Sedation is a practice frequently used in multiple settings outside the operating room. Currently, it is employed by different interdisciplinary groups. It is imperative to ensure safe, standardized practices and proper training of the personnel involved.
ObjectivesTo update available information, create minimum standards of care for performing high-quality sedation procedures based on the best available evidence. These standards lead to safe and effective care practices, reducing unjustified variability.
Materials and methodsA group of different health professionals with experience in sedation outside the operating room and representatives of scientific and patient societies developed the guideline. Through a non-formal consensus of thematic and methodological experts, the content, scope and objectives, study populations, and questions to be examined were all defined. A systematic review of the literature was performed to formulate the recommendations with graduated levels of evidence in accordance with GRADE methodology.
ResultsA summarized version of the Clinical Practice Guideline with recommendations for the administration of sedation as part of diagnostic or therapeutic procedures outside the operating room in patients over the age of 12.
ConclusionsThe Clinical Practice Guideline presents evidence-based recommendations in order to standardize the procedure, improving the quality of care, and reducing morbidity of patients that require sedation outside of the operating room.
La sedación es una práctica frecuentemente utilizada en múltiples escenarios fuera de salas de cirugía y que actualmente se realiza por diferentes grupos interdisciplinarios; es perentorio la adquisición de prácticas seguras, estandarizadas y un entrenamiento adecuado del personal involucrado.
ObjetivosActualizar la información disponible, generar estándares mínimos de atención para realizar procedimientos de sedación de alta calidad basada en la mejor evidencia disponible que redunde en prácticas de atención seguras y efectivas, disminuyendo la variabilidad no justificada de la misma.
Materiales y métodosSe conformó un grupo con diferentes profesionales de la salud con experiencia en sedación fuera de quirófano y representantes de sociedades científicas y de pacientes que desarrollaron la guía. A través de un consenso no formal de expertos temáticos y metodólogos, se definió su contenido, alcances y objetivos, población, preguntas a desarrollar y se realizó una revisión sistemática de la literatura para formular las recomendaciones con niveles de evidencia graduados de acuerdo a la metodología GRADE.
ResultadosSe presenta una versión resumida de la Guía de Práctica Clínica con recomendaciones para la administración de sedación como parte de los procedimientos diagnósticos o terapéuticos fuera del quirófano en pacientes mayores de 12 años.
ConclusionesLa Guía de Práctica Clínica presenta recomendaciones basadas en la evidencia con el propósito de estandarizar el procedimiento, mejorar la calidad de atención y disminuir la morbilidad de los pacientes que requieran sedación fuera del quirófano.
These clinical practice guidelines are aimed at physicians (general practitioners and specialists, including anesthesiologists) and dentists caring for hospitalized and ambulatory patients requiring sedation outside the operating room. In addition to proposing recommendations based on the best available evidence, it aims to establish safe and effective interventions for the management of patients over the age of 12 requiring sedation as part of diagnostic or therapeutic procedures performed outside of the operating room in order to provide the highest quality of care and to reduce unjustified variability in the practice.
Introduction and methodologySedation is a practice frequently used in invasive medical and dental procedures, such as imaging diagnostics, invasive radiology, cardiac catheterization and endoscopies.1 To develop this guideline, we followed a rigorous, valid and reproducible methodological design to prevent and control bias and support an interdisciplinary perspective with ample space for participation and discussion.
The interventions considered in the guideline were: (1) patient preparation; (2) the professional who will administer the sedation; (3) monitoring; (4) pharmacological interventions; and (5) immediate care post-sedation. In addition, an evidence-based curriculum is proposed which contains the necessary competencies of staff who administer sedation in patients over 12 years of age outside the operating room. This curriculum is presented in another article of this same issue.
To develop the recommendations, it was necessary to systematically recover and study all the available information, critically review its validity, and grade its quality. Another important aspect was producing secondary results based on primary studies, systematic reviews or meta-analyses, and promoting discussion panels to resolve controversy. The resulting CPG was subjected to expert academic and methodological peer review that ensured its quality and relevance.
To learn in greater detail about the methodology implemented during the development of these clinical practice guidelines, readers may view the methodological annex or access to the full version of the guideline at the web-appendix.
General recommendationsI. What are the safest and most effective interventions for the preparation of patient over the age of 12 that require sedation outside the operating room?a. Patient evaluationThe systematic search of the literature did not reveal studies that addressed this clinical question. Expert recommendations strongly support an evaluation of all patients before a procedure under sedation and analgesia. The evaluation should include: medical history with background of coexisting diseases and surgical background, previous sedation and general anesthesia, medications, allergies, fasting state, dental status, and presence of prostheses. The physical examination should include: evaluation of the airway, cardiovascular and respiratory status, and any relevant aspects of the patient history.2,3
During the pre-sedation evaluation, it is fundamental to identify patients with a risk of presenting adverse events, including: patients with cardiovascular or respiratory risks or compromise of the airway; those with liver or kidney disease, morbid obesity, obstructive sleep apnea syndrome; those with a risk of bronchoaspiration, precedents of adverse events during previous sedations, and those over the age of 75.4,5 These patients, and others classified as ASA IV/V, which have 5–7 times more risk of adverse events related to sedation compared with ASA I/II patients, will require evaluation and management by a specialist in anesthesiology and a setting with the appropriate conditions for managing complications.6–8
b. Informed consentThe search of the literature did not yield studies that addressed this clinical question. Expert recommendations strongly support explaining the procedure to the patient, including risks, benefits and alternatives in the case of deep sedation. In cases of moderate sedation, this explanation is also supported.2 Informed consent for sedation and/or analgesia for the procedure must be obtained from the patient or, when the patient is under the age of majority or has another limitation, from a legal representative. This is a requirement of current legislation.3 The person performing the procedure must inform the patient in writing of the nature of the procedure; other instructions, including fasting; and what they can expect during the procedure, including possible side effects.6
| Recommendation | Summary |
| Strong for | It is recommended that the professionals performing the clinical evaluation before the procedure be trained in sedation and that they document their findings in the clinical history. Very low quality evidence ⊕OOO |
| During the pre-sedation clinical evaluation, the following aspects should be considered: • Current medical condition and any surgical problem. • Height and weight. • Medical history (including precedents associated with sedation or anesthesia). • Current or past use of medications (including allergies). • Functional class. • Airway and cardiopulmonary evaluation. • Psychological condition and signs of anxiety. | |
| Strong for | The use of sedation outside of the operating room is not recommended in patients classified as ASA III and IV. Very low quality evidence ⊕OOO |
| An anesthesiologist must be consulted in special cases (signs of difficult airway, obstructive sleep apnea or difficult ventilation, patients with severe alteration of psychomotor development) or when the patient does not tolerate the procedure under sedation. | |
| Recommendation | Summary |
| Strong for | Obtaining informed consent is recommended for the use of sedation outside the operating room. Very low quality evidence ⊕OOO |
| The patient and the legal representative (for underage patients) must be allowed to make an informed decision based on verbal and written information provided to them about the proposed sedation technique, the alternatives to the use of sedation, the risk of having to cancel the procedure, and the risks and benefits resulting from the intervention. | |
The search in the literature did not yield studies that addressed this clinical question. Recommendations from experts show that the setting in which sedation is administered must have at least one reliable oxygen source, suction equipment, resuscitation equipment, and emergency medications.9
| Recommendation | Summary |
| Strong for | In settings in which sedation is administered outside the operating room, having the necessary tools and space available to perform basic and/or advanced cardiopulmonary resuscitation (depending on the type of medication used) is recommended. Very low level evidence ⊕OOO |
A systematic review of the literature, with an AMSTAR score of 5/11,10 evaluated the effectiveness of checklist use for the prevention of medical complications after elective or emergency procedures. Seven observational studies were retrieved with a total of 37339 participants. Five of the included studies used the World Health Organization (WHO) checklist, while another used the Association of Peri-Operative Registered Nurses (AORN) checklist.
