Following the introduction of the laryngeal mask in the 1980s, several similar devices have been developed, changing paediatric airway management under anaesthesia. This reflective article describes and examines the different paediatric supraglottic airway devices currently available in the U.K.
MethodologyThis perspective article is based on a narrative review of articles that discussed paediatric supraglottic airway devices using Cochrane, meddling, Ovid and pubmed databases as well as text books.
ResultsAs new devices continue to be developed with a clear indication of clinical relevance and genuine advantages, so high quality research will also continue to be required in order to improve features in new devices.
Con posterioridad a la introducción de la máscara laríngea en los años 1980 se han venido desarrollando varios dispositivos similares, los cuales han cambiado el manejo de la vía aérea pediátrica en anestesia. Este artículo de reflexión describe y examina los distintos dispositivos supraglóticos para la vía aérea pediátrica disponibles actualmente en el Reino Unido.
MetodologíaEste artículo de opinión se basa en una revisión de los artículos que tratan el tema de los dispositivos supraglóticos para la vía aérea pediátrica, realizada en las bases de datos Cochrane, Medline, Ovid y Pubmed y también en los libros de texto.
ResultadosA medida que se desarrollan dispositivos nuevos con indicación clara de pertinencia clínica y ventajas genuinas, se necesitarán también estudios de alta calidad a fin de mejorar las características de dichos dispositivos.
Airway management is an essential skill in paediatric anaesthesia. A challenging airway, inadequately prepared for, or sub-optimally managed, can be a determinate factor in morbidity and mortality in paediatric anaesthesia. The Laryngeal Mask Airway (LMA) and other supraglottic airway devices has led to important changes in airway management in routine and emergency procedures.
Before the introduction of the LMA in 1988 there were only two options for airway management in children and adults: the endotracheal tube or facemask.
Maintaining adequate ventilation with the facemask can be, at times, difficult to perform and when ineffective can lead to hypoxia and hypercapnia. A variety of facial, oral and pharyngeal anatomical features as well as body habitus can all contribute to difficulty in achieving good facemask ventilation in adults and children.
An endotracheal tube (ETT) provides a definitive, secured airway, but has drawbacks. Endotracheal intubation is an advanced skill to master, carrying with it risks of dental, laryngeal and tracheal damage, in addition to the risk of hypoxia when it is unexpectedly difficult or impossible to intubate the trachea.
In 1981, Dr. Archie Brain conducted experiments using plaster models of cadaveric larynx.1 He noticed that when an airtight seal is made around the posterior larynx with an elliptical-shaped cuff inflated in the hypopharynx, air could be conducted to the airways.2
By attaching a length of tubing to the rubber cuff of a Goldman dental mask he developed the early LMA prototype that was first used on a patient in 1981. By 1983 Brain was able to report the first trial of clinical use of the LMA in 23 patients, including 16 women undergoing laparoscopic surgery who were successfully ventilated through the LMA while paralyzed.3,4 He experimented with several materials, conducted 700 patient studies and used more than 200 prototypes, before launching it in 1988. The first commercially available LMA was introduced in Britain in 1988 and in the United States of America in 1991.
By 2006 the LMA had been used in an estimated 200 million patients and more than 2500 academic papers published.1 In 2003, the cLMA's patent expired, with the bars patent expiring in 2008. Several companies started to design and develop different devices based on the cLMA but as single-use devices. Aside from commercial factors, concerns over infection risk, by a prion disease (causative agent of the new variant Creutzfeldt–Jakob disease (vCJD), was the determinant factor for the development of single-use devices at the end of the 1990s.1
Cost, U.K. sterilization law, material disposal and environmental factors, amongst other things, have influenced the different supra-glottic airway devices (SGAD) produced over the last 10 years. With the expiration of the classic LMA patent and after an impressive flood of new devices, the number of new product designs appears to have now stabilized.
In this perspective article, we examine the different supraglottic airway devices currently available for paediatric use, describing their specific features and characteristics. It is based on a narrative review of articles that discussed paediatric supraglottic airway devices using Cochrane, meddling, Ovid and pubmed databases as well as text books.
BackgroundIn contrast with the endotracheal tube (ETT), the airway seal achieved with the LMA is less secure and therefore its use is limited in certain situations, including patients at high risk of regurgitation and patients requiring high ventilatory pressures or prolonged ventilation. However, for routine airway management, the LMA has become an essential tool. By the mid 1990s, it was estimated that the LMA was used in as many as 30% of all general anaesthetics worldwide.3–7 The LMA has an important role in the management of expected and unexpected difficult airway. It can be used as a channel for tracheal intubation with an ETT over a fibreoptic bronchoscope (FOB), as well as a rescue device when other means of achieving ventilation have failed or are not possible. There are several large studies reporting efficacy and safe use in neonates,8,9 trauma victims,8,10 women undergoing caesarean section,8,11 cardiac arrest situations and out of hospital emergencies.8,12 The Difficult Airway Society (UK), American Society of Anesthesiologists and the Australian and New Zealand College of Anaesthesia now include the LMA in their difficult airway guidelines.12–14
The first paediatric LMA was a smaller reproduction of the LMA produced for adults15 with no particular consideration given to the different airway anatomy of infants and children. In spite of this, the LMA performed well in this population with the classic LMA having a reported success rate of 95–98% in achieving adequate ventilation in children.9,16
LMA classification and sizingDepending on the presence or absence of a gastric channel, the available supraglottic airway devices (SGAD), are classified in to one of two groups (first or second generation devices). The first generation devices lack the gastric access channel, the mask rests above the glottis with an airway tube attached to it. In contrast, the second generation devices, integrate a channel for gastric access that permits venting as well as the possibility to insert a gastric tube.
