In pregnancy, women experience pain for reasons other than labor or delivery. Painful syndromes may occur during pregnancy, or chronic pain may become more acute, and patients should be offered treatment, always bearing in mind maternal and fetal safety.
ObjectiveTo conduct a review of the scientific literature on the management of non-obstetric pain during pregnancy and the potential implications of the different pharmacological and interventional therapies available.
Materials and methodsNon-systematic review in the following databases: Medline, Pubmed, FDA and Drugs with a search of relevant articles in English.
ResultsThe articles related to the various types of therapies for pain management during pregnancy were selected.
ConclusionThe comprehensive approach to the management of conditions that may produce pain during pregnancy requires the use of medications that are not always 100% safe. Treatment must be inter-disciplinary and humanized, and consider the implications for the mother and fetus, optimizing, whenever possible, non-pharmacological therapeutic options.
La mujer embarazada experimenta dolor por causas diferentes al trabajo de parto o al parto. Durante el embarazo pueden presentarse síndromes dolorosos agudos o se agudizan dolores crónicos que deben ser tratados, asegurándose siempre de mantener la seguridad para la madre y el feto.
ObjetivoRealizar una revisión de la literatura científica acerca del manejo del dolor de causas no obstétricas durante el embarazo, y las posibilidades e implicaciones de las diferentes terapias disponibles tanto farmacológicas como intervencionistas.
Materiales y métodosRevisión no sistemática. Se consultaron las siguientes bases de datos: Medline, Pubmed, FDA y Drugs, en búsqueda de artículos en inglés relevantes.
ResultadosSe seleccionaron los artículos correspondientes a los diferentes tipos de terapia disponibles en el manejo del dolor durante el embarazo.
ConclusiónEl manejo integral de las patologías que pudieran generar dolor durante el embarazo requiere del uso de medicamentos que no siempre son 100% seguros. Su tratamiento debe ser interdisciplinario y humanizado, teniendo en cuenta las implicaciones para la madre y el feto, y mientras sea posible, optimizando alternativas terapéuticas no farmacológicas.
Pregnancy involves physiological and body changes that favor the onset of painful diseases or may intensify pre-existing painful conditions. Even though non-obstetrical pain in pregnancy is common, in our search of pain management of pregnant women, we found abundant literature focusing on analgesia for labor, while pain management during the rest of gestation has been neglected.
Most cases of acute pain in pregnancy are treated, first of all, by ruling out an obstetric cause. The use of common analgesics, a few days of rest, and patient education about pain and the effect on the fetus, are usually enough in terms of treatment.
Consequently, the problem arises when pain does not improve, becomes chronic and, worse still, when the patient with a history of chronic pain becomes pregnant, because the therapeutic armamentarium is immediately reduced, either because of the availability of safe drugs or because of lack of knowledge of the problem.
The most important implication is the potential risk of toxicity or teratogenicity of the pharmacological agents or the interventions used for pain relief.
The following are some considerations to bear in mind in pregnant women who complain of pain.
MigraineMigraine of onset during pregnancy is rare and occurs only in 3% of patients, typically during the first trimester.1
However, it is common to find pregnant women with a past history of migraine. In general, migraine improves during pregnancy, in particular during the first trimester. Studies report an improvement range of 43–86%, depending on the type of migraine; for example, there is a higher rate of improvement in cases of menstruation-associated migraine.2
Initial management must focus on non-pharmacological therapies such as relaxation exercises, acupuncture, biofeedback and behavioral cognitive therapy. When those therapies fail, the first line drug treatment is paracetamol.
Triptanes must be avoided in the third trimester because of the slight increase in the risk of uterine atony and peripartum hemorrhage. The literature has not demonstrated or ruled out a relationship of teratogenicity.
Low-dose ergot derivatives have a high teratogenic risk, and high doses may provoke uterine contractions and miscarriage.
Caffeine may produce intra-uterine growth retardation (IUGR), fetal death and premature delivery.
