |
Demographic characteristics of the sample according to age, height, weight, body mass index (BMI) and left ventricular ejection fraction (LVEF). The standard deviation of each variable (r) is shown.
The variables studied were classified into three groups: cardiac function, vascular function and fluid content (see Tables 2–4).
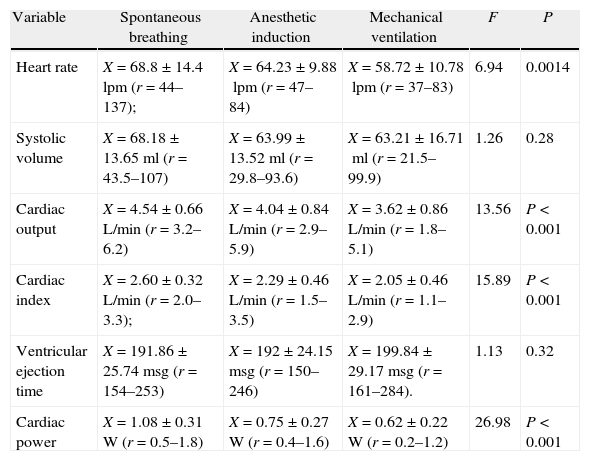
Cardiac function results.
| Variable | Spontaneous breathing | Anesthetic induction | Mechanical ventilation | F | P |
| Heart rate | X=68.8±14.4lpm (r=44–137); | X=64.23±9.88lpm (r=47–84) | X=58.72±10.78lpm (r=37–83) | 6.94 | 0.0014 |
| Systolic volume | X=68.18±13.65ml (r=43.5–107) | X=63.99±13.52ml (r=29.8–93.6) | X=63.21±16.71ml (r=21.5–99.9) | 1.26 | 0.28 |
| Cardiac output | X=4.54±0.66L/min (r=3.2–6.2) | X=4.04±0.84L/min (r=2.9–5.9) | X=3.62±0.86L/min (r=1.8–5.1) | 13.56 | P<0.001 |
| Cardiac index | X=2.60±0.32L/min (r=2.0–3.3); | X=2.29±0.46L/min (r=1.5–3.5) | X=2.05±0.46L/min (r=1.1–2.9) | 15.89 | P<0.001 |
| Ventricular ejection time | X=191.86±25.74msg (r=154–253) | X=192±24.15msg (r=150–246) | X=199.84±29.17msg (r=161–284). | 1.13 | 0.32 |
| Cardiac power | X=1.08±0.31W (r=0.5–1.8) | X=0.75±0.27W (r=0.4–1.6) | X=0.62±0.22W (r=0.2–1.2) | 26.98 | P<0.001 |
The table shows the average, the standard deviation and variable range (r) at each measurement point in time and the F and P values with ANOVA test and a 95% confidence interval.
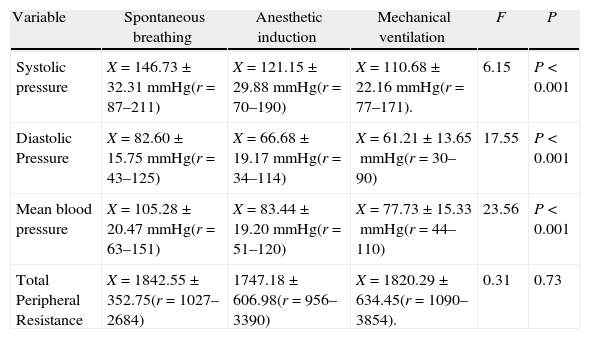
Vascular function results.