The selection of the correct size of LMA is determined by the patients’ weight. The size 1 has been recommended for neonates and infants up to 5kg but has also been used in preterm neonates weighing less than 1kg.15 Importantly, in the neonatal and preterm subset the incidence of airway problems seems higher (delayed airway obstruction and dislodgement).15 There is a suggestion from fibreoptic studies that the epiglottis may fold in on the LMA, especially in small infants, occluding the larynx.15 However, despite this, there appears to be no interdependence between the fibreoptic view and the clinically patent airway.15 This suggests that despite glottic impingement with the LMA, the remaining area could be large enough to permit satisfactory gas flow.
In the 10–20kg group the recommended size is 2, but in some patients the 2.5 may provide a better seal. This is, however, at the expense of higher cuff seal pressure, which may compromise venous drainage.17,18
The size 2.5 is recommended for patients between 20 and 30kg with patients of 30–50kg recommended a size 3.
As the incidence of childhood obesity increases, more overweight children are presenting for routine surgery. In these children it can be difficult to select the appropriately sized LMA. In children over 40kg an LMA sized by weight alone may have excessively high sealing pressures,8 a device may be more appropriately sized by age and predicted weight.
Every LMA size has a manufacturer recommended volume of air for inflation. Across all sizes of LMA's, hyperinflation of the cuff above that recommended by the manufacturer may result in compression of the structures around the pharynx. There are documented paediatric cases of hypoglossal and recurrent laryngeal nerve paralysis.15 This can also present when over inflation is used to compensate for leak when the LMA used is too small for a child. (Table 1)
Summary of characteristics of supraglottic airway devices available in children.
| Devices | Paediatric size by weight | Easy of insertion | Other features | Concerns | Indication |
|---|---|---|---|---|---|
| Classic Laryngeal Mask Airway | 1.0: <5kg | 99–100% first attempt | No gastric drain | Size 1.5 poor fit | Common for fibreoptic intubation |
| 1.5: 5–10kg | Low leak pressure | ||||
| 2.0: 10–20kg | |||||
| 2.5: 20–30kg | |||||
| 3.0: 30–50kg | |||||
| Flexible | 1.0: <5kg | Reinforced tube | Otorhinolaryngology, ophthalmology, Dental | ||
| 1.5: 5–10kg | |||||
| 2.0: 10–20kg | |||||
| 2.5: 20–30kg | |||||
| 3.0: 30–50kg | |||||
| Cobra Perilaryngeal Airway | 0.5: 2.5–7.5kg | 100%first attempt | Gastric inflation | ||
| 1.0: 7.5–15kg | |||||
| 1.5: 16–30kg | |||||
| 2.0: 31–60 | |||||
| Proseal | 1.0: <5kg | 99% first attempt | Gastric drain | Laparoscopic surgery | |
| 1.5: 5–10kg | |||||
| 2.0: 10–20kg | |||||
| 2.5: 20–30kg | |||||
| 3.0: 30–50kg | |||||
| Sepreme | 1.0: <5kg | 97% first attempt | Gastric drain | Narrow airway tube | |
| 1.5: 5–10kg | Bite block | ||||
| 2.0: 10–20kg | |||||
| 2.5: 20–30kg | |||||
| 3.0: 30–50kg | |||||
| i-Gel | 1.0: 2–5kg | 91% first attempt | Gastric drain | Dislodgement in | Good fibreoptic view |
| 1.5: 5–12kg | Bite block | Size 1 | |||
| 2.0: 10–25kg | |||||
| 2.5: 25–35kg | |||||
| 3.0: 30–60kg | |||||
| Air_Q | 0.5: <4kg | 99% first attempt | No gastric drain | Accommodates cuffed endotracheal tube | |
| 1.0: 4–7kg | |||||
| 1.5: 7–17kg | |||||
| 2.0: 17–30kg | |||||
| 2.5: 0–50kg | |||||
The LMA permits positive airway pressure ventilation due to the low pressure it exerts in the larynx. When the inspiratory pressure is higher than 20cm H2O there is an increased risk of gastric inflation and regurgitation.15 This risk will increase further if the LMA is poorly positioned. Pressure control ventilation can produce lower inspiratory pressures when compared to volume control ventilation where pressure is not regulated, particularly in infants and children.17
The use of muscle relaxants will facilitate effective mechanical ventilation by increasing thoracic compliance but prolonged periods of mechanical ventilation (more than 5h) via LMA have shown, albeit in animal studies, to produce undesirable effect such as, lingual oedema, capillary and epithelial breakdown.18
Removal of the LMAAlthough it was initially suggested that the LMA should be removed once the airway reflexes were restored and the patient was able to obey commands i.e. open the mouth on request, in paediatrics it is recognized practice to remove the LMA “deep” (with obtunded laryngeal reflexes).15 Anaesthetists who support this approach, describe fewer complications such as laryngospasm, coughing, clenching and desaturation. Secretions can be suctioned before the laryngeal reflexes return.15 However, there is no available evidence to support this technique.
Paediatric supraglottic airway devicesThere are seven different paediatric Supraglottic Airway Devices (SADs) currently available in the U.K.
Classic LMA (cLMA™).
Flexible LMA (fLMA™).
LMA-Unique™.
LMA -Proseal (PLMA™).
iGel™.
LMA Supreme™.
Cobra Perilaryngeal Airway (CobraPLA™).
AirQ™.
Good quality randomized control trials with adequate power to detect difference in outcomes are limited in paediatric anaesthesia practice.
According to the World Heath Organization, 250 million anaesthetics are administered worldwide every year.8 The incidence of aspiration during general anaesthetic is hard to establish. The bulk of evidence comes from adult studies (not directly applicable to the paediatric population), case series and from continuous use rather than high quality evidence. This creates a bias against newer devices, therefore any statements in relation to safety and efficacy must be taken carefully. Future large randomized control trials should focus in outcomes during different types of procedures.
