To compare the cost–effectiveness of three different formulations indicated for moderate and severe acute pain, commercialized in Colombia [acetaminophen 500mg+codeine 30mg (AC), acetaminophen 500mg+hydrocodone 5mg (AH) and acetaminophen 325mg+tramadol 37.5mg (AT)].
Materials and methodsCost–effectiveness analysis using the NNT as the health outcome indicator. The costs were evaluated in two specific settings: Institutional Channel (IC), representing the cost for the Colombian Ministry of Health (SISMED 2011); Retail Channel (RC), representing consumer prices, obtained from the IMS annual average for 2011, plus an adjustment to include the average profit margin for pharmacies (10%). The incremental cost effectiveness ratios (ICER) were calculated for the three formulations and the two settings (IC and RC). The intervention values are expressed in Colombian pesos (COP).
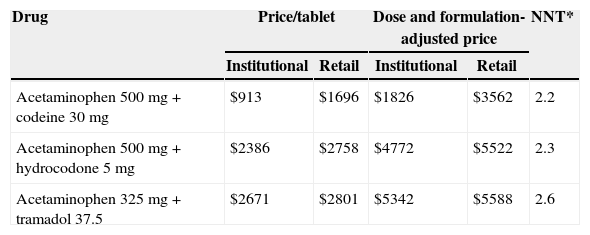
ResultsThe prices/NNT for each formulation were $1816 COP/2.2 for AC, $4772 COP/2.3 for AH and $5342/2.6 for AT. Using these data and taking AC as the comparator, the ICER for the other formulations shows the following results: in the RC, $5065 COP for AT and $19,600 COP for AH; in the IC setting, $8790 COP for AT and $29,460 COP for AH. The probabilistic sensitivity analysis demonstrated that the majority of simulation results fell between the 1st and 4th quadrants of the cost–effectiveness matrix, using AC as a reference.
ConclusionThe analysis, from the payer and patient perspectives, demonstrates that the AC formulation has a lower cost and is more effective in reducing pain within the first 4–6h after administration, compared with the AH and AT formulations in their specific indications.
Comparar diferencias en costo-efectividad de 3 formulaciones comercializadas en Colombia (acetaminofén 500 mg + codeína 30 mg [AC], acetaminofén 500 mg + hidrocodona5 mg [AH] y acetaminofén 325 mg + tramadol 37,5 mg [AT]) indicadas para el tratamiento del dolor agudo moderado-severo.
Materiales y métodosAnálisis de costo-efectividad, usando el NNT como medida de desenlace. Los costos fueron evaluados en 2 canales específicos: canal institucional (CI), representado por los costos relacionados con el medicamento consignados en SISMED 2011,y canal al por menor (CM), que representa los precios al consumidor tomados de IMS promedio anual 2011 más margen de farmacia (10%). Se calcularon las razones de costo-efectividad incremental (RCEI) para las 3 formulaciones en cada canal (CI y CM). Los valores de las intervenciones fueron expresados en pesos colombianos.
ResultadosLos precios/NNT para cada formulación fueron $1.816 COP/2,2 para AC, $4.772COP/2,3 para AH y $5.342/2,6 para AT. Con base en estos datos y tomando AC como el comparador, las RCEI para las otras formulaciones fueron: en el CM, $5.065 COP para la formulación AT y $19.600 COP para la formulación AH; en el CI, $8.790 COP para la formulación AT y$29.460 COP para la formulación AH. El análisis de sensibilidad probabilístico evidencia que las observaciones simuladas se ubican entre el primer y el cuarto cuadrante del plano de costo-efectividad, tomando como referente AC.
ConclusiónEl análisis, desde la perspectiva del tercer pagador y el paciente, permite concluir que la formulación AC tiene un menor costo y mayor efectividad para reducir el dolor en las primeras 4-6 h, comparada con AH y AT es sus indicadores específicos.