Based on this systematic review, it was possible to establish that, compared with not using checklists, the use of these instruments reduced the probability of complications of any type (RR 0.64; CI 95% 0.57–0.72) as well as the frequency of surgical site infections (RR 0.54; 95% CI 0.40-0.72), without its use is related to respiratory infection (RR 1.03; CI 95% 0.73–1.45), unexpected return to surgery rooms (RR 0.76; CI 95% 0.56–1.02) or death posterior to the procedure (RR 0.79; 95% CI 0.57–1.11).
The quality of the evidence was very low due to limitations in the risk of bias, the applicability, the accuracy and the consistency of the results.
e. Selection criteriaThe literature search did not yield reviews that addressed the clinical question. Nonetheless, two cohort studies were recovered that analyzed factors that influence the success of conscious sedation in patients receiving interventions outside the operating room.11,12
The first study,12 with 300 participants who underwent upper endoscopies or diagnostic sigmoidoscopy, evaluated the factors related to an appropriate level of sedation.
Based on this study, it was possible to establish that the factors that influence the success of sedation in patients outside of the operating room are age (p=0.0004, greater pain reported in young patients, lower level of sedation in older subjects), gender (p=0.0062, lower cooperation in female patients), body mass index (p=0.0495, lower cooperation in slim patients), having received sedation previously (p=0.030, greater pain reported in patients without previous sedation experience), duration of the procedure (p=0.026, lower cooperation with longer procedures) and the need of biopsy during the intervention (p=0.049, greater pain reported in patients undergoing biopsies). The duration of the procedure was not associated with a higher or lower frequency of successful sedation (p=0.31).
The second study11 evaluated the factors that influence sedation in a cohort of 180 participants who underwent upper endoscopies or colonoscopies. Based on this study, it was possible to establish that the factors that influence the success of sedation in patients outside the operating room were the degree of anxiety prior to the procedure (OR 3.29; 95% CI 1.49–7.26) and the presence of anxious personality traits (OR 2.92; 95% CI 1.32–6.46). The other analyzed variables showed no statistically significant association. The quality of the evidence was very low due to some limitations in the risk of bias and the accuracy of the results.
| Recommendation | Summary |
| Strong for | It is recommended that clinicians identify the factors in the clinical history that may influence the success of sedation to reduce pain and increase patient cooperation during the procedure. These include: age, gender, body mass index, duration of procedure, degree of anxiety, and anxious personality traits. Very low quality evidence ⊕○○○ |
A systematic review of the literature, with a AMSTAR score of 9/11,13 evaluated the optimal duration of preoperative fasting and the type and volume of ingestion permitted in adults scheduled for ambulatory surgery under general anesthesia. 22 controlled clinical trials were recovered with a combined total of 2270 participants. Based on this systematic review, the following could be determined:
- i.
Preoperative fasting compared to the ingestion of liquids or solids
When preoperative fasting was compared against the ingestion of liquids during the 120–180min prior to the procedure, the restriction of the ingestion of solid food was not associated with higher or lower gastric content in milliliters (MD −0.84ml; 95% CI −2.77 to 1.08) or with differences in the estimated gastric pH (MD 0.14 points; 95% CI −0.04 to 0.31). When fasting was compared against the ingestion of solids, the consumption of solid foods was not associated with a higher gastric volume (MD 0.88ml; 95% CI −7.68 to 9.44) or with substantial differences in the pH values compared to the preoperative fasting strategy (MD −1.15 points; 95% CI −4.09 to 1.79). Finally, when preoperative fasting was compared with the unlimited ingestion of liquids, no statistically significant differences in terms of volume (MD 0.33ml; 95% CI −4.38 to 5.05) or gastric pH (MD 0.19 points; 95% CI −0.01 to 0.39) were observed either. No serious adverse events occurred. The quality of the evidence was very low due to limitations in risk of bias, applicability, and consistency of results.
- ii.
Ingestion of solids versus ingestion of liquids
One study compared the safety and effectiveness of allowing the consumption of solid food versus liquids in this population. When compared against the consumption of liquids, the consumption of solid food was not associated with a higher or lower volume (MD −1.7ml; 95% CI −12.44 to 9.04) or with substantial changes in gastric pH (MD 0.3 points; 95% CI −1.13 to 1.73). The quality of the evidence was very low due to limitations in the risk of bias, the applicability and the accuracy of the results.
- iii.
Preoperative fasting versus ingestion by type of liquid
When preoperative fasting was compared against the ingestion of water before the procedure, the restriction of food ingestion was not associated with a higher or lower gastric content in milliliters (MD −2.51ml; 95% CI −4.6 to 0.42) or with a difference in estimated gastric pH (MD 0.09 points; 95% CI −0.1 to 0.29). When fasting was compared to the ingestion of coffee with milk, the consumption of coffee and milk was not associated with a higher gastric volume (MD 1.3ml; 95% CI −6.37 to 8.97).
Finally, when preoperative fasting was compared to the ingestion of fruit juice, no statistically significant differences in terms of gastric volume were observed either (MD 0.5ml; 95% CI −6.5 to 7.5). No adverse events occurred. The quality of the evidence was very low due to limitations in the risk of bias, the applicability, and the accuracy of the results.
| Recommendation | Summary |
| Weak for | Preoperative fasting from solids for at least 6h is suggested in patients requiring sedation outside the operating room. Very low quality evidence ⊕○○○ |
| Weak for | No preoperative fasting is suggested when nitrous oxide is administered as the only sedative without premedication in patients requiring sedation outside the operating room. Very low quality evidence ⊕○○○ |
| Weak for | Allowing preoperative ingestion of clear liquids is suggested up until 2 hours prior to sedation outside of the operating room. Very low quality evidence ⊕○○○ |
| In patients with pathologies that alter gastric clearance, such as obesity, diabetes mellitus, gastroesophageal reflux, fasting from liquids and solids for at least 8h before the procedure should be considered. | |
| In the case of emergency procedures in patients without fasting, the decision to proceed with the use of sedation must be made taking the urgency and the medication used during the intervention into account. | |
A systematic review of the literature, with an AMSTAR score of 10/1114 evaluated the effectiveness of the use of non-pharmacological interventions to increase the cooperation of children submitted to ambulatory medical or dental procedures under general anesthesia.
28 controlled clinical trials were recovered, yielding a total of 2681 participants under the age of 17. Of the 28 clinical trials included in this review, 12 evaluated the presence of parents as an intervention; in 13, the intervention was received by the child or the parent-child pair. Meanwhile, in 3, the parents were the object of the intervention. The majority of the children received inhaled anesthesia with oxygen, nitrous oxide and sevoflurane. When the presence of parents or the use of hypnosis was compared against the administration of midazolam as a sedative prior to the procedure, the use of these non-pharmacological interventions did not reduce anxiety (standardized mean difference (SMD) 0.03; 95% CI −0.14 to 0.2 and RR 0.59, 95% CI 0.33–1.04, respectively), time (MD −0.94; 95% CI −2.41 to 0.53 in min) or the frequency of delirium (RR 0.66; 95% CI 0.37–1.18) after induction. Nevertheless, greater cooperation was observed during induction when it was administered using a mask (RR 1.27; 95% CI 1.06–1.51 compared with intravenous induction), but not with low sensory stimulation (RR 0.66; 95% CI 0.45–0.95).