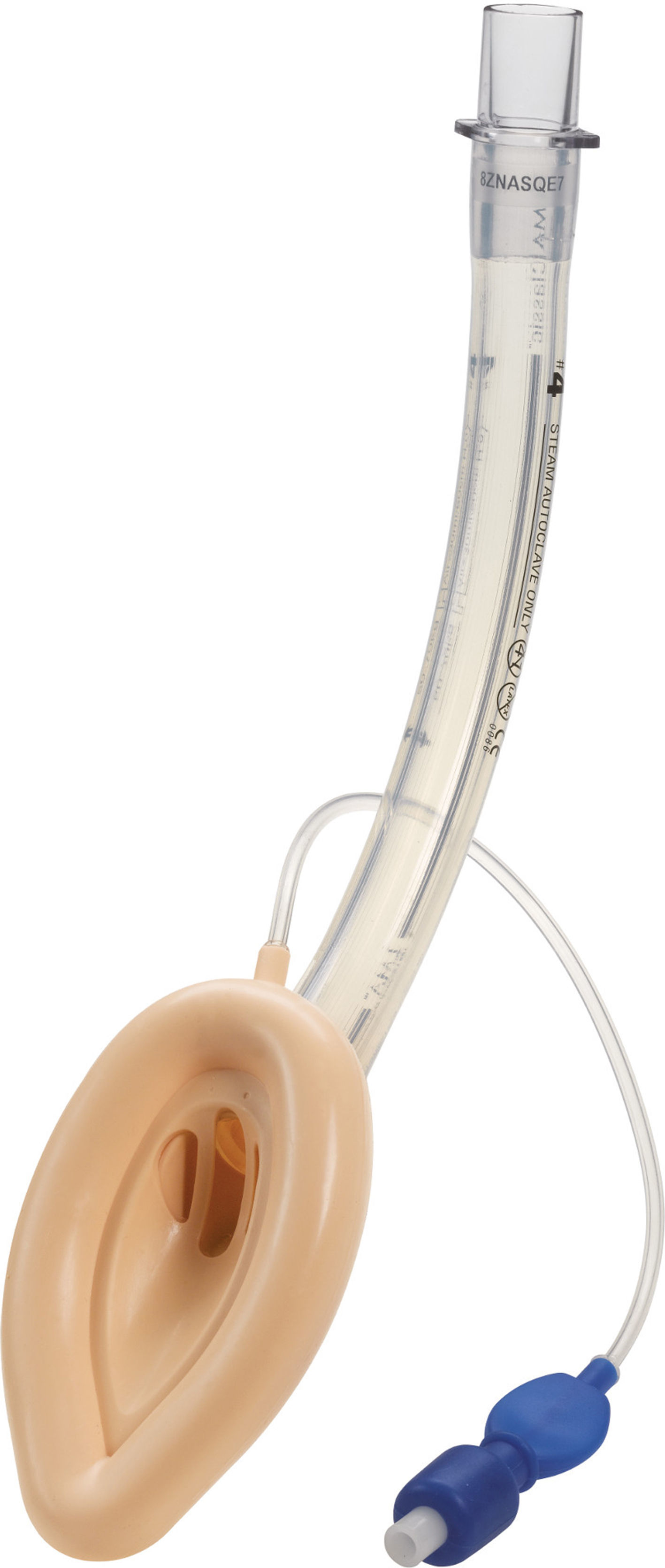
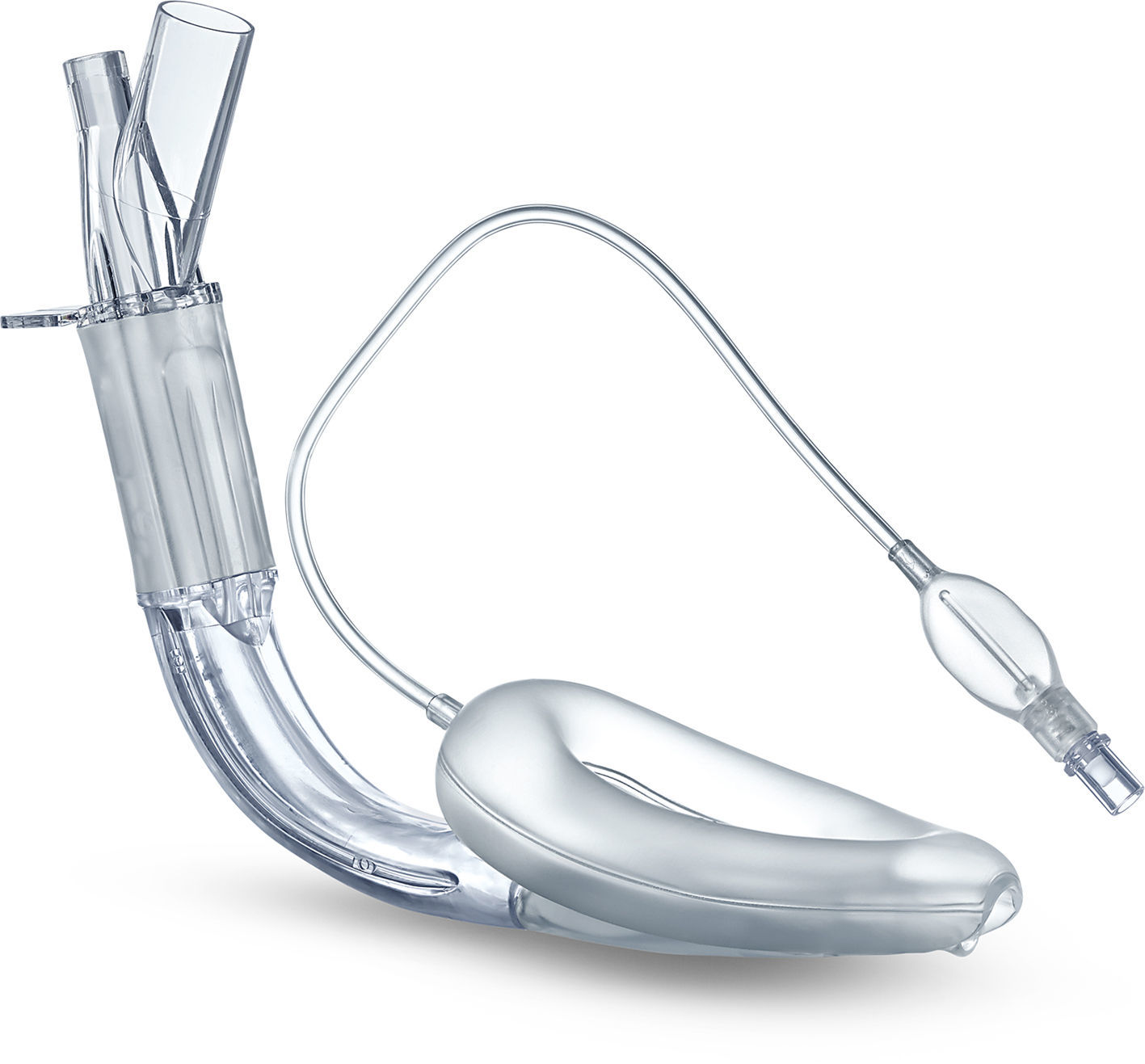
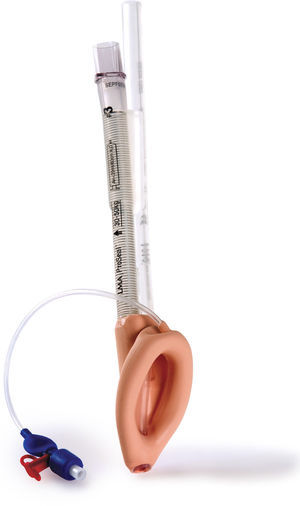
Classic LMA (cLMA™)The classic laryngeal mask airway (cLMA) (Figs. 1 and 2) consists of an oval soft silicone mask that sits over the larynx with an integrated stem that extends to allow attachment to the anaesthetic circuit. It has two thick silicone rubber strands (grilles or bars) designed to prevent the epiglottis falling into and occluding the lumen. As they are made from silicone rubber, cLMA's can be autoclaved and reused.19
In paediatric patients the use of SGAD may have advantages over endotracheal tubes during anaesthesia of children with recent upper respiratory tract infections. In a randomized trial Tait et al. compared the use of the cLMA with the endotracheal tube. The cLMA group had significantly fewer respiratory complications compared to the endotracheal tube group.20
The main reported complications with the use of the cLMA are laryngospasm and obstruction due to epiglottic folding. Small infants seem to have a higher complication rate of up to 47%.9–11,20
In children the airway leak pressure is higher than in infants,21,22 this could raise concerns over the ability to provide positive pressure ventilation safely with a classic LMA in this group. Leakage around the cuff can potentially produce gastric distention and in turn increase the risk of aspiration.