Beta-blockers have not been shown to be teratogenic. Low fetal weight has been reported as a result of a moderate reduction of placental blood flow from low maternal cardiac output. When used during the peripartum period, it is important to pay attention to the newborn because of the risk of inducing bradycardia, hypotension, hypoglycemia, hypothermia, respiratory distress and apnea. Of this group of drugs, propranolol, metoprolol, nadolol and timolol are considered category C, while atenolol is considered category D.3
Angiotensin converting enzyme inhibitors are considered toxic for the fetus and should not be used during pregnancy. They may cause limb contractures, craniofacial malformations, pulmonary hypoplasia, renal defects, oligohydramnios, IUGR, patent ductus arteriosus, anuria, neonatal hypotension and death.4
Muscle-skeletal painBack pain may occur in two-thirds of all pregnancies, and pelvic pain in one-fifth.5 The physiological changes that take place during this period, including fluid retention, joint laxity and displacement of the center of gravity, explain the high prevalence of these conditions during pregnancy.6 The two types of pain worsen sleep disorders, and affect work and the woman's daily life.5,7
The most frequent causes of muscle-skeletal pain include upper lumbar pain (10%), lower lumbar pain (41%) and sacroiliitis (48%).7 Pubic symphysis pain is less frequent, although it is more disabling.8,9
In pregnancy-related posterior pelvic pain (PRPP), laxity of the sacroiliac joints has been proposed as a cause. Some propose the asymmetric laxity of the joint. Relaxin, previously implicated as a cause of this form of pain, has been ruled out in recent research.6,10,11
Fortunately, the frequency of disc disease is not higher in pregnancy, but nerve root compression and the presence of a cauda equina (1:10,000) may require neurosurgical or interventional procedures such as percutaneous discectomies or epidural injections,12 with the consequences that they may bring both for the mother as well as the fetus.
In 2008, there was a Cochrane review of the randomized controlled studies available for assessing response to interventions for the prevention and treatment of pelvic and back pain during pregnancy. The interventions described included exercises in the water, pelvic belts, the Ozzlo cushion, physical therapy, acupuncture, TENS and acetaminophen. The conclusion was that no treatment is 100% effective, and that the combination of standard prenatal care together with exercise led to a slight reduction in the pain reported by the patients.5,13–15 Moreover, it was determined that pain worsened as gestation advanced.
Neuropathic painThe most common conditions that produce neuropathic pain in pregnant women include carpal tunnel syndrome, meralgia paresthetica, low intercostal nerve compression, and pain in the scar of a previous cesarean section.
In general, carpal tunnel syndrome in pregnancy has been attributed to physiological changes, among which fluid retention favors nerve trapping, especially during the second trimester. The treatment includes using splints during the night, physical therapy, local anesthetic infiltration and slow-release steroids and, in extreme cases, surgical decompression.15–17 Fortunately, symptoms usually go away after delivery in most cases.
Pregnancy, together with obesity, diabetes and certain postures favors meralgia paresthetica. Local infiltration of steroids and anesthetics, physical therapy, surgical decompression, and expectant management have all been described.11,12
Distended abdominal tissues could also be a source of pain because of traction on subcostal nerves and previous C-section scars.14,15
The results of the Collaborative Perinatal Project suggest that fetal risk is minimal when low-dose single injections of local anesthetics and slow-release steroids are used during the second or the third trimester.18
Women with pre-existing chronic painIn the United States, at least 50% of women become pregnant unexpectedly and, in Colombia, figures are not very different. This creates a risk for the fetus, given that exposure to teratogenic factors may occur during the first few weeks and, by the time pregnancy is recognized, it is already too late. This represents an even bigger risk for women suffering from chronic pain.
The current use of some of the medications given to pregnant women with chronic pain is based on what is known from studies conducted in patients with depressive disorder or epilepsy. In these disorders, the risk of not using or discontinuing the medication is greater than that of using it. Unlike these conditions, neuropathic pain does not imply a major direct risk for the life of the patient, and the approach to treatment must be as safe as possible for the mother and the fetus. Moreover, the mother must play an active role in decision-making regarding her management and should be fully informed of the uncertainty that exists regarding many of the treatments available at the present time.