The quality of the evidence was very low due to limitations in the risk of bias, the applicability, and the accuracy of the results.
| Recommendation | Summary |
| Weak against | The routine use of non-pharmacological interventions in adults requiring sedation outside the operating room is not recommended. Very low quality evidence ⊕OOO |
| Weak for | The use of non-pharmacological interventions, such as induction with mask and the presence of parents, is recommended in the pediatric population requiring sedation outside the operating room to increase cooperation during the procedure. Very low quality evidence ⊕OOO |
- -
Sedation administered by non-anesthesiologists versus sedation administered by anesthesiologists.
The search recovered one systematic review and one controlled, randomized clinical trial that evaluated the safety and effectiveness of the intervention in question. The systematic review of the literature, with a AMSTAR score of 3/1115 consisted of a meta-analysis of indirect comparisons aimed at evaluating the safety and effectiveness of non-anesthesiologist administration of propofol (NAAP) against anesthesiologist administration of propofol (AAP) in patients who underwent elective endoscopy procedures, such as endoscopic ultrasound, endoscopic retrograde cholangiopancreatography, or enteroscopy.
In the meta-analysis (16 studies included, 2953 participants),15 the sedation was administered by non-anesthesiologists. In half of the cases, propofol combined with an adjuvant agent (midazolam, fentanyl or meperidine) was used to induce sedation. In the second meta-analysis (10 studies included, 2374 participants), the sedation was administered by anesthesiologists, and in 2 studies no adjuvant agent was used together with propofol. Finally, for both meta-analyses, the sedation was delivered by an anesthesiologist or by a nurse certified in anesthesiology under the guidance of an anesthesiologist, or by a nurse guided by a gastroenterologist, or by a non-anesthesiologist physician. Based on this meta-analysis, it was possible to establish that patients receiving sedation by an anesthesiologist report higher satisfaction after the procedure (10-point visual analog scale, 1 being unsatisfied and 10, very satisfied. Non-anesthesiologists: score 7.22; 95% CI 7.17–7.27. Anesthesiologists: score 9.82; 95% CI 9.76–9.87), at the expense of a greater frequency of intervention in the airway (non-anesthesiologist: 3.5%; 95% CI 2.6–4.7. Anesthesiologists: 13.3%; 95% CI 11.8–15.0). Moreover, sedation administered by anesthesiologists increased the satisfaction of the endoscopist of the procedure (10-point visual analog scale, 1 being unsatisfied and 10, very satisfied. Non-anesthesiologists: 6.02; 95% C1 5.94–6.11. Anesthesiologists: 9.06; 95% CI 8.91–9.21). This was not reflected in a higher or lower frequency of episodes of hypoxemia (non-anesthesiologists: 13.3%; 95% CI 11.7–15.2. Anesthesiologists: 14.3%; 95% CI 1.8–15.9) when the sedation is administered by a health professional trained in an area other than anesthesiology.15 The quality of the evidence was “very low” due to some limitations in the risk of bias, consistency, applicability, and accuracy of the results.
The controlled, randomized clinical trial16 evaluated the safety and effectiveness of sedation administered by gastroenterologists, compared to sedation administered by anesthesiologists. The study recruited 154 participants between the ages of 20 and 80 who underwent endoscopic submucosal dissection as part of treatment for early gastric cancer.
The sedation of the intervention group was administered by a gastroenterologist-endoscopist different from the one performing the procedure. The sedation in the control group was administered by an anesthesiologist. Based on this clinical trial, it was possible to establish that compared to sedation administered by an anesthesiologist, sedation administered by a gastroenterologist was not accompanied by a higher or lower frequency of episodes of hypotension (RR 1.04; 95% CI 0.17–6.05) or airway interventions (RR 1.02; 95% CI 0.47–2.22). However, it did lead to a higher number of patients experiencing inadvertent deep sedation (RR 1.66; 95% CI 1.20–2.29) and a lower frequency of patients achieving complete recovery within the first 5min (RR 0.53; 95% CI 0.40–0.69). Nevertheless, the patients assigned to endoscopist administered sedation group reported greater satisfaction with the care received (RR 1.80; 95% CI 1.07–3.02) at the expense of lower satisfaction from those performing the procedure (RR 0.56; 95% CI 0.42–0.75).
The quality of evidence was low due to some limitations in the risk of bias, consistency, applicability and accuracy of the results.
| Recommendation | Summary |
| Strong for | It is recommended that the team administering sedation outside the operating room include at least two health professionals, one in charge of administering sedation and monitoring the patient to ensure safety during the procedure. Very low quality evidence ⊕OOO |
| Strong for | When nitrous oxide is administered as the only sedative without premedication, it is recommended that the health professional performing the procedure also be able to administer and monitor sedation. Very low quality evidence ⊕OOO |
| Strong for | It is recommended that the administration of sedation outside the operating room be under the supervision of a health professional with formal training to increase satisfaction with the care provided and to ensure safety during the procedure. Very low quality evidence ⊕OOO |
| Strong for | Health professionals administering sedation with propofol are recommended to have formal training in the use of this intervention to increase satisfaction with the care provided and to ensure safety during the procedure. Very low quality evidence ⊕OOO |
A systematic review of the literature, with an AMSTAR score of 8/11,17 evaluated the safety of the use of clinical monitoring alongside capnography for the surveillance of patients requiring sedation outside the operating room. 6 controlled clinical trials, with a combined total of 2524 patients, were recovered that evaluated the intervention in question. In 5 of the studies recovered, propofol was administered as a sedative in the adult population, while one study used midazolam or ketamine for sedation in the pediatric population. In 3 of the studies included in this systematic review, the intervention was administered by anesthesiologists, by non-anesthesiologist physicians, or by nurses specialized in anesthesia. When compared against clinical monitoring only, the patients assigned to the clinical monitoring and capnography group experience a lower frequency of hypoxemia without this having an impact on a higher frequency of airway intervention (RR 0.58; 95% CI 0.26–1.27).
Very low quality of evidence due to limitations in the risk of bias, consistency and the accuracy of the results.
| Recommendation | Summary |
| Strong for | The use of basic and clinical monitoring in patients requiring sedation outside the operating room is recommended to reduce the frequency of hypoxemia. Very low quality evidence ⊕○○○ |
| Strong for | When nitrous oxide is administered as a single agent, the implementation of clinical monitoring and at least pulse-oximetry with an auditory signal and heart rate is recommended. Very low quality evidence ⊕○○○ |
| The information obtained during clinical and/or basic monitoring must be registered in the clinical history. | |
| Weak for | The use of capnography is recommended when this resource is available to reduce the frequency of hypoxemia in patients requiring sedation outside the operating room. Very low quality evidence ⊕○○○ |
| Capnography should be used for patients with a substantial risk of over-sedation (combined use of sedatives or propofol), when a direct evaluation of ventilation is not feasible, and for procedures involving manipulation of the airway. | |
| Strong for | After ending the procedure, continued monitoring is recommended until the patient is alert, hemodynamically stable, and has a clear airway and adequate airway and respiratory reflexes. Very low quality evidence ⊕○○○ |
A systematic review of the literature, with an AMSTAR score of 7/11,18 compiled the psychometric characteristics of the different scales used to assess the level of sedation in hospitalized adults in the Intensive Care Unit (ICU). We retrieved 40 studies with more than 27 scales for a total of 4088 participants. For this CPG, the information from the five scales that had the largest number of studies and which, in turn, correspond to those used most frequently in the Colombian context, was analyzed. However, this article presents the analysis of the psychometric characteristics of the two scales selected to be implemented in the CPG by the group of thematic experts. For more information regarding other instruments analyzed, we invite the reader to consult the GPC in its complete version.