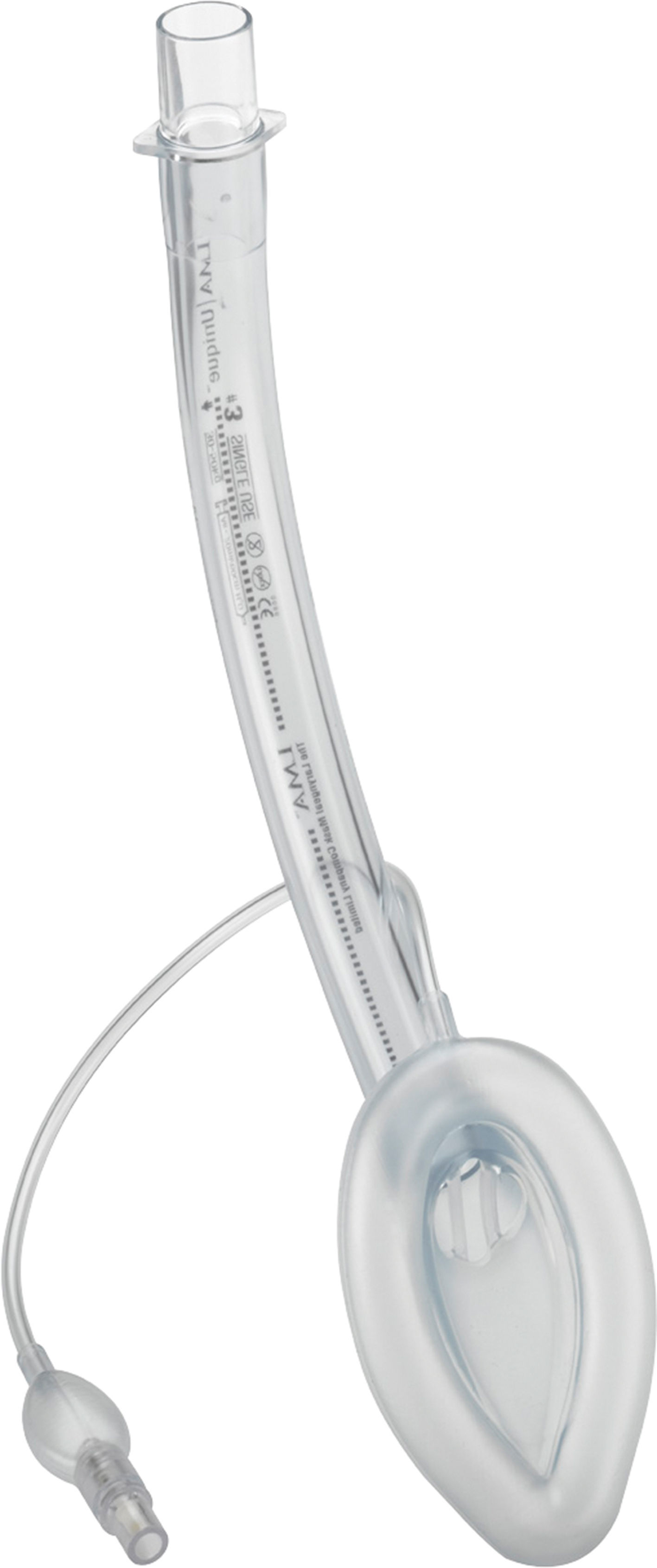
Flexible LMA (fLMA™)The flexible (or reinforced) LMA (Fig. 3) is similar to the cLMA but it has a thinner, narrower and longer breathing tube reinforced with a spiral of steel wire to add flexibility and reduce the risk of kinking.19
The flexible LMA (fLMA) was first used in 1990. It was specially developed for ENT and dental anaesthesia. Its role has extended to multiple areas including head and neck and ophthalmic anaesthesia. Some large paediatric studies have evaluated its efficacy22,23 and have shown that in cases such as adenotonsillectomy its performance is similar to or better than endotracheal tubes in terms of improved surgical access and prevention of airway soiling.24–26
Webster et al.26 studied the prototype of the fLMA and compared it with endotracheal intubation in 99 children requiring adenotonsillectomy. This study concluded that the fLMA was a safe alternative to tracheal intubation in adenotonsillectomy. The fLMA did not limit surgical access and protected the lower respiratory tract from soiling with aspirated blood. They also concluded that there was reduced need for assisted ventilation and that the haemodynamic response to laryngoscopy was reduced in this group. The incidence of post-extubation complications was reduced but not eliminated in the LMA group.