| Variable | Spontaneous breathing | Anesthetic induction | Mechanical ventilation | F | P |
| Systolic pressure | X=146.73±32.31mmHg(r=87–211) | X=121.15±29.88mmHg(r=70–190) | X=110.68±22.16mmHg(r=77–171). | 6.15 | P<0.001 |
| Diastolic Pressure | X=82.60±15.75mmHg(r=43–125) | X=66.68±19.17mmHg(r=34–114) | X=61.21±13.65mmHg(r=30– 90) | 17.55 | P<0.001 |
| Mean blood pressure | X=105.28±20.47mmHg(r=63–151) | X=83.44±19.20mmHg(r=51–120) | X=77.73±15.33mmHg(r=44–110) | 23.56 | P<0.001 |
| Total Peripheral Resistance | X=1842.55±352.75(r=1027–2684) | 1747.18±606.98(r=956–3390) | X=1820.29±634.45(r=1090–3854). | 0.31 | 0.73 |
The table depicts the average, the standard deviation and the variable range (r) at each point in time measured and the F and P values with ANOVA test and a 95% confidence level.
Results of thoracic fluid contents.
| Variable | Spontaneous breathing | Anesthetic induction | Mechanical ventilation | F | P |
| X=127.91±41.32(r=43.1–237.2) | X=127.91±41.32(r=42.9–236.5) | X=124.68±39.33(r=41.5–234) | 0.09 | 0.91 |
The table depicts the average, standard deviation and variable range (r) at each point in time measured and F and P values with ANOVA test and a 95% confidence level.
In a healthy heart the systolic volume and the cardiac output are to a large extent governed by the diastolic filling of the left ventricle (Preload). During positive pressure mechanical ventilation intra-aortic pressure increases in the inspiratory phase, and this may lead to a reduced venous, and subsequent drop in the cardiac output and oxygen delivery in a healthy patient. So the recommendation has been the pharmacological reestablishment of the systolic volume and fluid maintenance of volemia.3
The transmission of pressure from the airway to the pulmonary parenchyma and the intrapleural space depends on the airway, lung and thoracic compliance. In a study by Chapin et al. (1979) measurements of the transmission of airway pressure into the pleural space under varying conditions in 10 pigs with induced anesthesia and assisted mechanical ventilation showed that with normal lung and thoracic compliance, pressure transmission to the pleural space was approximately 50% of the applied pressure, while under reduced thoracic compliance the pressure was 75%. In contrast, increased thoracic compliance reduced the ability to transfer the applied pressure. If lung compliance decreases, the pressure transmission into the pleural space will decrease as well instead of increasing.4 The conclusions of the Chapin trial are solid arguments to study the heart/lung interaction and its reliance on intra-thoracic pressures, since the effects on venous return, cardiac output and vascular resistance depend on the transmission of pressures to the vascular bed.
Moreover, changes in the intra-aortic pressure impact directly the right atrial transmural pressure. During spontaneous breathing in inspiration, the right atrial transmural pressure drops and the pressure gradient between the vena cava and the atrium itself increases, thus facilitating blood flow or venous return. In positive pressure breathing the pleural pressure raises and this increases the atrial pressure, reducing the gradient for the return flow; thus the right ventricular filling during inspiration under mechanical ventilation drops. Furthermore, changes in pulmonary volume will result in changes mainly on the alveolar vessels so that at the beginning of inspiration, while the alveolar volume increases, the blood contained in the capillaries is forced to flow forward: then, toward the end of inspiration with positive pressure, if the pressure is high, the compression on the capillaries increases flow resistance with two outcomes: (1) retrograde reduction of the ventricular emptying, rise in the right atrial pressure and (2) a higher resistance to blood flow reduced the left atrial filling and hence the left ventricle. Depending on the amount of volemia a higher right ventricular volume may displace the septum to the left, further restricting the left ventricular filling and generating a lower cardiac output. This is called the ventricular interdependence.