- -
The Ramsey Sedation Scale (RSS): Scale composed of 6 domains that evaluate the degree of agitation, anxiety and response to physical or auditory stimulus. For this instrument, a high score identifies patients who are under the effects of sedation, since a low score is obtained when the patient is anxious.18 The psychometric properties of this scale were evaluated in 13 studies of which 12 were cohort studies and 1 was a controlled clinical trial, giving a total of 701 participants.
For the most part, studies used two evaluators including health professionals with different training profiles, among them professional nurses, physiotherapists, postgraduate students and specialist physicians. None of the retrieved studies assessed the internal consistency. 7 studies, however, evaluated reliability. The reliability ranged from 0.94 to 0.28 (p≤.001) using the k-statistic and from 0.93 to 0.85 when using weighted-k (p≤.001).
The validity of the scale was analyzed using different statistics and showed good correlation. When the Pearson correlation coefficient was used, the coefficient ranged from 0.79 to 0.91 (p≤0.01). This is compared to the Harris Scale, the Newcastle Scale, the Auditory Evoked Potentials (AEP), Sedation-Agitation Scale (SAS), Richmond Agitation Sedation Scale (RASS) and Glasgow Coma Scale (GCS). When compared to the use of auditory potentials evoked using the Kendall's tau coefficient, a positive correlation of 0.71 (p≤0.05) was obtained. This finding was like that observed in the Vancouver Interaction and Calmness Scale (VICS), SEDIC (Spanish Society of Documentation and Scientific Information) and the Visual Analog Scale (VAS) scales in which a Spearman's correlation coefficient was obtained of 0.68–0.77 (p≤0.01). When compared with the bispectral index and the Observer's Assessment of Alertness and Sedation (O/AAS) scale, a negative correlation was observed using Pearson correlation coefficient with a score between −0.62 to −0.89 (p≤0.01). The utility of the instrument was evaluated using a 10-point Likert scale applied to nurses assigned to the intensive care unit with a mean of 4.67–6.11 points.
Quality of evidence low because of limitations in sensitivity to change and utility.
- -
Richmond Agitation Sedation Scale (RASS): Scale composed of 10 items that evaluate the response to physical and auditory stimuli (+4 to −5). Positive scores identify restless patients and negative scores identify sedated patients.18 The psychometric properties of this scale were evaluated by 7 cohort studies, for a total of 579 participants. The staff who participated in the studies were clinical experts, general practitioners, postgraduate students and professional nurses.
Internal consistency was reported by two studies.18 The first one evaluated the validity of the criterion using 411 paired observations in the first 96 participants, a satisfactory internal consistency was observed even for different levels of consciousness (p≤0.001). The second study also documented an acceptable internal consistency between the domains that conform this tool, reporting a Cronbach's α of 0.770. The reliability of the instrument was analyzed in 6 studies in which the instrument was applied by nurses or physicians in the ICU; a positive inter-rater agreement was documented with a Pearson correlation coefficient of between 0.86 and 0.973 with a non-significant variance between groups (F=0.18, p=0.57).
To evaluate the validity of the scale it was compared to the Sedation-Agitation Scale (SAS), the Glasgow Coma Scale (GCS), the bispectral index and the Visual Analog Scale. The retrieved studies showed a positive correlation with a Pearson correlation coefficient of between 0.63 and 0.92 (p≤0.001). When compared to The Ramsey Sedation Scale (RSS) conflicting results were found with a Pearson correlation coefficient of between −0.78 and 0.79. For the construct validity, the scale was evaluated against the instrument “Attention screening examination tool”, and a positive correlation was documented with a Pearson correlation coefficient of 0.78 (p≤.001). The same is true when it is compared against the RSS, in which a Spearman's correlation coefficient of 0.98 (p≤0.001) was obtained.
Only one of the retrieved studies evaluated the sensitivity to change using a variation in the medication dose variation at 8h. A negative correlation was observed, documenting a Pearson correlation coefficient of −0.31 (p≤.001). Finally, the utility of the instrument was evaluated using a 10-point Likert scale with a mean of 7.72–8.40 points.
The quality of evidence was low because of limitations in validity and sensitivity to change.
| Recommendation | Summary |
| Strong for | It is recommended that professionals who administer sedation outside the operating room evaluate depth levels to improve the quality, safety and satisfaction of the patient led to sedation, based on the parameters established in the scales. Low quality evidence ⊕⊕○○ |
| Strong for | The use of the Ramsey Sedation Scale (RSS) is recommended as the first alternative to evaluate the depth of sedation in patients outside the operating room. Low quality evidence ⊕⊕○○ |
| Weak for | The use of the Richmond Agitation Sedation Scale (RASS) is recommended as an alternative to the RSS for the evaluation of the depth of sedation in patients outside the operating room. Low quality evidence ⊕⊕○○ |
A systematic review of the literature, with an AMSTAR score of 8/11,19 evaluated the effectiveness and safety of bispectral index (BIS) monitoring in patients requiring sedation during endoscopy of the digestive tract.
10 controlled clinical trials were retrieved for a total of 1039 participants that evaluated the intervention of interest. In eight of the studies included in the systematic review, sedation was administered with propofol alone or in combination with other sedatives (midazolam, meperidine, remifentanil and fentanyl). The procedures performed in the 10 studies were endoscopic retrograde cholangiopancreatography, endoscopic submucosal dissection, endoscopic ultrasound, and colonoscopy. Five studies report that sedation was administered by a nurse specialized in anesthesiology, two by an anesthesiologist, one by a non-anesthesiologist physician, and two were not specified.
When comparing sedation level surveillance with the Modified Observer's Assessment of Alertness/Sedation (MOAAS) scale, the group of patients assigned to BIS monitoring experienced a lower total consumption of propofol (SMD −0.15mg/kg/H; 95% CI −0.28 to −0.01), but this was not reflected in a shorter recovery time (SMD 0.14min, 95% CI −0.07 to 0.36) or in a shorter duration of the procedure (SMD 0.13min; 95% CI −0.03 to 0.29). As for adverse effects, the use of BIS did not reduce the frequency of episodes of desaturation (OR 0.79, 95% CI 0.51–1.24) or the number of episodes of hypotension (OR 0.95, CI 95% 0.51–1.77) or bradycardia (OR 0.31, 95% CI 0.09–1.06). Finally, the use of BIS also did not increase patient (SMD 0.03, 95% CI −0.23 to 0.29) or physician (SMD 0.19, 95% CI −0.18 to 0.55) satisfaction.
Very low quality of evidence due to limitations in accuracy and consistency of results.
IV. Which are the safest and most effective pharmacological interventions?a. Nitrous oxide versus traditional sedation agentsA systematic review of the literature (AMSTAR 8/11)20 evaluated the safety and effectiveness of nitrous oxide administration for sedation of patients outside the operating room. We retrieved 7 controlled clinical trials for 507 adult patients. When compared to the traditional sedatives group (midazolam plus meperidine or ketobemidone, propofol or meperidine), participants who received nitrous oxide experienced a similar possibility of successfully completing the procedure (OR 0.58, 95% CI 0.25–1.33) and hypotension episodes (OR 0.45, 95% CI 0.08–2.65), but a lower frequency of episodes of hypoxemia (0% vs 21% with midazolam plus meperidine, p=0.01) and a shorter recovery time [28min vs. 51 with midazolam plus fentanyl (MD −23min 95% CI −28.6 to −17.4)], not so when compared to propofol [28min vs. 28min; p=0.86].