Williams and Bailey25 randomized 104 children ASA grade I and II, that presented for elective tonsillectomy and/or adenoidectomy to have either a fLMA or a RAE ETT. They concluded that the fLMA provides a safe airway until laryngeal reflexes are fully recovered.
Despite existing evidence, in paediatric ENT anaesthesia the use of tracheal tubes remains most common practice.22,23
The classic and flexible LMA have dramatically changed paediatric anaesthetic practice and are currently the SGADs most commonly used in paediatric anaesthesia. They have conclusive evidence given by several good quality studies, they are both safe and efficacious and they are the point of reference when studying new devices. They have an invaluable role in the airway management of children presenting with respiratory tract infections and in cases of predicted and unpredicted difficult airway. The main limitation is in small infants in whom a poor seal, gastric inflation or leak of gastric contents may occur.21,22
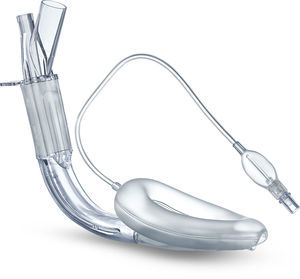
Cobra PLA™The Cobra Perilaryngeal airway (CobraPLA) (Fig. 4) was launched in 2003. It has a distal flexible tip whose shape gives name to the device, is single use and is manufactured in 8 sizes for use in neonates to patients over 140kg in weight.
A soft plastic head is designed to sit in the hypopharynx sealing it, with the anterior surface lying at the laryngeal inlet. Proximally, it has an inflatable balloon that elevates the base of the tongue and aids sealing of the oropharynx.19