Teratogenicity and toxicityWhen pregnancy is suspected, the first thing to do is to minimize the use of drugs and optimize non-pharmacological therapies, as long as it is possible. When the mother needs medication, the physician must be aware of the potential harmful effects for the mother, the fetus and the course of pregnancy.
Protein binding, liposolubility, molecular weight and the rate at which drugs are metabolized are all determinants of the degree to which they are present in placental and fetal circulation. Except for large molecules like insulin and heparin, for example, almost all medications cross to the placental circulation to a certain extent. The time of highest teratogenic risk is during organogenesis, between weeks 4 and 10. Before that time, there is an “all or none effect”, that is to say that the embryo dies (many times unnoticed), or pregnancy continues with no harm to the fetus. After week 10, drug effects may affect fetal organs, reduce the amount of amniotic fluid, generate IUGR, delay delivery, or trigger syndromes or create fetal pulmonary hypertension.
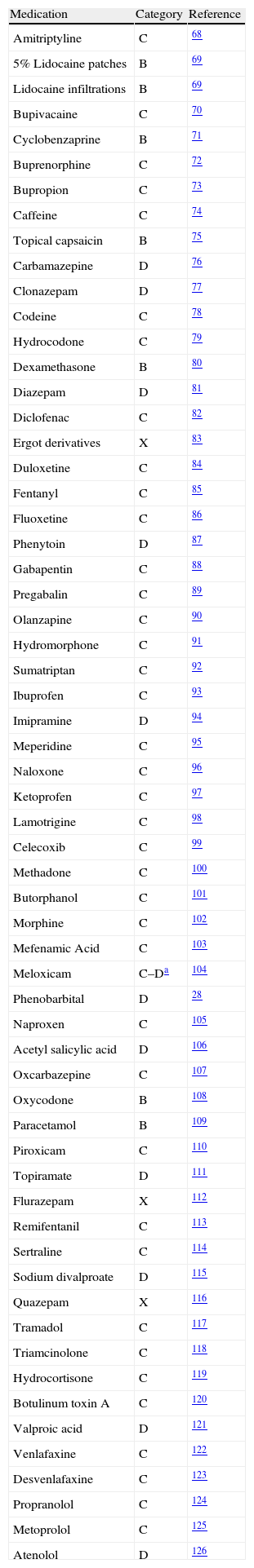
The US Food and Drug Administration (FDA) recommends a classification based on the studies available to determine drug risk versus benefit in pregnancy. Each medication is classified under one of five categories, according to the results of animal or human studies:
Category A: Adequate studies in pregnant women have not shown risk for the fetus in the first trimester of pregnancy and there is no evidence of risk later in pregnancy. Category B: Animal studies have not shown adverse effects on the fetus, but there are no adequate clinical trials in pregnant women. Category C: Animal studies have shown an adverse effect on the fetus, but there are no adequate clinical studies in pregnant women. The drug may be useful in pregnant women despite potential risks. Category D: There is evidence of risk for the human fetus, but potential benefits of use in pregnant women may be acceptable despite potential risks. Category X: Animal or human studies show fetal abnormalities, or the reports of adverse reactions indicate evidence of fetal risk. The risks involved are clearly greater than potential benefits.19
Each medication is classified individually and there may even be drugs of different categories in the same group (Table 1). However, due to the difficulty and the ethical implications of conducting trials in pregnancy, the FDA created a registry for the exposure to medication during pregnancy, and has proposed a new additional classification that considers four components: risk to the fetus, clinical considerations (risk of inadvertent exposure), decision to prescribe (indication and time during pregnancy), and data section (detailed discussion of the existing literature). To this effect, it is collecting information from women, companies or doctors about the effects perceived by the pregnant women when they were taking a medication. Existing registries include autoimmune diseases, asthma, cancer, epilepsy, HIV/AIDS, and transplants.19
Drug categorization according to fetal risk.