The quality of the evidence was very low due to limitations in the risk of bias and in the accuracy of the results.
b. Propofol versus traditional agentsA systematic review of the literature (AMSTAR score 7/11),21 evaluated the effectiveness and safety of sedation outside the operating room with propofol. 22 clinical trials were retrieved with 1798 patients scheduled for endoscopy of the upper gastrointestinal tract, ERCP or colonoscopy. When compared to the use of traditional agents (midazolam, meperidine, scopolamine, fentanyl or pentazocine), patients assigned to the propofol group had a similar frequency of hypoxemia (OR 0.79, 95% CI 0.58–1.09), hypotension (OR 1.46, 95% CI 0.92–2.31), apnea (OR 0.60, 95% CI 0.27–1.32) and duration of the procedure (MD 0, 37, 95% CI −0.04 to 0.78). However, those who received propofol did experience a shorter recovery time (MD −19.8min, 95% CI −27.7 to −11.9), a better level of sedation (OR 4.78, 95% CI 2.56–8.9) and greater satisfaction with the care received (OR 1.55, 95% CI 0.36–6.57).
The quality of the evidence was low because of some limitations in the risk of bias, precision and consistency of results.
c. Propofol against other traditional agentsA systematic review of the literature, with AMSTAR score 8/11,22 evaluated the safety and effectiveness of sedation of patients with propofol alone or in combination with other agents. The review recovered 10 studies with 818 patients in emergency rooms undergoing cardioversion and orthopedic procedures.
- i.
Propofol plus fentanyl versus ketamine plus midazolam
A quasi-experimental controlled clinical trial,23 analyzed the effectiveness and safety of propofol plus fentanyl for sedation of 113 patients in the emergency room. Compared with the administration of ketamine and midazolam, patients receiving propofol plus fentanyl had shorter recovery (MD −33.4min, 95% CI −26.1 to −40.8) at the expense of more frequent episodes of hypoxemia (OR 5.49 95% CI 1.72–17.49). There was no difference in the frequency of apnea (0/59 for propofol plus fentanyl versus 0/54 for the ketamine and midazolam group, p=1.0), agitation (OR 8.09, 95% CI 0.41–160.27), laryngospasm (OR 0.36; 95% CI 0.01–8.97) or vomiting (OR 5.67; 95% CI 0.27–120.73).
The quality of the evidence was very low due to limitations in the risk of bias, the precision of the results and the applicability of the evidence.
- ii.
Propofol versus midazolam combined with fentanyl.
A randomized controlled clinical trial24 evaluated the safety and effectiveness of propofol sedation in 86 patients in the emergency room.
When compared with the combined use of midazolam and fentanyl, patients assigned to propofol sedation reported a shorter recovery time (MD −21.7min, 95% CI −28.7 to −14.7), without this being reflected in a higher rate of successful procedures (RR 4.03, 95% CI 0.40–40.38), episodes of hypotension (PD 2.6%, 95% CI −4.8% to 10.1%), hypoxemia (PD 3.1%, 95% CI −9.9% to 16%), apnea (OR 1.58, 95% CI 0.53–4.77) or minor adverse events (PD 2.1%, 95% CI 4.3–8.5%).
The quality of the evidence was very low because of limitations in the risk of bias and the accuracy of the results.
- iii.
Propofol versus ketamine
A controlled clinical trial25 evaluated the effectiveness and safety of sedation in 97 patients with propofol in the emergency room. When compared to the administration of ketamine, sedation with propofol outside the operating room was associated with a similar frequency of hypotension (OR 0.94, 95% CI 0.13–6.94), apnea (OR 1.60; 95% CI 0.71–3.57) and hypoxemia (OR 1.11, 95% CI 0.34–3.59). However, patients assigned to the propofol group did report a shorter recovery time (MD −9.38min, 95% CI −13.48 to −5.28) and a lower frequency of agitation during recovery (OR 0, 15; 95% CI 0.05–0.50).
The quality of the evidence was very low because of some limitations in the risk of bias and the accuracy of the results.
A systematic review of the literature, AMSTAR score 7/1126 assessed the safety of the use of propofol combined with other agents in patients outside the operating room. We retrieved 9 controlled clinical trials that recruited a population older than 17 years undergoing endoscopic ultrasonography, ERCP or colonoscopy outside the operating room. Of these 9 studies, 5 of them, with 999 participants, compared the use of propofol combined with traditional agents (midazolam, midazolam with ketamine and pentazocine or remifentanil) against the administration of propofol as a single agent. When compared to propofol monotherapy, the use of propofol in combination with other traditional agents did not increase the frequency of hypoxemia (RR 0.93, 95% CI 0.30–2.92), hypotension (RR 1.32, 95% CI 0.30–2.92), apnea (RR 2.81, 95% CI 0.27–29.07) or episodes of cardiac arrhythmias (RR 2.61, 95% CI 0.23–29.99).
The quality of the evidence was very low due to some limitations in the consistency and precision of the results.
e. Dexmedetomidine versus MidazolamA systematic review of the literature, with an AMSTAR score of 5/11,27 estimated the safety and effectiveness of the use of dexmedetomidine for sedation outside the operating room. We retrieved 9 controlled clinical trials with 469 participants, who underwent endoscopy of the upper gastrointestinal tract, colonoscopy, ERCP or endoscopic submucosal dissection.
When compared to the use of midazolam alone or in combination with other traditional agents, patients assigned to receive dexmedetomidine experienced a lower frequency of suspension of the procedure (OR 0.07, 95% CI 0.01–0.45), and a better level of sedation (standardized mean difference [SMD] 0.40 points, 95% CI, 0.11–0.69 on the Ramsay scale). However, no difference was noted in the frequency of hypoxemia (OR 0.45, 95% CI 0.10–2.11), hypotension (OR 1.37, 95% CI 0.52–3.64) Or in the recovery time (MD −2.5min, 95% CI −7.3 to 2.3).
The quality of the evidence was very low due to limitations in the risk of bias, precision and inconsistency of the results.
f. Oral agentsA systematic review of the literature, with AMSTAR 10/11 score,28 analyzed the safety and effectiveness of the use of oral sedatives in patients undergoing minor dental procedures. 9 of 36 studies retrieved in the review used oral midazolam, nitrous oxide, chloral hydrate and meperidine.
- i.
Oral midazolam versus placeb.
Five controlled clinical trials with 182 participants estimated the effectiveness and safety of the use of oral or nasal midazolam for sedation of patients 4–10 years of age in dental procedures. Compared with placebo, patients assigned to the midazolam group performed better during the procedure (MD 2.98, 95% CI 1.58–4.37), at the expense of a higher frequency of amnesia episodes (RR 4.17 95% CI 2.07–8.37).
The quality of the evidence was very low because of some limitations in the risk of bias, the precision of the results and the applicability of the evidence.
- ii.
Oral chloral hydrate
A controlled clinical trial of 60 patients assessed sedation with chloral hydrate in patients younger than 10 years of age during dental procedures. Compared with placebo, patients who received chloral hydrate did not perform better during the procedure (RR 1.33, 95% CI 0.80–2.22), but did present a higher frequency of episodes of airway obstruction at a dosage of 60mg/kg chloral hydrate (27%).
The quality of the evidence was very low due to some limitations in the risk of bias, the precision of the results and the applicability of the evidence.