Consequently, in patients undergoing high-risk surgical procedures – in this particular case myocardial revascularization – hemodynamic monitoring is of the essence and becomes a key tool to guide treatment. Non-invasive monitoring devices have been developed overcoming the pulmonary artery catheter limitations and Fick's method to measure systolic volume. Bioreactance is a non-invasive method that delivers information about the hemodynamic profile, circumventing the risks, the costs and expertise required in thermodilution and other minimally invasive methods. Understanding the functional principles of bioreactance helps in identifying its advantages and disadvantages.5
This trial used a non-invasive cardiac output monitor based on the measurement of thoracic reactance. The machine operates under the electrical principles of bio-impedance but with filter optimization. The bioreactance monitor is based on the analysis of frequency change and the oscillation of the electrical signal generated as the current traverses the thoracic cavity. Validation trials have been completed comparing the bioreactance monitor against thermodilution and other minimally invasive techniques. Bioreactance has shown acceptable accuracy with higher precision as compared to other devices.6,7 In the population studied, CO monitoring based on thoracic electrical resistance has shown a good correlation with thermodilution. In the case of more heterogeneous populations such as septic patients and trauma, further analyses are needed.8
In accordance with the epidemiology and risk factors for coronary disease, the demographic profile of the 38 patients is consistent with the literature. 87% were males and the most common risk factors were: hypertension 71%, Dyslipidemia 60%, Diabetes mellitus 36%, and cigarette smoking 31%. All 38 patients had been receiving treatment for their respective conditions. Following is a discussion of the hemodynamic variables monitored with bioreactance.
Cardiac functionThe heart rate decreased linearly at the three time points measured. This is achieved with betablockers therapy for coronary disease and during the induction of anesthesia from the negative chronotropic and inotropic pharmacological effect of the drugs administered. For instance, the combination of Fentanyl, Midazolam and Vecuronium causes bradycardia and hypotension.9
In this study, the decrease in cardiac output during mechanical ventilation may be associated with the peak effects of the anesthetic induction agents. The lower heart rate under mechanical ventilation is also associated with the pulmonary insufflation reflex with a tidal volume >15ml/kg.10 In this trial, the average tidal volume was 8.3ml/kg and the subjects were under the effects of anesthesia; however, there was a minor but significant decrease (10 beats/min) in heart rate during mechanical ventilation that could be related to parasympathetic action.
It has been stated that the systolic volume during mechanical ventilation may be compromised mainly as a result of changes in the preload due to the rise in intra-thoracic pressure. This reduces the venous return of the right atrium. Such decrease in the preload initially compromises the cardiac output with right ventricular involvement. Furthermore, the relative left ventricular compliance may decrease when the septum is displaced as a result of an increased right ventricular post-load (pulmonary vascular compression). However, the post-load to the left ventricle may decrease if the pressure gradient increases between the ventricle and the thoracic aorta and between the latter and the extra-thoracic aorta, as a result of the ITP.11 Thus, the deleterious effect on the pre-load of an ailing heart may be compensated lowering the left ventricular post-load so that the changes in the average systolic volume can be minimized. Notably, the significant change in systolic volume occurred during the induction of anesthesia instead of when the mechanical ventilation was established. This may be explained because of the vasodilation caused by the anesthetic agents and the muscle relaxant.
The reduced cardiac output may be the result of the drop in its two component factors; i.e. CO=HR×SV. The determining components of SV are: preload, postload and contractility. Under mechanical ventilation the preload of the left ventricle is affected by the lower systemic venous return and by the drop in the right ventricular systolic volume that may result in a drop in CO.12 The left ventricular function is also evaluated in terms of: (1) Systolic Volume; (2) Cardiac Output; (3) Ventricular Exertion; and (4) Cardiac Power.13 These measurements are difficult to make in clinical practice directly through the Frank-Starling relationship (length–tension); thus the ventricular function curves that relate the end diastolic volume or the end diastolic pressure as indicators of preload versus the product of the ventricular discharge are used: systolic volume, cardiac output, ventricular exertion or cardiac power. Under this approach, it is important to discriminate the factors that may lower the CO.
The lineal decrease of the CO at the three points in time measured was not the result of changes in systolic volume since, as already mentioned, the systolic volume remained constant. On the other hand, the cardiac output dropped significantly at the three measurement points. Consequently, it may be suggested that the decrease in cardiac output was related to the deleterious effect on chronotherapy of the drugs during the induction of anesthesia.