Pain from multiple causes is the most frequent complaint from patients seeking medical care. Irrespective of the cause, timely, effective and multidimensional control of acute pain is a priority for patients, practitioners and the healthcare systems alike.1 Despite the limitation of the evidence available, it is well known that adequate management of acute pain improves recovery, reduces comorbidities, and lowers the risk of the pain becoming chronic.2 Multiple options are available at the present time for the management of acute pain. Traditionally, analgesic combinations have been used to control acute and chronic pain and, increasingly, multimodal analgesia is recommended for the control of acute postoperative pain.3,4 The most recent guidelines from the American Society of Anaesthesiologists recommend the use of multimodal techniques (e.g. two or more analgesics or other synergistic drugs) in order to provide better analgesia with lower doses, thus preventing adverse events.5 The recommendations include a combination of opioids with a local anaesthetic or a medication of another pharmacologic class. Added to the above, there is evidence showing that the administration of opioid analgesics at fixed intervals is more effective for pain relief than dosing as needed. Moreover, there are dose formulations for slower drug release that offer more lasting and steady level of analgesia.
The estimated market value of analgesics in 2009 was more than $27 billion dollars in the United States, of which the share of opioids was almost 30%.6 In Colombia there are no estimates, but although the price of analgesic drugs is lower compared to other therapeutic classes, the high prevalence of acute pain and the frequent use of these medications may have an important impact on the budget of any health system.
Aside from the clinical aspects of drug effectiveness and safety, patients, clinicians and decision-makers in the healthcare system must consider practical scenarios that enable them to use the available evidence and the right context to make more appropriate decisions.7 Considering that there is growing use of opioids for the relief of moderate to severe pain,8 there is a need to determine the cost–effectiveness ratio of the different drug combinations available for the management of acute pain in Colombia. This would inform decision makers and optimize the use of health resources. Based on the above, the objective of this analysis was to compare the cost–effectiveness of: (1) acetaminophen 500mg+codeine 30mg (AC); (2) acetaminophen 500mg+hydrocodone 5mg (AH); and (3) acetaminophen 325mg+tramadol 37.5mg (AT) in the management of moderate to severe acute pain in Colombia, given that these are the drugs used most often for the management of this type of pain in this country.
Materials and methodsA cost–effectiveness analysis was conducted using the number needed to treat (NNT) as an outcome measure. The NNT is defined as the number of people needed to treat in order to avoid a specific event.9 In the case of pain control, the interpretation of this number may vary, although it is generally reported as the number needed to treat in order to achieve a predetermined percent reduction in the score reported by the patient on the Visual Analogue Scale (VAS). In order to arrive at conservative estimates and to be consistent with the published literature,9 this study used NNT outcomes that showed a reduction of at least 50% in the pain reported by the patient within 4–6h after the drug was administered, according to the Oxford Analgesic League efficacy report published in 2007.10 Therefore, the values used in the analyses correspond to data extracted from the published literature. The NNT was used as an outcome measure given that it measures benefit when the intervention has an immediate effect.11. Depending on the source,10 the NNT is constructed using a placebo group as comparator, making the estimation of the difference in effectiveness between the fixed combinations of acetaminophen and opioids similar to an indirect comparison. Given that the NNTs obtained in the literature do not describe the values used to calculate them, we had to use their value as the sole reference and calculate NNT difference among the different fixed-dose combinations.
Given that the formulations available in Colombia of the three drugs assessed in this article are not identical to those reported in the Oxford Analgesics League efficacy table,12 the following assumptions were considered in order to adjust the model to the information available:
- 1.
For acetaminophen 500mg+codeine 30mg and acetaminophen 325mg+tramadol 37.5mg, it was determined that the NNT of each tablet of these combinations is equivalent to two tablets of the same combination. For the acetaminophen+codeine combination, the Oxford Analgesia Table provides the NNT for the acetaminophen 1000mg+codeine 60mg formulation, while the formulation available in Colombia is acetaminophen 500mg+codeine 30mg, hence the need to multiply the number of tablets times two in order to be able to use the NNT.
- 2.