| Medication | Category | Reference |
| Amitriptyline | C | 68 |
| 5% Lidocaine patches | B | 69 |
| Lidocaine infiltrations | B | 69 |
| Bupivacaine | C | 70 |
| Cyclobenzaprine | B | 71 |
| Buprenorphine | C | 72 |
| Bupropion | C | 73 |
| Caffeine | C | 74 |
| Topical capsaicin | B | 75 |
| Carbamazepine | D | 76 |
| Clonazepam | D | 77 |
| Codeine | C | 78 |
| Hydrocodone | C | 79 |
| Dexamethasone | B | 80 |
| Diazepam | D | 81 |
| Diclofenac | C | 82 |
| Ergot derivatives | X | 83 |
| Duloxetine | C | 84 |
| Fentanyl | C | 85 |
| Fluoxetine | C | 86 |
| Phenytoin | D | 87 |
| Gabapentin | C | 88 |
| Pregabalin | C | 89 |
| Olanzapine | C | 90 |
| Hydromorphone | C | 91 |
| Sumatriptan | C | 92 |
| Ibuprofen | C | 93 |
| Imipramine | D | 94 |
| Meperidine | C | 95 |
| Naloxone | C | 96 |
| Ketoprofen | C | 97 |
| Lamotrigine | C | 98 |
| Celecoxib | C | 99 |
| Methadone | C | 100 |
| Butorphanol | C | 101 |
| Morphine | C | 102 |
| Mefenamic Acid | C | 103 |
| Meloxicam | C–Da | 104 |
| Phenobarbital | D | 28 |
| Naproxen | C | 105 |
| Acetyl salicylic acid | D | 106 |
| Oxcarbazepine | C | 107 |
| Oxycodone | B | 108 |
| Paracetamol | B | 109 |
| Piroxicam | C | 110 |
| Topiramate | D | 111 |
| Flurazepam | X | 112 |
| Remifentanil | C | 113 |
| Sertraline | C | 114 |
| Sodium divalproate | D | 115 |
| Quazepam | X | 116 |
| Tramadol | C | 117 |
| Triamcinolone | C | 118 |
| Hydrocortisone | C | 119 |
| Botulinum toxin A | C | 120 |
| Valproic acid | D | 121 |
| Venlafaxine | C | 122 |
| Desvenlafaxine | C | 123 |
| Propranolol | C | 124 |
| Metoprolol | C | 125 |
| Atenolol | D | 126 |
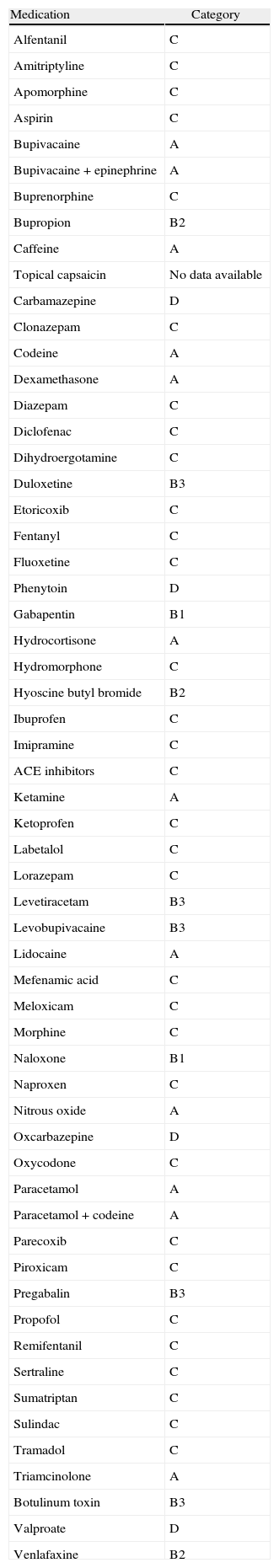
Australia has a different classification system (Table 2)20 that takes into consideration the known harmful events for the developing fetus, including birth defects, effects that may or may not be reversible, and problems later in life. Overdose and occupational exposure to medications are not taken into consideration, and the classifications as A, B1, B2, B3, C, D, and X are not hierarchical (Table 3).