The ventricular ejection time (VET) is the time elapsed from the opening of the aortic valve and its closure during the cardiac cycle and to a large extent it contractility and postload-dependent. Weissler et al. (1968) evaluated the ventricular function based on the systolic intervals and found that in heart failure patients, the pre-ejection time (PET) was longer and hence the ventricular ejection time was shorter than normal.14 No significant differences were found in the study with regards to the VET at the three time points measured. However, the average VET under mechanical ventilation was a bit higher than at other time points. The rise in VET during mechanical ventilation may be the result of a drop in the postload of the left ventricle, derived from the effects of positive pressure on the aorta, although the postload-lowering pharmacological effects on vascular reactivity also have an impact. These conclusions however require specific contractility studies (electromechanical coupling dynamics) that are beyond the scope of this research.
The cardiac power (CP) is the heart's ability to deliver energy to the arterial system and to maintain the blood circulation. CP is usually a prognostic indicator of cardiac function.15 Studies for measuring the heart's functional reserve in patients with cardiac failure showed that CP correlates well with exercise performance and it is a good prognostic indicator in these patients.16 In patients with coronary disease, the cardiac power may be affected and the reserve to respond to stress situations such as mechanical ventilation. In patients with coronary disease the cardiac power may be affected as well as the reserve to respond to stress situations such as mechanical ventilation. In the 38 patients studied, the cardiac power dropped at each time point measured. This may be associated with: (1) the negative inotropic effects of the drugs used; (2) the blood pressure drop; and (3) the increase in the ventricular ejection time with no significant disruption in the systolic volume, as previously shown.
Vascular functionArterial pressures (systolic, diastolic and mean) behaved similarly. Basically, the decrease in pressure occurred during the induction of anesthesia and this may be explained by the dual pharmacological effect: (1) the inotropic impact, and (2) vasodilatation with the resulting reduced vascular resistance.9 During mechanical ventilation the blood pressure did not drop further, showing that the most important decrease occurred during the passage from spontaneous breathing to the moment of pharmacological anesthetic induction.
In the bioreactance monitor, the measurement of peripheral resistance is the ratio between the mean arterial blood pressure obtained using the oscillometric non-invasive method and the cardiac output estimated as the product of SV and HR. As seen in Table 3, there are no significant differences among the three measurement time points. The small variation in blood pressure and the total peripheral resistance when comparing the anesthetic induction and the effects of mechanical ventilation may be the result of: (1) the sympathetic stimulus caused by the intubation maneuvers; and (2) the maintenance of anesthesia with Sevofluorane started at the end of intubation – Sevoflourane is the agent with the least vasodilatation effects as compared to the IV agents administered at the start of induction and because of their short half life could be in the elimination phase at the time of making the observation.
Thoracic fluid contentIntravascular and extravascular fluids contain a considerable amount of electrolytes (i.e. sodium, chlorine, potassium, calcium and others) which are the components of electrolytic solutions. These solutions are excellent electrical conductors; more electrolytes imply higher conductance, while less electrolytes represent lower conductance. The bioreactance monitor measures the changes in the alternate applied high frequency changes, concurrently with the changes in applied voltages through the tissues. In other words, it measures the bioimpedance (Z) which is the instant resistance to the current flow (|Z|=|P|/|Q|).
Impedance 0 (Z0) reflects the thoracic resistance to electrical conductance. This is a measurement that depends on the thoracic fluid content since the higher the fluid content, the lower the impedance and vice versa. The opposite of impedance (resistance) is called conductance (1/Z0), which is directly proportional to the thoracic fluid content (TFC) – electrolytic solution.