For the acetaminophen 500mg+hydrocodone 5mg combination, there is no information in the Oxford Analgesics League and no information was obtained from the pharmaceutical laboratory that sells the molecule in Colombia. For these reasons, and because of the evidence available in the literature, the following assumptions were considered for analysing the model:
- (a)
The level of analgesia provided by one dose of 75mg of tramadol+650mg of acetaminophen is comparable to that provided by 10mg of hydrocodone+650mg of acetaminophen.13
- (b)
Given the formulations of tramadol+acetaminophen and hydrocodone+acetaminophen marketed in Colombia, it may be said that the equivalence ratio in terms of tablets is one tablet of tramadol 37.5mg+acetaminophen 325mg to one tablet of hydrocodone 5mg+acetaminophen 500. The equianalgesia assumption for these two combinations is consistent with the NNT value of the former, which ranges between 2.3 and 3.0, according to the Oxford table. In order to make cost/effectiveness comparisons between the different drugs for the hydrocodone+acetaminophen combination, the lower limit of 2.3 for that interval was used.10 This is the most conservative assumption. However, both values were used in the sensitivity analyses.
- (a)
In terms of the safety of these drugs, considering that the model was built with the purpose of evaluating their effect on the management of acute pain only, the assumption was that the probability of adverse events and the cost associated with their management using these drugs acutely are similar among the different comparators and do not affect the model. This is also a conservative assumption, considering that there are indeed differences regarding adverse effects, particularly when the use of these drugs becomes more chronic. One such example is that of hydrocodone, which has a higher risk of inducing opioid abuse and dependence.
Pain measurement Visual Analogue ScalePain intensity is quantified through the use of self-reporting scales. The most widely used among these is the Visual Analogue Scale (VAS), which measures perceived pain on a scale from 1 to 10 (0–100mm), where 1 is “absence of pain” and 10 is “the worse imaginable pain”. The VAS can be used to assess not only pain intensity but also how pain evolves. In a systematic review of 74 studies assessing the use of various tools for pain measurement and reporting, it was found that 56 (75.7%) of these studies used the VAS to measure and report changes in pain and defined as clinically relevant differences those absolute score differences between 10 and 40mm, representing a percent improvement difference of 15–50% in pain perception.14
Cost estimationBearing in mind that the highest cost associated with the management of acute pain is the price of the analgesics15 and that there is low probability of variation of other costs among the three options in this model, this analysis included only the direct costs of the drugs in order to evaluate the cost–effectiveness ratio of the combinations. Two scenarios were built around the source of pricing:
- -
Institutional Channel (IC), representing the institutional price, that is, the mean weighted price paid by Health Management Organizations and Healthcare Organizations.
- -
Retail Channel (RC), representing the retail price usually paid by the patient.
For the fist scenario (IC), the prices of AC (acetaminophen 500mg+codeine 30mg), AT (acetaminophen 325mg+tramadol 37.5mg) and AH (acetaminophen 500mg+hydrocodone 5mg) were taken from the Ministry of Health Integrated Drug Price System (SISMED) price list for 2011. For the second scenario using retail prices (RC), we used the price information per tablet from the IMS Consulting Group,16 with a twelve-month sliding average for November 2011, adjusted for a 10% pharmacy profit margin (Table 1).
Cost and effectiveness of various opioid combinations: acetaminophen–codeine, acetaminophen–hydrocodone and acetaminophen–tramadol adjusted to the Colombian market. Institution and retail price scenarios.
| Drug | Price/tablet | Dose and formulation-adjusted price | NNT* | ||
|---|---|---|---|---|---|
| Institutional | Retail | Institutional | Retail | ||
| Acetaminophen 500mg+codeine 30mg | $913 | $1696 | $1826 | $3562 | 2.2 |
| Acetaminophen 500mg+hydrocodone 5mg | $2386 | $2758 | $4772 | $5522 | 2.3 |
| Acetaminophen 325mg+tramadol 37.5 | $2671 | $2801 | $5342 | $5588 | 2.6 |
NNT: number needed to treat.