Australian risk categories for drugs during pregnancy.a
| Categories | Definition |
| A | Medications taken by a large number of pregnant women and women of child bearing age, with no observation of a proven increased frequency of malformations or of other direct or indirect harmful effects on the human fetus. |
| B1 | Medications that have been used only by a limited number of pregnant women and women in child bearing age, with no observation of an increase in the frequency of malformations or other direct or indirect harmful effects on the human fetus. Animal studies have not shown evidence of an increased incidence of fetal damage. |
| B2 | Medications that have been used only by a limited number of pregnant women and women in child bearing age, with no observation of an increase in the frequency of malformations or other direct or indirect harmful effects on the human fetus. Animal studies are inadequate or may be missing, but existing data do not show evidence of an increased incidence of fetal damage. |
| B3 | Medications that have been used only by a limited number of pregnant women and women in child bearing age, with no observation of an increase in the frequency of malformations or other direct or indirect harmful effects on the human fetus. Animal studies have shown evidence of an increased incidence of fetal damage, and their significance is considered uncertain in human beings. |
| C | Medications that, due to their pharmacological effects, have caused or are suspected to cause, harmful effects on the human fetus or the neonate, without causing malformations. Those effects may be reversible. |
| D | Medications that have caused or are suspected of having caused or expected to cause a higher incidence of human fetal malformations or irreversible damage. These medications may also have adverse pharmacological effects. |
| X | Drugs with a high risk of producing permanent fetal damage and that should not be used during pregnancy or when the possibility of pregnancy exists. |
Source: Taken with permission from the Australian categorisation system for prescribing medicines in pregnancy, 2011, Therapeutic Goods Administration, used by permission of the Australian Government<http://www.tga.gov.au/hp/medicines- pregnancy-categorisation.htm>.
Australian categorization of medications according to teratogenic risk.
| Medication | Category |
| Alfentanil | C |
| Amitriptyline | C |
| Apomorphine | C |
| Aspirin | C |
| Bupivacaine | A |
| Bupivacaine+epinephrine | A |
| Buprenorphine | C |
| Bupropion | B2 |
| Caffeine | A |
| Topical capsaicin | No data available |
| Carbamazepine | D |
| Clonazepam | C |
| Codeine | A |
| Dexamethasone | A |
| Diazepam | C |
| Diclofenac | C |
| Dihydroergotamine | C |
| Duloxetine | B3 |
| Etoricoxib | C |
| Fentanyl | C |
| Fluoxetine | C |
| Phenytoin | D |
| Gabapentin | B1 |
| Hydrocortisone | A |
| Hydromorphone | C |
| Hyoscine butyl bromide | B2 |
| Ibuprofen | C |
| Imipramine | C |
| ACE inhibitors | C |
| Ketamine | A |
| Ketoprofen | C |
| Labetalol | C |
| Lorazepam | C |
| Levetiracetam | B3 |
| Levobupivacaine | B3 |
| Lidocaine | A |
| Mefenamic acid | C |
| Meloxicam | C |
| Morphine | C |
| Naloxone | B1 |
| Naproxen | C |
| Nitrous oxide | A |
| Oxcarbazepine | D |
| Oxycodone | C |
| Paracetamol | A |
| Paracetamol+codeine | A |
| Parecoxib | C |
| Piroxicam | C |
| Pregabalin | B3 |
| Propofol | C |
| Remifentanil | C |
| Sertraline | C |
| Sumatriptan | C |
| Sulindac | C |
| Tramadol | C |
| Triamcinolone | A |
| Botulinum toxin | B3 |
| Valproate | D |
| Venlafaxine | B2 |
Source: Authors.