Summary: recommendations of clinical practice guidelines.
| Recommendation | Summary |
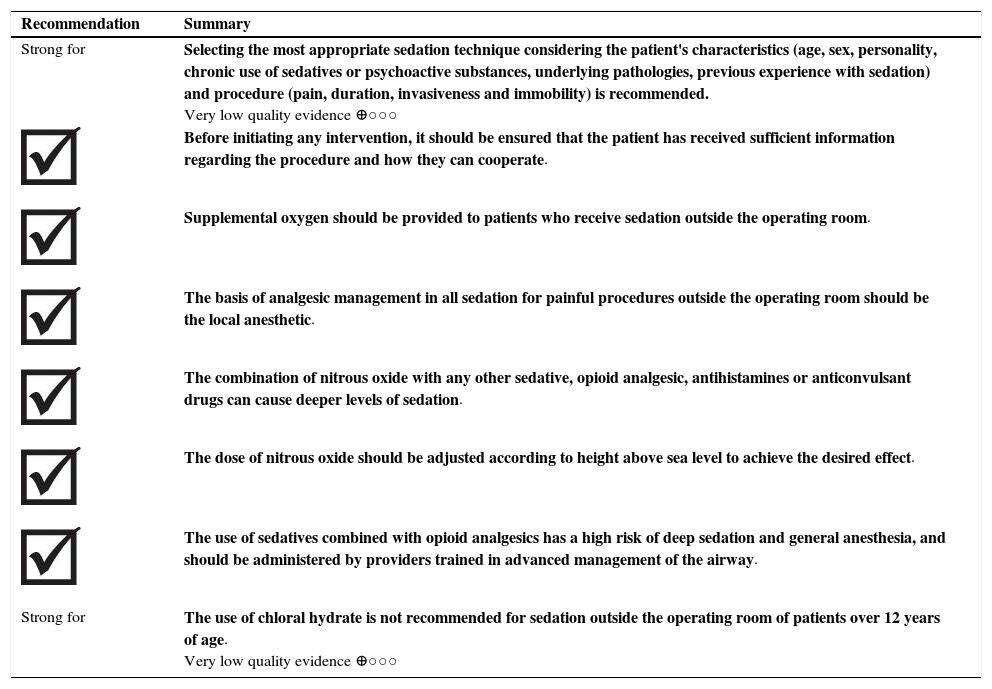
| Strong for | Selecting the most appropriate sedation technique considering the patient's characteristics (age, sex, personality, chronic use of sedatives or psychoactive substances, underlying pathologies, previous experience with sedation) and procedure (pain, duration, invasiveness and immobility) is recommended. Very low quality evidence ⊕○○○ |
| Before initiating any intervention, it should be ensured that the patient has received sufficient information regarding the procedure and how they can cooperate. | |
| Supplemental oxygen should be provided to patients who receive sedation outside the operating room. | |
| The basis of analgesic management in all sedation for painful procedures outside the operating room should be the local anesthetic. | |
| The combination of nitrous oxide with any other sedative, opioid analgesic, antihistamines or anticonvulsant drugs can cause deeper levels of sedation. | |
| The dose of nitrous oxide should be adjusted according to height above sea level to achieve the desired effect. | |
| The use of sedatives combined with opioid analgesics has a high risk of deep sedation and general anesthesia, and should be administered by providers trained in advanced management of the airway. | |
| Strong for | The use of chloral hydrate is not recommended for sedation outside the operating room of patients over 12 years of age. Very low quality evidence ⊕○○○ |
Clinical scenario: endoscopic procedures (gastroenterology, gynecology, pneumology, urology, etc.).
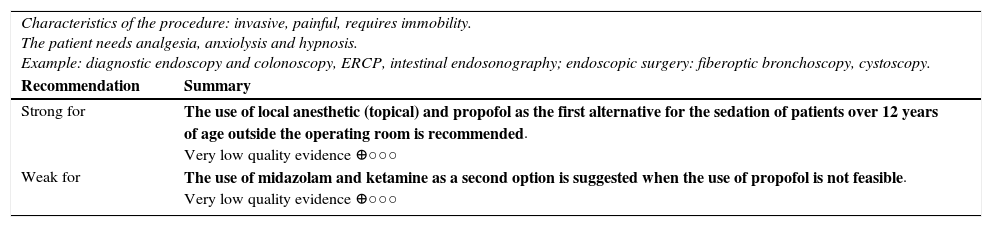
| Characteristics of the procedure: invasive, painful, requires immobility. The patient needs analgesia, anxiolysis and hypnosis. Example: diagnostic endoscopy and colonoscopy, ERCP, intestinal endosonography; endoscopic surgery: fiberoptic bronchoscopy, cystoscopy. | |
| Recommendation | Summary |
| Strong for | The use of local anesthetic (topical) and propofol as the first alternative for the sedation of patients over 12 years of age outside the operating room is recommended. Very low quality evidence ⊕○○○ |
| Weak for | The use of midazolam and ketamine as a second option is suggested when the use of propofol is not feasible. Very low quality evidence ⊕○○○ |
Clinical scenario: dental procedures.
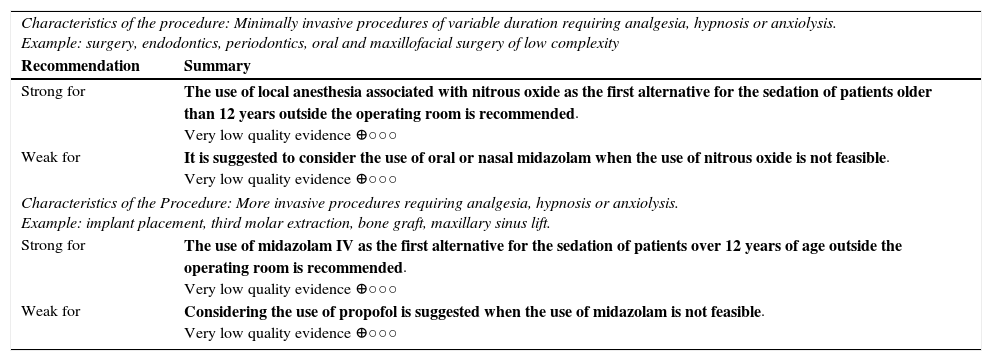
| Characteristics of the procedure: Minimally invasive procedures of variable duration requiring analgesia, hypnosis or anxiolysis. Example: surgery, endodontics, periodontics, oral and maxillofacial surgery of low complexity | |
| Recommendation | Summary |
| Strong for | The use of local anesthesia associated with nitrous oxide as the first alternative for the sedation of patients older than 12 years outside the operating room is recommended. Very low quality evidence ⊕○○○ |
| Weak for | It is suggested to consider the use of oral or nasal midazolam when the use of nitrous oxide is not feasible. Very low quality evidence ⊕○○○ |
| Characteristics of the Procedure: More invasive procedures requiring analgesia, hypnosis or anxiolysis. Example: implant placement, third molar extraction, bone graft, maxillary sinus lift. | |
| Strong for | The use of midazolam IV as the first alternative for the sedation of patients over 12 years of age outside the operating room is recommended. Very low quality evidence ⊕○○○ |
| Weak for | Considering the use of propofol is suggested when the use of midazolam is not feasible. Very low quality evidence ⊕○○○ |
Clinical Scenario: Diagnostic Imaging and Interventional Radiology.