The cost–effectiveness analysis was performed using the treatment with AC as reference. This analysis is the third-party payer perspective. Given that no cost/effectiveness threshold has been defined for Colombia, acceptability curves were built with different values of willingness to pay, understanding willingness as the amount of money a patient is willing to pay in order to attain a one unit increase in the clinical effectiveness achieved with an intervention.17 To do this, incremental cost/effectiveness ratios had to be built, estimating the cost difference and the clinical effectiveness differences of the alternatives to be evaluated, to arrive at the result of how much is paid for every additional unit of clinical effectiveness.12
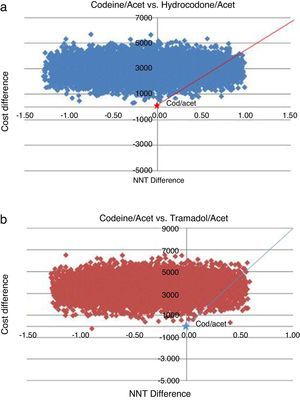
Finally, probabilistic sensitivity analyses were performed using Monte Carlo simulations with 10,000 random number repetitions derived from a normal distribution of the parameters of the model, taking the mean price values, their minimum and maximum values, and the mean NNT value, with their respective confidence intervals.
The Office® Excel 2010 software was used for the simulation. The SPSS® Version 15 was used for preparing the acceptability curves.
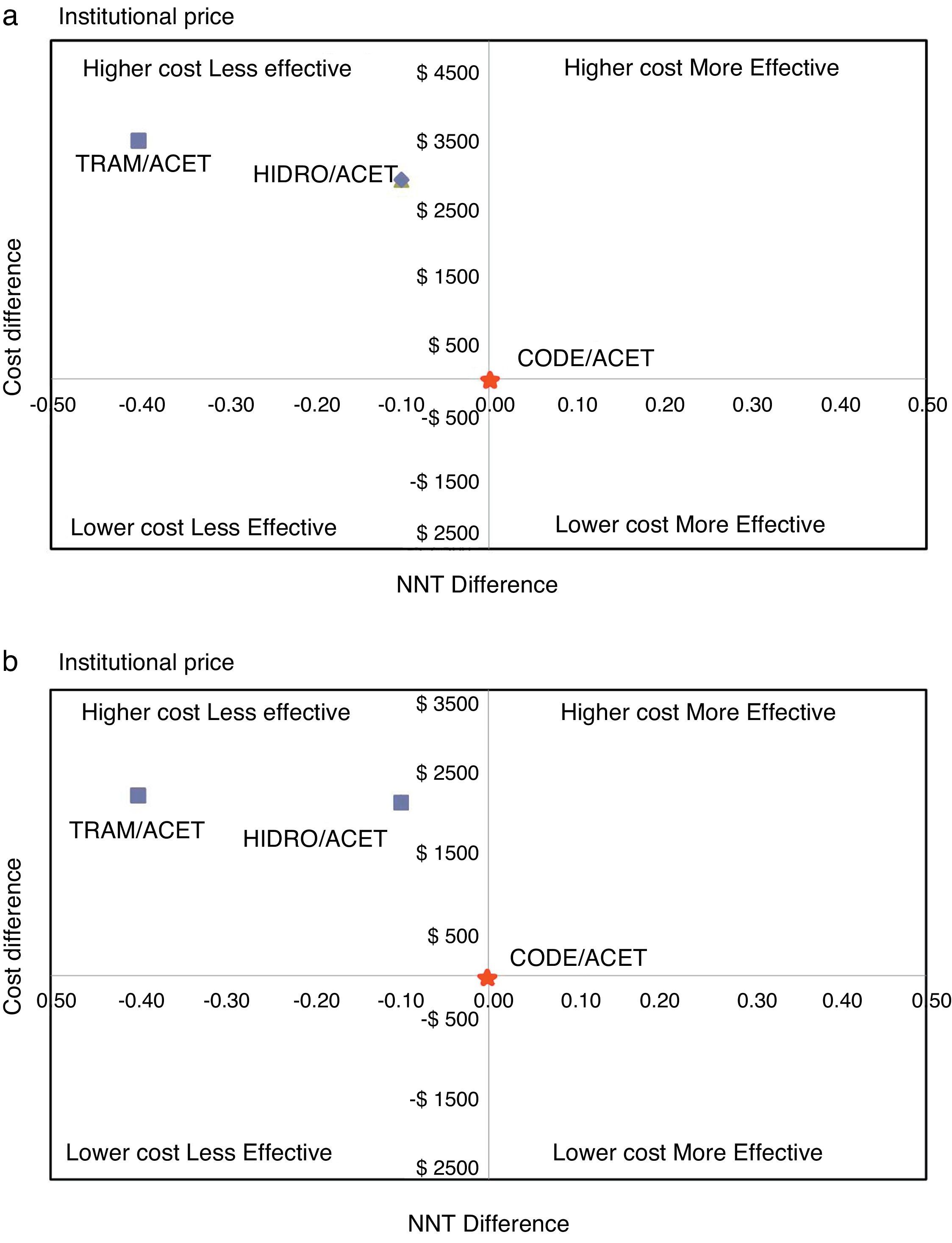
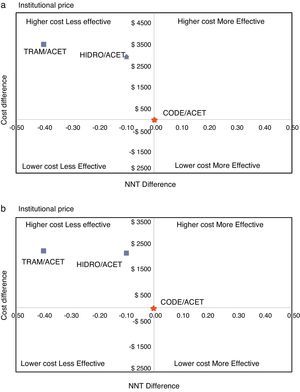
ResultsTable 1 shows the estimated cost of the treatment for each combination, and their effectiveness measured as NNT. In the first scenario (IC), using values corresponding to the mean institutional purchase price and their respective NNTs, the specific estimates for AH ($4772, NNT 2.3) and AT ($5342, NNT 2.6) showed that these combinations were more costly and less effective than AC ($1816, NNT 2.2) (Fig. 1). In the second scenario (RC), using the retail price, there was an overall increase in the price of drugs that reduced the difference among the cost–effectiveness ratios. However, the specific estimates showed that AC again emerged as the cost-effective alternative, compared to the other two alternatives (Fig. 1). Using the AC combination as the reference treatment, the incremental cost–effectiveness ratio (ICER) is the cost difference between the most costly and the least costly intervention divided by the difference in clinical effectiveness of the interventions considered: is equal to $19,600 (IC) and $29,460 (RC) Colombian pesos per dose of AH, and $5065 (IC) and $8790 (RC) for AT. Although these values appear to be relatively low, it is important to note that this is the estimate of the additional cost per dose that the patient would incur in order to increase the level of effectiveness (reduce the NNT by 0.1). In average, a patient with acute pain may require 3 daily doses and 2 or 3 days of treatment, leading to an important increase in cost differences.
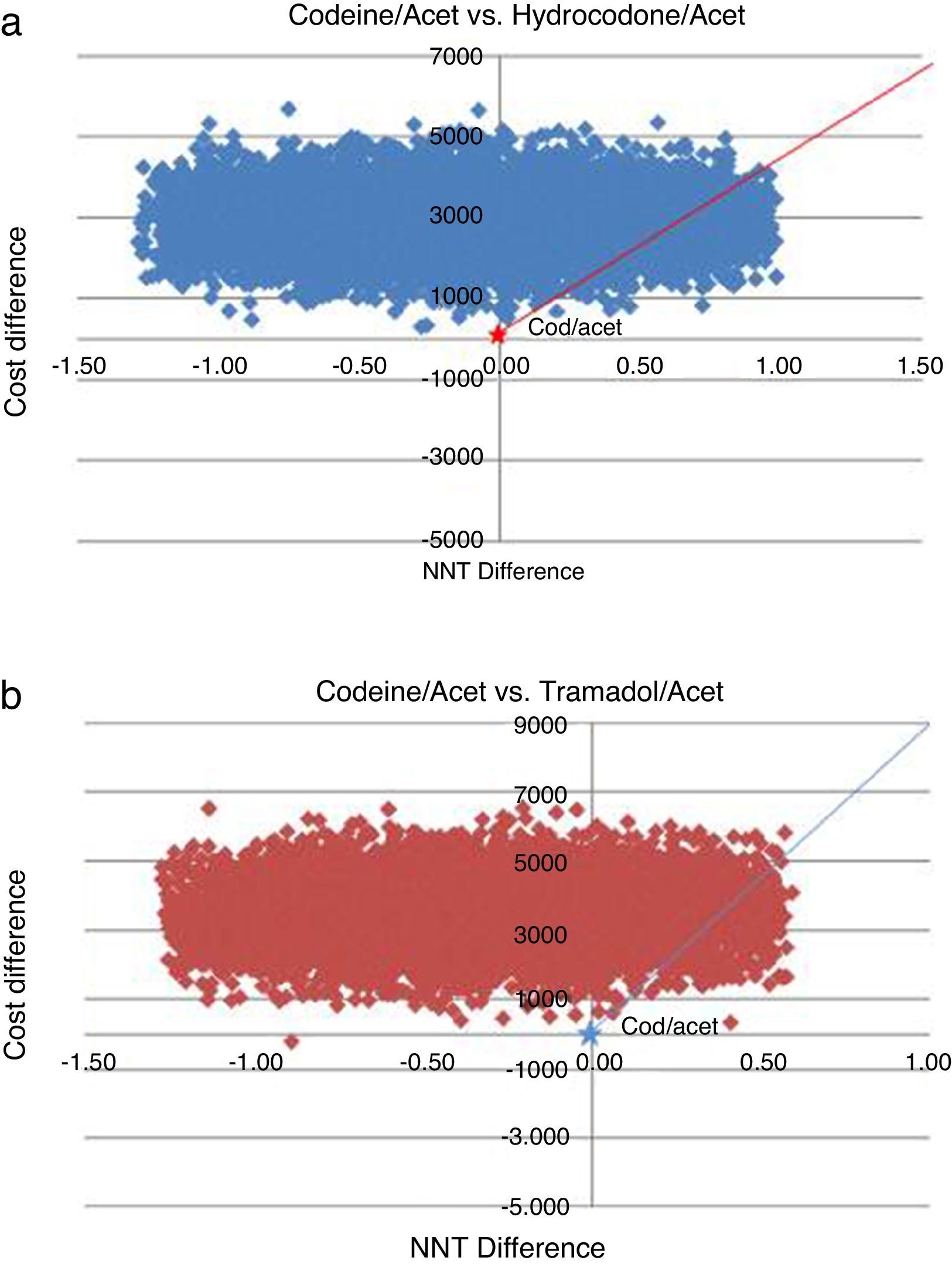
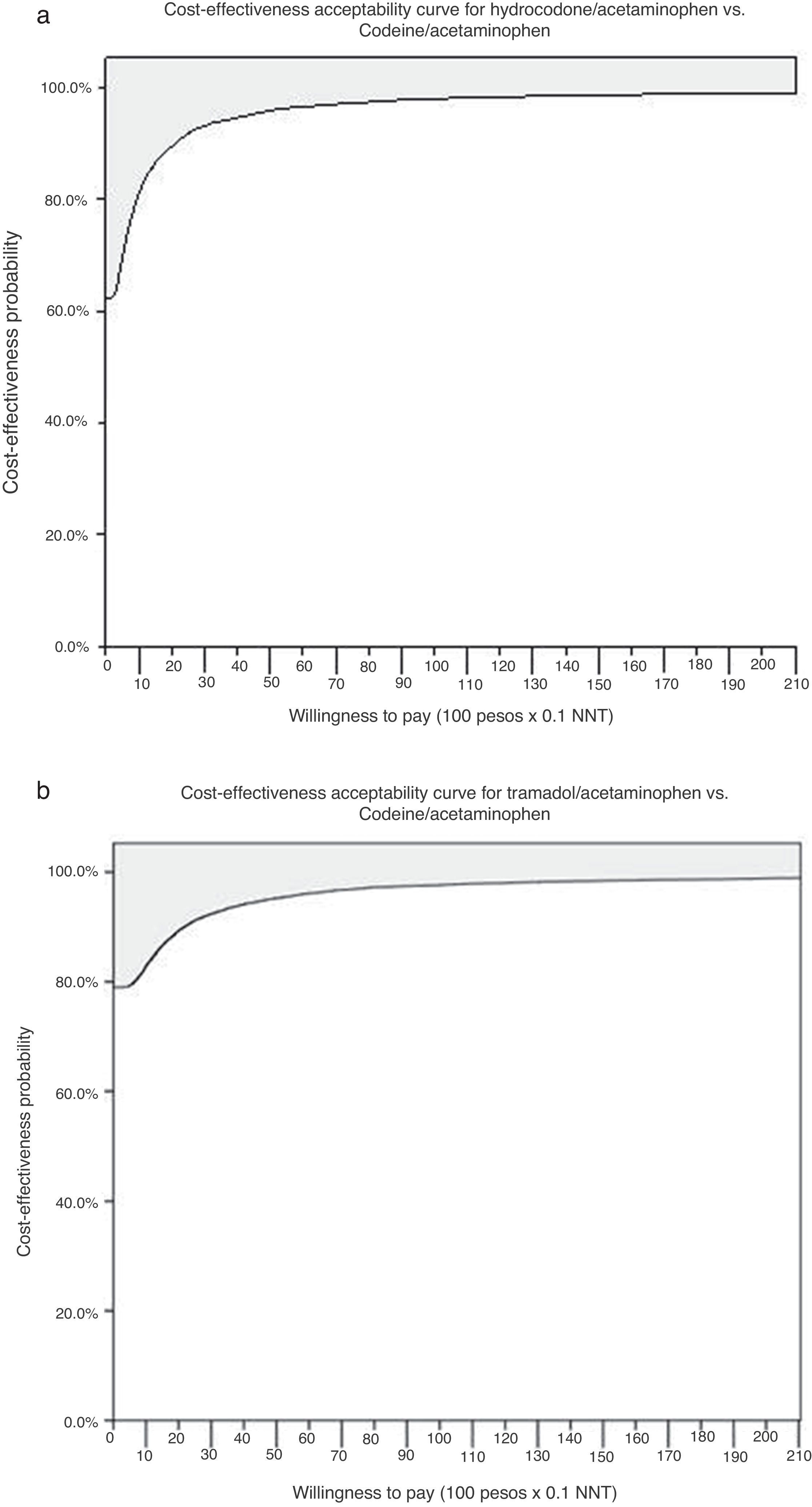
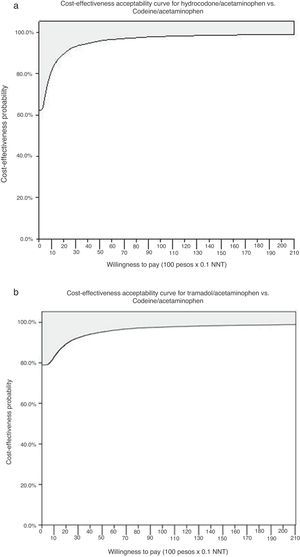
The probabilistic sensitivity analysis showed that the simulated observations fall between the first and the fourth quadrant of the cost–effectiveness plane (Figs. 1 and 2). The above is interpreted in the sense that the comparator is more costly than the reference treatment, with a relative benefit distribution from lower (fourth quadrant) to higher (first quadrant). In these circumstances, when results appear scattered over different quadrants, the recommendation is to use acceptability curves, which estimate the probability of the comparator treatment being more or less effective under a cost threshold that usually represents willingness to pay. The results show that, using AC as reference, the AH combination is more costly and less effective, in 60% of the simulations; in 40% of the remaining simulations, depending on the threshold selected in accordance with the willingness to pay to reduce the NNT by 0.1, the additional cost is $10,000 for 10%, and up to $40.000 pesos for 40% (Fig. 3). In the case of AT, using AC as reference, close to 80% of the simulations fell in the fourth quadrant, meaning that AT is a more costly and less effective alternative. In 20% of the remaining simulations, depending on the threshold selected in accordance with the willingness to pay to reduce the NNT by 0.1, the additional cost is around $15,000 pesos (Fig. 3).