An adult study by Cook27 had to be stopped early as there were 2 cases of aspiration during low risk surgery. There are, however additional adult studies where a similar performance between the cobra and the classic LMA are reported.28–30
In paediatric practice, the CobraPLA has been compared with the LMA Unique in studies by Szmuk5,25 and by Gaitini31 with 200 and 80 children respectively. No difference was reported regarding ease of insertion of either device, but Gaitini31 reported higher leak pressures with the CobraPLA when compared to the LMA Unique. In a study by Passariello, forty children between one and ten years of age which weight was between 10 and 35kg and graded ASA 1 or 2 were scheduled for minor surgical procedures.32 Gastric inflation rates of 21% were reported in the CobraPLA group, despite ventilation pressures of 20cmH2O or less.
There is no evidence that supports a clear advantage of the Cobra PLA over the classic LMA.8
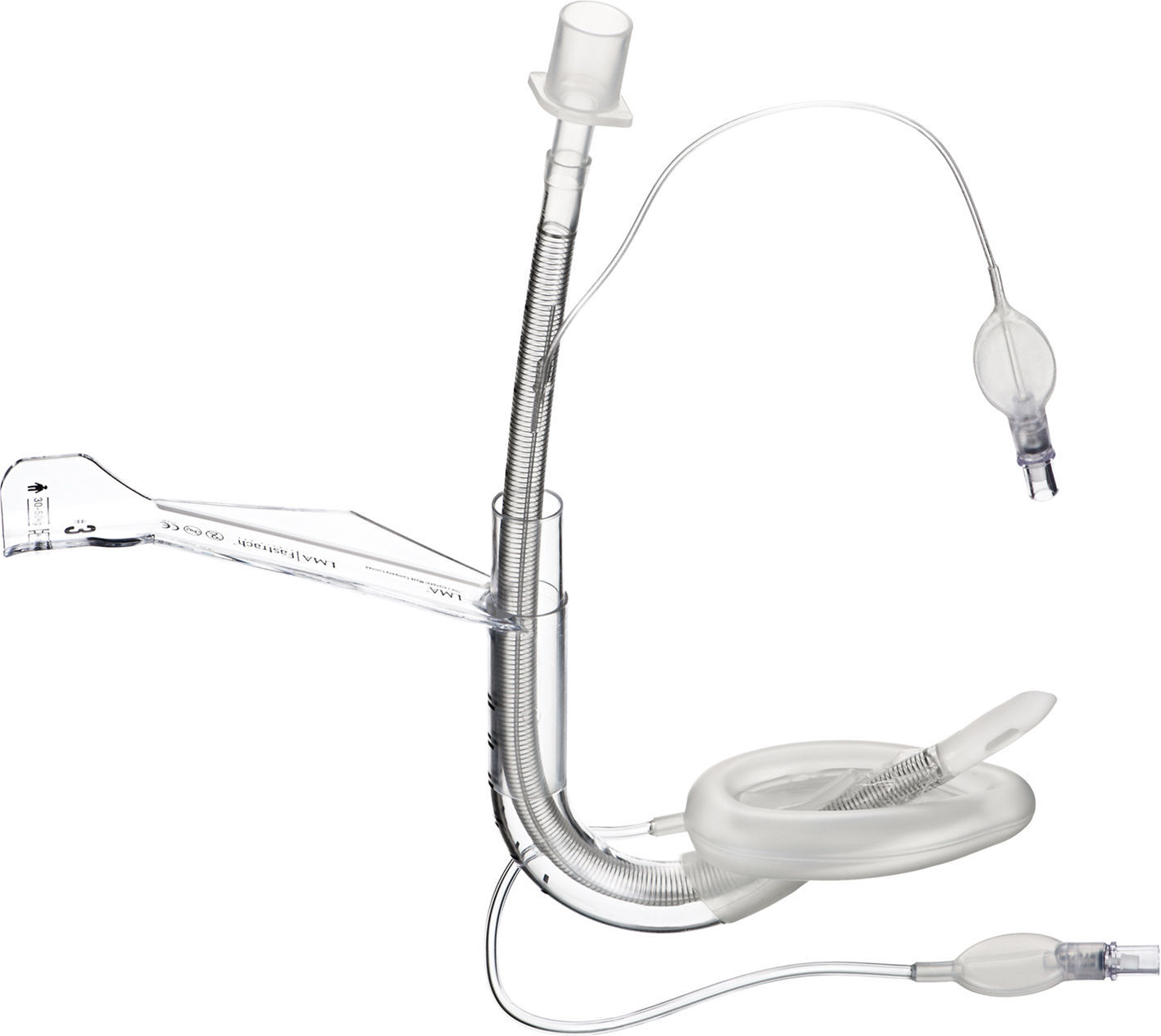
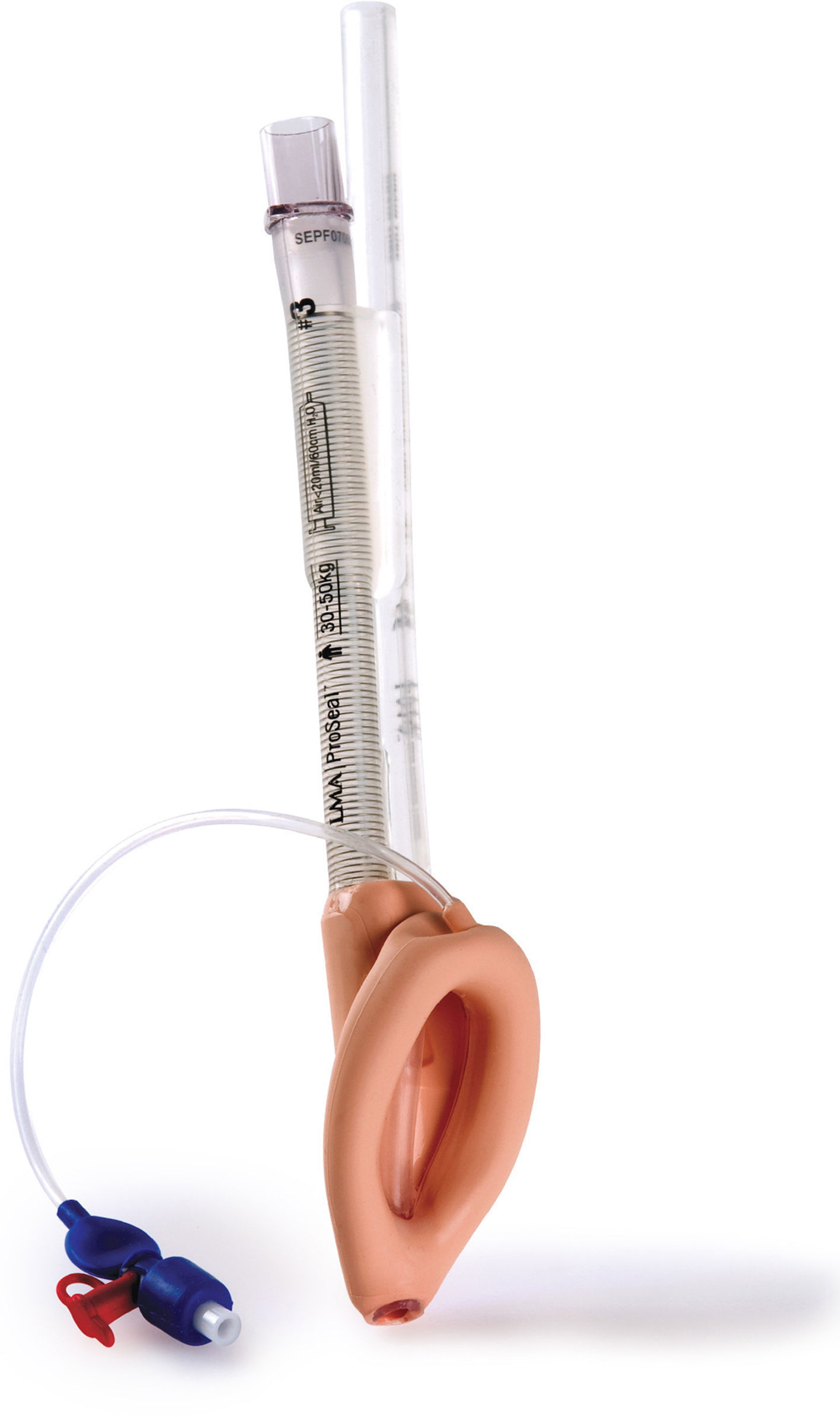
LMA ProSeal (PLMA)™The LMA Pro-Seal (PLMA) (Fig. 5) was first used in the UK in 2000 and was launched for paediatric use in 2007. Design modifications aimed to improve performance during controlled ventilation, improve safety by reducing the risk of aspiration and facilitate the ability to diagnose misplacement of the tip.19
The main differentiating feature from the cLMA is the presence of a gastric channel that runs alongside the airway tube. This can act as a placement channel for a gastric tube. There is an integral bite block and the mask shape is larger than the classic LMA.