It is worth noting that this is done for information purposes, and that data shown may vary based on the results of present or future research, and that medications may change category according to the reports. Consequently, the recommendation is to check the FDA or the Australian websites periodically for updates on adverse events, in order to reduce risks.20,21
The following are a few recommendations on the use of medications.
Non-steroidal anti-Inflammatory agentsThe use of NSAIDS must be avoided in the first trimester because of the risk of miscarriage, and during the second and the third trimester because of the risk of oligohydramnios or premature closure of the ductus with an effect on fetal circulation.18,22Dipyrone, taken out from the US market, is still available in some European and Latin-American countries. It has been studied in Brazil with no association found with major fetal malformations, IUGR, intra-uterine death, or pre-term delivery.23–25 In contrast, in other studies it has been associated with Wilms tumors in children exposed in utero, and there are several case reports suggesting its association with oligohydramnios and closure of the ductus arteriosus when used in the third trimester.23–26
Low-dose (60–80mg/day) acetyl salicylic acid does not create an important risk for the mother or the fetus, but higher doses have been associated with a greater risk of intra-cranial hemorrhage in neonates born before week 35.27
Anti-epileptic agentsAnti-epileptic agents during pregnancy must be used at the lowest effective dose. There are known effects related to major (cardiac, urogenital, central nervous system, cleft lip and palate) and minor fetal malformations, IUGR, microcephaly, and cognitive deficits. They may produce harmful effects up until day 70 after conception.28,29
What is clear about these drugs is that the lowest effective dose should be used, and that mono-therapy is better than poly-therapy.
Valproic acid is contraindicated both as mono-therapy and as part of poly-therapy. The dose at which it is used is directly associated with the risk of fetal malformations. A dose greater than 1.1g/day has a 30% risk of malformations while the risk with a dose under 1.1g/day is less than 3.2%.30,31
Carbamazepine must be used as mono-therapy, and has not been associated with cognitive disorders. In studies with this medication, it was concluded that carbamazepine had the lowest risk, as mono-therapy, of producing major congenital malformations.32
Anticonvulsants such as topiramate have also been associated with the possibility of producing major malformations such as cleft lip and palate. Gabapentin has been associated with minor malformations and it is contraindicated with breastfeeding because 100% crosses into the milk. These two drugs have shown increased toxicity when they are part of poly-therapy. The most recent publications suggest that, more important than poly-therapy itself is to analyze what the drug combination is.13,33
Pregabalin is frequently used in chronic neuropathic pain, but there are no human studies that can help determine its safety in pregnancy. In rats, it has been associated with low weight fetuses and bone problems.32
Anti-depressantsAnti-depressants are used frequently in the treatment of chronic pain, either as adjuvants in analgesic management, or as primary treatment of the concomitant depression. In pregnant women, depression is found in up to 12% of the population.34
As far as tricyclic antidepressants (TCAs) are concerned, adverse side effects and the possibility of lethal overdosing are well known. In pregnancy, no direct association has been reported with fetal malformations, although there are reports of fetal withdrawal syndrome due to TCA suppression.35
Cyclobenzaprine is a tricyclic amine used as muscle relaxant. Its use has been authorized by the FDA for the short-term (maximum 2 weeks) symptomatic treatment of muscle spasm pain. Studies in rats and rabbits using doses 20 times higher than those used in humans did not produce effects on the fetus, but the lack of controlled trials in humans limits the routine use of this drug. It should be used only when the benefit outweighs the risk.36
A higher risk of heart disease has been reported with exposure to paroxetine.37,38 There are no human studies with duloxetine, although there are reports of neonatal withdrawal syndrome and serotoninergic syndrome in babies who have been exposed. No conclusions can be derived to date about safety in pregnant women.39
In recent publications, fluoxetine has not been associated with fetal malformations and, if well tolerated by the mother, its use could be less risky than discontinuation.