Different studies have shown evidence of how the management of acute pain is underestimated. The inadequate control of pain points to the need of generating knowledge regarding the better use of analgesic drugs, with the right doses and intervals for improved therapeutic management.24
After a search in the world literature of articles on the cost–effectiveness analysis of the use of opioid combinations with acetaminophen as a fixed dose for the management of acute pain, we did not find any articles on the use of those combinations. However, there is literature comparing the use of opioids with fixed combinations, focused on showing differences in terms of effectiveness and adverse effects between the treatments included in the comparisons. This highlights the need to conduct an in-depth study of the clinical effects of fixed combinations of opioids plus acetaminophen in acute pain, and the potential financial impacts derived from the use of these medications for the patients as well as the healthcare systems.
In this cost–effectiveness analysis of fixed combinations of acetaminophen and opioids for the management of moderate or severe acute pain in Colombia, from the perspective of the health system, it was found that the AC formulation has a lower cost and is more effective in reducing moderate-to-severe acute pain within the first 4–6h after the administration of a single dose, compared to AH and AT. The additional simulations performed as part of the sensitivity analysis indicate that the AC combination offers the higher probability of being cost-effective when compared to the other two options available in Colombia. It important to distinguish cost–effectiveness, which assesses the “value” of the interventions, from the budgetary impact, which determines the effect on the existing resources and the ability to pay.
The results enable us to conclude that, despite the fact that all of the combinations are potentially cost-effective depending on the cost–effectiveness threshold determined by the decision-maker, these differences may result in an important additional cost if the benefits mentioned above are to be realized. For example, if an insurer has 10,000 patients requiring one of these medications for the management of acute pain in one year, the additional cost for managing the patients and achieving the same pain reduction benefit may range between $588,000,000 (IC) and $883,800,000 (RC) for AH, and $151,950,000 (IC) and $263,700,000 (RC) for AT, using the AC combination as the reference treatment.
The results of this study have limitations that need to be considered. First, given the various causes of pain and the absence of evidence segmented by type of pain for the Colombian population, our treatment efficacy estimates are based on pain intensity categories (mild, moderate and severe) and not on the nature or origin of the pain. The assumption in this study is that adverse events for each drug are equivalent and, consequently, adverse events are not taken into consideration for the cost and effectiveness measurements. Future prospective analyses that include individual information and that may capture patient and drug clinical characteristics might overcome these limitations.
Second, some important treatment effects that may influence treatment selection beyond efficacy and reported costs may have been underestimated. A case in point is the treatment with hydrocodone, with a higher risk of leading to opioid abuse and dependence18–23 which would result in greater use of resources for the system, and a potential decline in patient quality of life in the long run, factors which have not been considered in our model.
Third, in the absence of specific information regarding the effectiveness and safety of these treatments for the Colombian population, the NNT calculation for commercial formulations available in the country are based on estimates and approximations that might affect specific results, although they were considered for the sensitivity analysis. In this regard, there is a need to conduct clinical studies in Colombia to inform about the efficacy and safety of these drugs in order to arrive at direct NNT estimates with the local formulations, as well as cost studies that allow for a comprehensive evaluation of the effect of using these drugs on the use and costs associated with other resources that were not considered in this analysis.
Ethical disclosuresProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
FundingFinanced by Sanofi-Aventis de Colombia S.A.
Conflicts of interestJuan Diego Misas is an employee of Sanofi-Aventis (Colombia).
Please cite this article as: Cristancho RA, Vecino AI, Misas JD. Evaluación de costo/efectividad de tres combinaciones fijas de acetaminofén y opioides para el manejo del dolor agudo en Colombia. Rev Colomb Anestesiol. 2015;43:87–94.