The paediatric and neonatal sizes do not have the additional dorsal cuff that is present in sizes 3–5.8
The PLMA also exerts less pressure against the mucosa than the cLMA, reducing the potential for mucosal trauma and damage.33,34
There are multiple studies supporting the use of the PLMA.35–37 All of these studies agreed that the proseal LMA is easy to insert first time in 84–94% of cases, provides for effective ventilation in 99–100% of cases and facilitates a clear fibreoptic view of the larynx in 86–92%.36,37
In a study, Sinha et al. compared the proseal LMA to the endotracheal tube in 60 children aged from 6 months to 8 years33 having laparoscopic surgery where the duration of carboperitoneum was less than 60min. In this study patients with active airway infections and children with known difficult airway or risk of aspiration were ere not included. The proseal LMA and the endotracheal tube were found to have similar ventilatory efficacy with no statistically significant difference in oxygen saturation or end tidal carbon dioxide measurements. The first time insertion success rate was 88% for the LMA.
This was a small study and further studies should include a variety of procedures and longer carboperitoneum times, before the proseal LMA can be advised to be used during elective laparoscopic and abdominal surgery in paediatric patients.
A number of randomized controlled trials have compared the proseal and classic LMA.21,22,38–40 These studies confirmed that the proseal LMA is easy to insert and maintains a good anatomical position, but they also suggest that the Proseal LMA has a higher oropharyngeal leak pressure when compared with the cLMA. Goldman22 performed fibreoptic examinations in 30 anesthetized, non-paralyzed infants aged 2–30 months and weighing 5–12kg. Although this was a relatively small study, he found that in three of five patients that had a reduction of maximal tidal volume, there was fibreoptic confirmation of larynx compression during the use of the PLMA compared to the cLMA.
Lopez et al.36 studied 60 infants and neonates undergoing elective surgery. They were divided into either size 1 proseal LMA or cLMA. They concluded that the PLMA provided safe and effective ventilation as well as allowing positive pressure ventilation.