Among selective serotonin reuptake inhibitors (SSRIs), some authors suggest that sertraline is the safest due to its low levels in blood and mother's milk. Although there is an absolute risk of malformations, it is low when compared to other SSIRs.40
OpioidsPregnant women receiving chronic opioid treatment should take the lowest effective dose or, if possible, discontinue its use. Opioids should never be discontinued abruptly because the ensuing withdrawal syndrome may produce uterine irritation and miscarriage or premature delivery.41,42
For opioid-dependent patients, weaning must be done using methadone of buprenorphine. Recent case series and one randomized clinical trial show some advantages of buprenorphine in terms of higher birth weight, lower prevalence and severity of the neonatal withdrawal syndrome and shorter hospital stay for the newborn. Another important finding is that the neonatal withdrawal syndrome is less when buprenorphine is started before delivery versus starting it in the post-partum period (26% vs. 60% respectively).43,44
There are reports of better neonatal results when opioids are weaned when they are in use for chronic treatment of pain than when it is a matter of addiction, in terms of less severe neonatal withdrawal syndrome and less effect on birth weight.45
In a case report about the analgesic management of a pregnant woman with upper limb compartmental syndrome, the patient received long-term morphine infusion. This treatment was associated with fetal cerebral and placental vasoconstriction, which improved after switching to a fentanyl infusion. Whenever opioid infusions are required, the suggestion is to use opioids lacking active metabolites.46,47
Reports on the use of fentanyl patches propose this as a treatment option during pregnancy and even while breastfeeding. However, it is important to note that neonatal withdrawal syndrome is still a problem.48
According to the FDA, most opioids are category B or C, but during pregnancy they become category D. The FDA classifies codeine in category C after a study with 563 pregnant women exposed to it during the first trimester found that 8 neonates had respiratory malformations. This figure is statistically significant.49 However, other reports that came later have not found that association, and in the Australian categories it is under category A.20
Meperidine must not be given in repeated doses because of normeperidine, a long half-life (18h) metabolite that accumulates after several doses and produces central nervous system excitation that results in manifestations such as myoclonus and generalized seizures.50 It is classified under category C.
BenzodiazepinesIn the first trimester, benzodiazepines are associated with a higher risk of congenital malformations, with an association between diazepam and a higher risk of cleft lip and palate and congenital inguinal hernia.51
There are other reports where diazepam has not been found to increase those risks but the recommendation is not to use it because of this history. The use of benzodiazepines in the peripartum period may produce fetal hypothermia, hyperbilirubinemia and respiratory depression. Moreover, neonatal metabolism of these drugs is very slow, with metabolites having been found in the baby's circulation even up to 10 days after a single dose given to the mother during the peripartum period.
Because of all these reasons, the use of these drugs is not recommended during the first trimester, peripartum or breastfeeding.
Other transdermal therapiesUnder the FDA classification, 5% lidocaine patches are category B. There are studies in rats with transdermal application of 30mg/kg of lidocaine, with no adverse effects for the fetus, but there are no human controlled trials to confirm this information. Consequently, this therapy must be used only when the practitioner considers that the benefit outweighs any risk.21
Interventional analgesiaWe did not find reports of the use of infusion pumps or spinal stimulators in pregnancy. However, there are several reports of patients who became pregnant while wearing one of those devices for chronic pain control.Regarding these patients, it is important to remember that uterine growth and skin and soft tissue distension that occur during pregnancy might displace the devices and alter their optimal functioning. Moreover, the presence of these devices has not been associated with difficulty controlling pain during labor. There are reports of epidural catheter placement in a woman in labor with cervical spine stimulator, and a woman with intrathecal morphine pump infusion. In these two cases, the catheter was placed with no complication and there was adequate control of pain.52–54
Management of these patients must be initiated during prenatal control in order to find the cause that led to the placement of the device, the anatomical location, the exact course of the electrodes (nerve stimulator) or of the catheter (intrathecal therapy pump), and to make decisions together with the obstetrician and the gynecologist about the different analgesic/anesthetic management possibilities (neuroaxial or another technique), either for normal delivery or for C-section.52
As mentioned above, there are no adverse events from the application of single-dose local anesthetic and slow-release steroids. In some cases, an analgesic block may be indicated.