It's important to keep in mind that the specific relationship between the TFC units (1/Ohms) and the litters of fluids in absolute terms is unknown. It is said that there is no such thing as a normal FTC value and hence this is a qualitative rather than quantitative measurement of the amount of electrolyte-containing solution in the chest or any body area studied. Hence, in the present study, the TFC measurement produced values ranging from 41.5 and 237.2, with no significant differences in the three points in time measured. This behavior is consistent with the procedure since during the measurement times the patients did not receive any representative amounts of fluids that could affect their volemia, nor were there any changes in position or intrathoracic pressures strong enough to affect the composition and distribution of body fluids.
Currently, several research studies are evaluating the volume response in ICU patients; TFC measurements seem to be a key tool for assessing volume resuscitation. In contrast, studies on mathematical models that explain the fluid mechanics and the hemodynamic response to changes in volemia17 may generate some headway in volume resuscitation protocols in the critical patient.
In 1975 Qvist et al., using the pulmonary artery catheter as a measurement instrument, found that by establishing MV in normovolemic dogs, the filling and cardiac output pressures decreased and there was no hemodynamic recovery in the first few hours. Finally, Qvist concluded that the changes in the cardiac index depended on the integrity of the right and left ventricular contractility.18 In 2004 Reuters et al., used the pulmonary artery catheter and the trans-esophageal ultrasound to compare the hemodynamic effects of mechanical ventilation in patients undergoing cardiac surgery before and after thoracotomy, and just as Chapin, they were able to show that the effects of mechanical ventilation are directly related to the compliance of the thoracic cage. This means that the cardiac output decrease resulting from the presence of positive pressures in the thorax dropped upon opening the chest.19 In 2013 Kristensen et al. (2013) made the observation that even at low positive pressure values during positive pressure mechanical ventilation, there was a decrease in the preload and in the cardiac output. This trial was done with cardiac magnetic resonance images.20
Thermodilution with pulmonary artery catheter has been the standard technique for measuring the CO in the clinic since it was introduced in 1970. Recent modifications to the device enable the measurement of the right ventricular function and the beat-by-beat right ventricular end-diastolic volume and the beat-by-beat right ventricular end of diastole volume; however, there are complications reported with increased morbidity and mortality. The trans-esophageal ultrasound estimates the CO by multiplying the blood flow rate×the cross sectional area of the aorta at the point of insonation. This is an accurate and reproducible technique but it is time-consuming and requires considerable expertise. Magnetic resonance (MR) is expensive and requires expertise to interpret the images. Hence, this measurement instrument may provide a more faithful determination of the changes resulting from the heart–lung interaction.
In this context, the difficulty to differentiate whether the changes in the hemodynamic profile of the 38 trial patients is a consequence of pharmacological and/or mechanical effects is directly related with the measuring instrument. However, the studies by Qvist, Reuters and Kristensen17–19 fail to specify the effects clearly related to the study drugs. Bioreactance generates real time measurements and is non-invasive, so it's ideal for clinical research. Nonetheless, it is necessary to do further studies and to continue to apply physiological methods under clinical situations to further our understanding about the heart–lung interaction.
This research work is within the realm of applied physiology and is intended to describe the heart–lung interaction in a real life situation. In this context, isolating the variables that impact such relationship and differentiating the effects strictly produced by the drugs from the effects of mechanical ventilation is not easy, since under the protocols of a conventional clinical practice it is practically impossible.
FundingDivision of Physiology, MS in Physiology, National University of Colombia.
Conflicts of interestThe authors have no conflicts of interest to declare.
AcknowledgementWe appreciate the support of the Departments of Anaesthesia, Cardiovascular Surgery and surgery rooms of Fundación Clínica Abood Shaio, who contributed with their knowledge and experience in conducting research.
Please cite this article as: Rodríguez IJ, Echeverry JC, Abello M, Cruz LE. Cambios en el perfil hemodinámico al instaurar la ventilación mecánica en pacientes con cardiopatía isquémica y enfermedad coronaria. Medición con biorreactancia torácica. Rev Colomb Anestesiol. 2014;42:76–82.