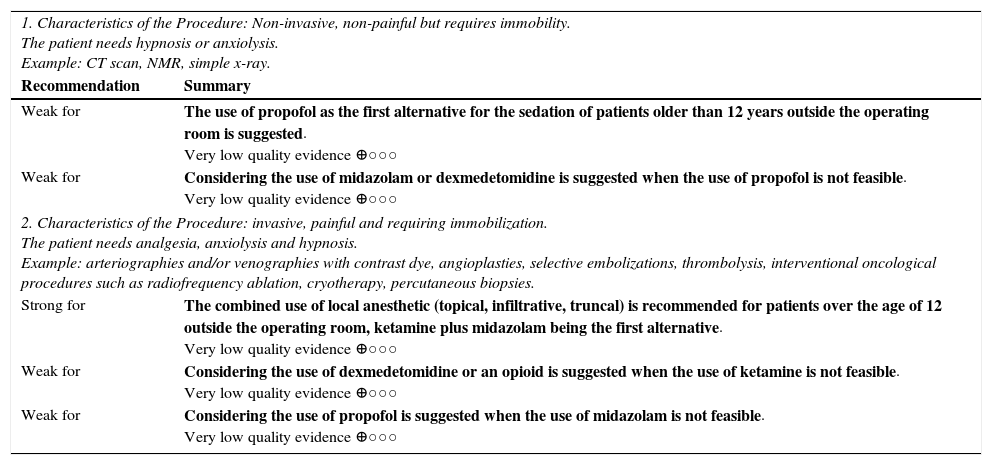
| 1. Characteristics of the Procedure: Non-invasive, non-painful but requires immobility. The patient needs hypnosis or anxiolysis. Example: CT scan, NMR, simple x-ray. | |
| Recommendation | Summary |
| Weak for | The use of propofol as the first alternative for the sedation of patients older than 12 years outside the operating room is suggested. Very low quality evidence ⊕○○○ |
| Weak for | Considering the use of midazolam or dexmedetomidine is suggested when the use of propofol is not feasible. Very low quality evidence ⊕○○○ |
| 2. Characteristics of the Procedure: invasive, painful and requiring immobilization. The patient needs analgesia, anxiolysis and hypnosis. Example: arteriographies and/or venographies with contrast dye, angioplasties, selective embolizations, thrombolysis, interventional oncological procedures such as radiofrequency ablation, cryotherapy, percutaneous biopsies. | |
| Strong for | The combined use of local anesthetic (topical, infiltrative, truncal) is recommended for patients over the age of 12 outside the operating room, ketamine plus midazolam being the first alternative. Very low quality evidence ⊕○○○ |
| Weak for | Considering the use of dexmedetomidine or an opioid is suggested when the use of ketamine is not feasible. Very low quality evidence ⊕○○○ |
| Weak for | Considering the use of propofol is suggested when the use of midazolam is not feasible. Very low quality evidence ⊕○○○ |
Clinical scenario: emergency services.
| Characteristics of the Procedure: invasive, painful of moderate to severe intensity and of short duration. The patient needs analgesia and anxiolysis. Example: fracture reduction, skin sutures, cardioversion, abscess drainage, thoracostomies, central venous accesses, washes and cures, etc. | |
| Recommendation | Summary |
| Strong for | The use of local anesthetic (topical, infiltrative, truncal) and ketamine plus midazolam as a first alternative is recommended for the sedation of patients over the age of 12 outside the operating room. Very low quality evidence ⊕○○○ |
| Weak for | Considering the use of propofol is suggested when the use of midazolam is not feasible. Very low quality evidence ⊕○○○ |
| Weak for | Considering the use of nitrous oxide or of a short-acting opioid is suggested when the use of ketamine is not feasible. Very low quality evidence ⊕○○○ |
The systematic search in the literature did not identify reviews that addressed the clinical question. Nevertheless, one controlled, non-randomized clinical trial16 with 220 participants was retrieved in which the decision of discharge based on the modified Post-Anesthetic Discharge Scoring System (PADSS) score was compared to the use of clinical criteria in patients from 18 to 75 years of age that received sedation for colonoscopies performed outside the operating room. The study recruited patients that were administered meperidine (40–60mg) and midazolam (2–5mg).
After the colonoscopy, the patients were moved to a recovery room where their clinical condition was evaluated every 20min until they were discharged. In the first 110 participants, the discharge decision was made when blood pressure, heart rate and SaO2 remained stable. The next 110 patients were discharged when they received two consecutive evaluations with a PDSS score ≥9.
When compared against clinical criteria, the use of the modified PADDS instrument was associated with a shorter recovery time (MD −36.4min; 95% CI −40.6 to −32.2). In the PADDS group, 37.2% of the participants were discharged within 60min; a finding that did not occur in any patient of the clinical criteria group (p=0.001). In terms of the frequency of adverse events, no complications or cases of hospitalization were documented. The patients assigned to the modified PADDS reported a lower frequency of drowsiness, weakness, abdominal distension and headache (RR 0.59; 95% CI 0.43–0.81).
Very low quality of evidence due to limitations in the risk of bias and the accuracy of the results.
| Recommendation | Summary |
| Weak for | The use of the PADDS instrument is suggested as an evaluation tool for patients requiring sedation outside the operating room in order to reduce recovery time and the frequency of side effects. Very low quality evidence ⊕○○○ |
| The post-sedation recovery site may be the same location in which the procedure was performed and should have access to oxygen, suction, appropriate monitoring, and resuscitation equipment. | |
| At the time of discharge, patients’ vital signs should have returned to their normal state, the patient should be alert with the ability to walk, and nausea, vomiting and pain should have been properly controlled. | |
The search did not produce reviews that addressed the clinical question. Expert recommendations strongly support assessment and monitoring or respiratory, cardiovascular and neuromuscular function, along with mental state, temperature, pain and the presence of nausea and vomiting before deciding on discharge.
All patients should be accompanied by a responsible person. Written recommendations for posterior care and alarm signs should be given to the patient.9
Curriculum recommendations about the competencies that the professional administering sedation to patients older than 12 years of age should have are published in another paper that can be consulted here.29
Clinical practice guideline development groupClaudia Cecilia Burbano-Paredes. Surgical physician from the Universidad Cooperativa; Anesthesiologist from the Universidad del Rosario.
Adriana María Rubiano-Pinzón. Surgical physician from the Pontificia Universidad Javeriana; Anesthesiologist from the Pontificia Universidad Javeriana.
Ángela Constanza Hernández-Caicedo. Surgical physician from the Universidad del Rosario; Anesthesiologist from the Universidad del Rosario.
Jairo Amaya-Guio. Surgical physician; Specialist in Obstetrics and Gynecology; Specialist in Epidemiology; Full Professor, Faculty of Medicine, Universidad Nacional de Colombia.
Carlos Fernando Grillo-Ardila. Surgical physician; Specialist in Obstetrics and Gynecology; Master of Epidemiology; Assistant Professor, Faculty of Medicine, Universidad Nacional de Colombia.
Martín Cañón-Muñoz. Surgical physician; Specialist in Family Medicine; Master of Clinical Epidemiology.
Diana María Ángel-Betancur. Surgical physician; Specialist in Pediatrics; Master of Epidemiology (cand), Universidad Nacional de Colombia.
Management TeamLuz María Gómez. Surgical physician; Anesthesiologist; Scientific Assistant Director of the S.C.A.R.E. (Colombian Society of Anesthesiology and Resuscitation).
Javier Eslava-Schmalbach. Surgical physician; Anesthesiologist; MSc in Epidemiology; PhD in Public Health; Manager of the S.C.A.R.E. Technological Development Center.
Literature Search TeamGrupo Cochrane STI. Bogotá, Colombia.
Patient RepresentativeMercy Yolima Martínez-Velásquez.