In interventional analgesia in the non-obstetrical population, most procedures are done under fluoroscopic guidance, and for this reason it is not recommended during pregnancy, and it is practically contraindicated in the first trimester. This requires the specialist to look for other alternatives to guide the procedures, including ultrasound and nuclear magnetic resonance. There are multiple reports in the literature of blocks guided by these two forms of imaging, with good results in terms of pain reduction.55
Radiofrequency is another procedure used often in the management of chronic pain. Since it does not require fluoroscopic guidance, it is safe for the fetus. There are case reports in the literature, in particular of ultrasound-guided radiofrequency in cardiac arrhythmogenic foci, where the benefit of the procedures outweighs any risk. Pain management studies should be done in pregnancy, which does not pose the same risk as cardiac arrhythmia.56
There are case reports of epidural infusions in pregnancy for periods of more than 72h in conditions of pubic symphysis or lumbosacral pain that did not respond to medical treatment.53,57
Physical therapyConditioning exercises and good posture are recommended in order to prevent low back pain.58 When pain is already present, there are reports of good results with relaxation exercises and education on sleeping positions.59
Aerobic exercises in water help improve pain because of reduced gravitational loads on maternal muscles.60
Regarding therapies based on massage, heat and cold, results are contradictory. There are case series reporting benefits, but a systematic review reported absence of strong evidence about the benefit of this therapy. Moreover, it is important to note that massage performed by people who are not qualified may be more risky.61
Transcutaneous electrical nerve stimulation (TENS) is also useful in localized muscle pain and it has no adverse effects on the fetus. It must be applied maintaining low current density and avoiding acupuncture points used for inducing labor.62 According to a Cochrane review, there is inconsistent and limited evidence to determine if it is useful as mono-therapy in low back pain.63 However, there is evidence that doing TENS is better than doing nothing in this form of pain.
AcupunctureAcupuncture has proved to be useful in muscle skeletal pain during pregnancy. In general, at least six sessions are required in the hands of a specialist in acupuncture, always avoiding uterine and cervical reference points in order not to trigger labor.64 Acupuncture also appears to be useful in the management of tension headache.65
Botulinum toxinThere is good evidence regarding the use of botulinum toxin in myofascial pain syndrome, neuropathic pain and joint pain.66
The data available in the literature regarding the use of botulinum toxin in pregnancy are case reports of women who received the toxin without knowing that they were pregnant. There were no harmful effects for the fetus in these cases where the application occurred during the first trimester.67
ConclusionsIt is important to remember that pain in pregnant women is not always obstetrical.
Pregnant women receiving treatment for chronic pain must continue to be treated, even during labor and delivery.
The comprehensive management of the conditions that may be a source of pain during pregnancy requires the use of medications that are not always 100% safe. Treatment must be multidisciplinary and humanized, bearing in mind the implications for the mother and the fetus.
Most of the medications and therapies used for the treatment of pain have not been tested in controlled trials during pregnancy. Existing data suggest that the use of any of them, even those classified under category B, must be weighed against the potential short- and long-term risk for the fetus. There are websites that can be used for registering adverse events from drugs during pregnancy; based on the entries, the risk categories are then updated. It is important to visit those websites periodically in order to learn about the potential risks when initiating or continuing any form of therapy. Aside from the teratogenic risk, it is important to consider the effects on fetal development during the rest of the pregnancy as well as during breastfeeding.
FundingThis review was financed with personal resources.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Roberto Díaz R, Lopera Rivera A. Manejo del dolor no obstétrico durante el embarazo. Artículo de revisión. Rev Colomb Anestesiol. 2012;40:213–23.