Peer ReviewersAna Marcela Torres-Amaya. Pharmaceutical chemist; Master of Clinical Epidemiology; Managing Editor Cochrane Sexually Transmitted Infections Group; Universidad Nacional de Colombia.
Liliana Suárez-Aguilar. Surgical physician; Specialist in Anesthesiology from the Universidad el Bosque FSFB; Specialist in University Teaching from the Universidad el Bosque.
Scientific Societies| Jorge Ernesto Rincón-Aguilar. Oral and Maxillofacial Surgeon. Pontificia Universidad Javeriana, Universidad del Bosque. | Colombian Society of Oral and Maxillofacial Surgery |
| Luz Ángela Moreno. Surgical physician; Specialist in Radiology; Master of Educación; Full Professor, Faculty of Medicine, Universidad Nacional de Colombia. Radiologist, Hospital Fundación de la Misericordia. | Colombian Association of Radiology |
| Mario Humberto Rey-Tovar. Surgical physican, Specialist in Gastroenterology-Digestive Endoscopy, Universidad del Rosario. | Colombian Association of Gastroenterology |
| Francisco José Hernández-Restrepo. Dentist and Specialist in Pediatric Dentistry from the Pontificia Universidad Javeriana. | Colombian Academy of Pediatric Dentistry |
| Claudia Paola Martínez-Rebolledo. Surgical physician, Specialist in Emergency Medicine from the Universidad del Rosario-Fundación Santafé de Bogotá. Instructor at the Universidad de los Andes. | |
| Colombian Association of Specialists in Emergency Medicine | |
| Elvia Karina Grillo-Ardila. Dentist from the Universidad Nacional de Colombia; Specialist in Pediatric Dentistry from the Universidad del Bosque. Associate Professor, Universidad Antonio Nariño. | Ethics and Research |
| Daniel Cortés-Díaz. Surgical physician; Specialist in Obstetrics and Gynecology; Specialist in Development Projects; Master of Gender Studies. Associate Professor, Faculty of Medicine, Universidad Nacional de Colombia. | Colombian Federation of Obstetrics and Gynecology |
| Lina María González-Gordon. Research Advisor, S.C.A.R.E. | Colombian Society of Anesthesiology and Resuscitation (S.C.A.R.E.) |
The Colombian Society of Anesthesiology and Resuscitation provided financial support during the production of this guideline, so recommendations and opinions expressed in this article are those of the authors and do not necessarily reflect the S.C.A.R.E.’s ones. The members of the development team, as well as health professionals participating as experts and external reviewers, signed a declaration of conflicts of interest.
FinancingThe development of the entirety of these CPG was financed exclusively by the Colombian Society of Anesthesiology and Resuscitation (S.C.A.R.E.). This organization did not influence the content, which was produced independently by the Guideline Development Group (GDG).
Conflict of interestAll of the members of the Development Group, as well as those who participated as experts or as external reviewers, previously declared in writing their conflicts of interest related to the production of this guideline. They declared that they were not involved as researchers in clinical trials in progress on this topic, that they did not receive donations or benefits from groups interested in the declarations, and that they do not pertain to professional groups with conflicts of interest.
Sedation is a practice frequently used for performing invasive and non-invasive medical and dental procedures, such as imaging, invasive radiology, cardiac catheterization, and endoscopy1.
A development group was formed with the participation of Anesthesiologists, Maxillofacial Surgeons, Emergency Specialists, Gastroenterologists, Gynecologists, Dentists, Pediatricians, Radiologists, Epidemiologists, experts in medical education, educational computing and Public Health. We worked with a patient who had undergone sedation outside the operating room for a therapeutic orthopedic procedure. She was fully aware of the condition and voluntarily agreed to the development of this guide. We obtained her consent through prior explanation and objective and adequate communication. The patient delivered feedback on the documents regarding scope and objectives, PICO questions and the final draft of the guide.
The guide does not consider critically ill patients requiring mechanical ventilation; patients requiring palliative care; patients requiring sedation as part of their treatment for mental illness; patients requiring sedation as a prior step for the administration of general anesthesia or as postoperative analgesia; patients requiring nocturnal sedation to sleep; patients with complications or history of complications arising from the administration of sedation outside the operating room or pregnant women. The Clinical Practice Guidelines (CPG) do not address interventions related to the diagnosis or treatment of complications arising from the use or administration of sedation, nor do they address the administration of sedation in patients with special conditions or in interventions other than diagnostic or therapeutic procedures.
Following the formulation of the guiding questions, a non-formal consensus was reached with thematic experts and methodologists who generated a definitive list of interventions to be included in the guide: 1) Preparation of patients. 2) The health professional that should administer sedation. 3) Patient monitoring during sedation. 4) Administration of sedation drugs, and 5) Immediate care following the use of sedation. We identified and prioritized the clinical outcomes of safety, effectiveness, quality of life and importance for patients. Following the proposal by the GRADE group2,3 the outcomes were classified on a scale from 1 to 9 as “critical,” “important non-critical” and “not important.”
In a meeting with representatives of the sedation group and the scientific societies that accompanied the development of the guide, through non-formal consensus and following a systematic and rigorous methodology, the scope and objectives, the target population and the clinical aspects of the guidelines were communicated and consolidated.
Through a systematic search of the literature in electronic databases of fifteen entities that develop clinical practice guidelines, in electronic repositories and in non-specialized search engines, 14 Colombian and international CPGs susceptible for adaptation were identified. Each of the identified guides was evaluated as adaptable independently by two methodological experts using a screening tool4. The final grade was the result of a consensus process by both evaluators.
A non-formal consensus meeting was held with the development team to determine if it was feasible to adapt one of the identified guides or develop a novo. Based on the results of the screening tool, it was considered that there was no guide to be submitted to a process of adaptation and incorporation of its evidence. Therefore, it was decided that the guide would be developeda novo. For the questions to be developed in the denovo guide, a search for systematic reviews, without language restrictions, was performed in the Cochrane Database of Systematic Reviews (CDRS), MEDLINE, EMBASE and LILACS and in the Colombian Journal of Anesthesiology, using a format that contained MESH keywords and terms related to clinical questions5.
The systematic reviews (SR) identified were evaluated using the AMSTAR checklist6. Each systematic review was evaluated with respect to its content, quality and clinical relevance to identify those of higher methodological quality that should be included within the guide. In the case of failure to identify high-quality systematic reviews, primary studies were assessed using the risk-of-bias tool suggested by Cochrane5.
The summary of the selected evidence was performed with the help of the GRADEpro program and the levels of evidence were graded according to GRADE (High, Moderate, Low and Very Low)2,3. In order to consolidate the recommendations and grade their strength and direction based on the GRADE methodology, a workshop was held with representatives of the S.C.A.R.E., the sedation group attached to S.C.A.R.E., epidemiologists and experts in medical education and educational computing, and public health representatives of the scientific associations and patients3.
Two independent reviewers independently assessed the final version of the guide prior to publication. One reviewer was an expert in methodology and the other was a clinical expert. The final document was reviewed and approved by the guideline's developer group.
Please cite this article as: Burbano-Paredes CC, Amaya-Guio J, Rubiano-Pinzón AM, Hernández-Caicedo ÁC, Grillo-Ardila CF. Guía de práctica clínica para la administración de sedación fuera del quirófano en pacientes mayores de 12 años. Rev Colomb Anestesiol. 2017;45:224–238.
Full list at bottom.
This paper comes from the complete version of “Clinical Practice Guidelines with recommendations for the administration of sedation as part of diagnostic or therapeutic procedures outside the operating room in patients over the age of 12” which is available on the web-appendix.