In summary, the PLMA could have improved performance characteristics over standard LMA's, especially where controlled ventilation is required. The body of evidence supporting the use of the proseal LMA is smaller than for the classic LMA, but its paediatric use may be particularly indicated, when controlled ventilation is required.41
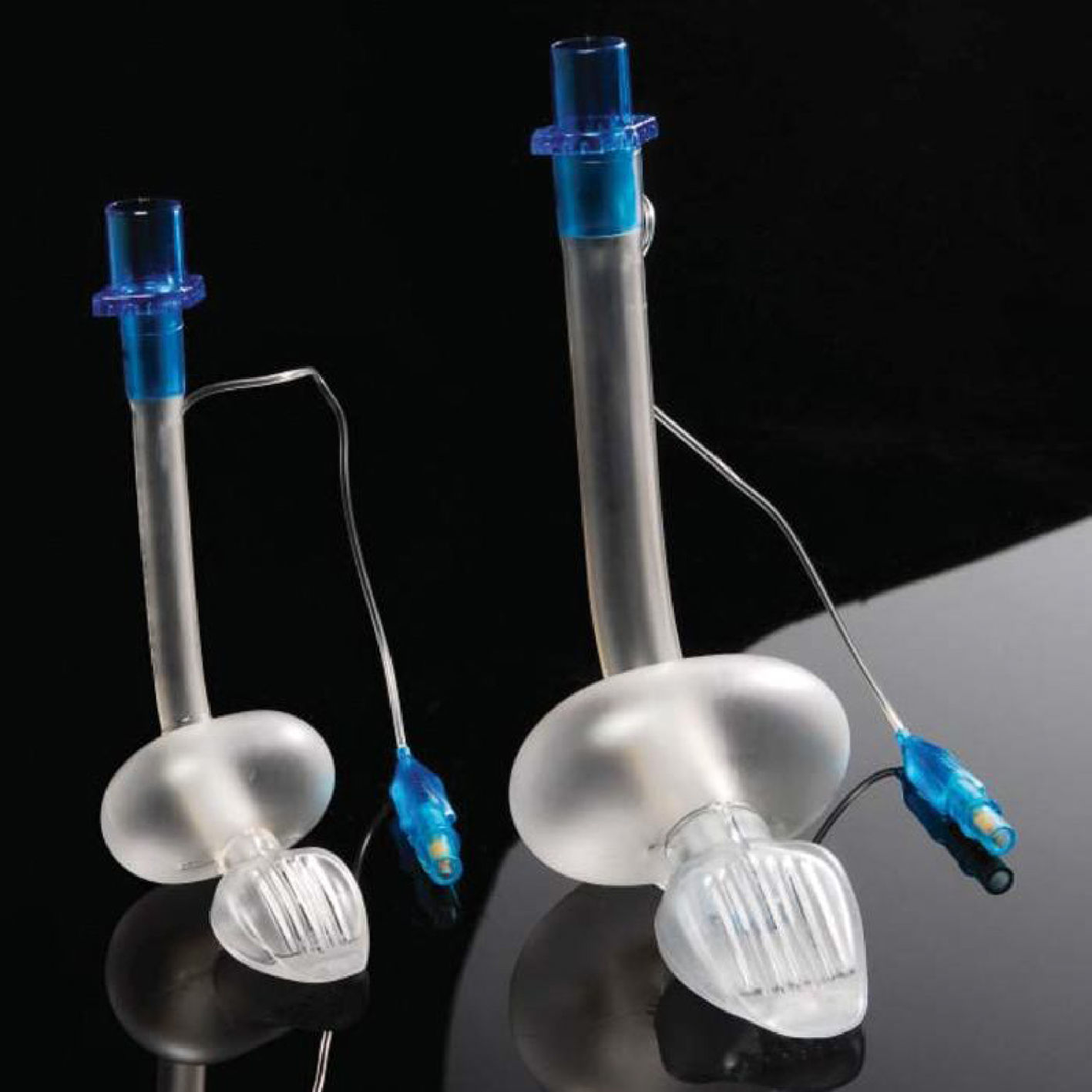
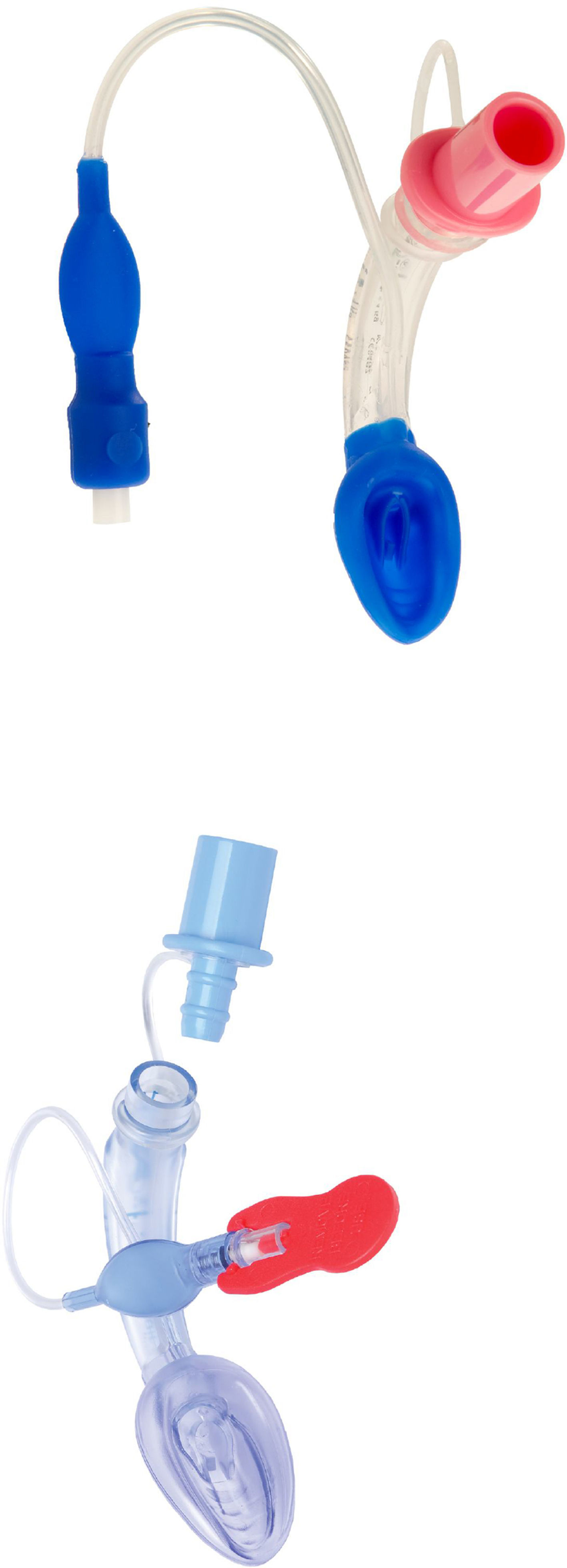
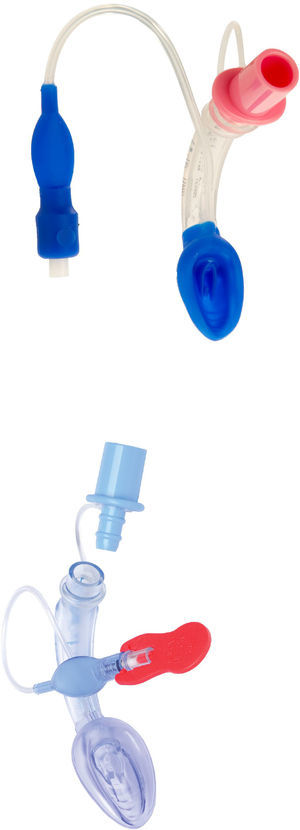
LMA Supreme™LMA Supreme (Fig. 6) was first used in 2009. This single-use PVC device was developed to amalgamate the most advantageous characteristics of the PLMA (improved seal, drain tube, integral bite block) with an easier insertion, as it is more curved and rigid. The ventilating orifices are placed laterally on the mask and in order to avoid entrapment of the epiglottis, it has overlying epiglottic fins.19
Two randomized trials by Jagannathan et al. compared the LMA Supreme with the LMA Unique (a disposable version of the cLMA). These studies concluded that the LMA Supreme provided an adequate port for gastric access reducing gastric inflation when using airway leak pressures comparable or higher than the ones used with the LMA unique.42–44
When comparing the igel with the LMA Supreme, the igel has a higher airway leak.42 Despite this, both devices are adequate to provide intermittent positive pressure ventilation and there are no dissimilarities in their clinical performance.
There is not enough evidence available to fully assess the paediatric performance of the LMA supreme. Trevistanuto et al.45 conducted an observational study with 40 anaesthetists using the LMA supreme, the classic and the proseal LMA. They subjectively appraised the characteristics and nature of ventilation as well as maximal inflation pressure. This study showed that the clinicians perceived a better quality of ventilation when using the LMA supreme. Jagannathan et al.46 evaluated successful first time insertion rates in an observational study of 100 children. They used sizes 1.0, 2.0 and 3.0 of the LMA supreme. Successful insertion was achieved in 100% of cases with first time successful insertion rate of 97%.
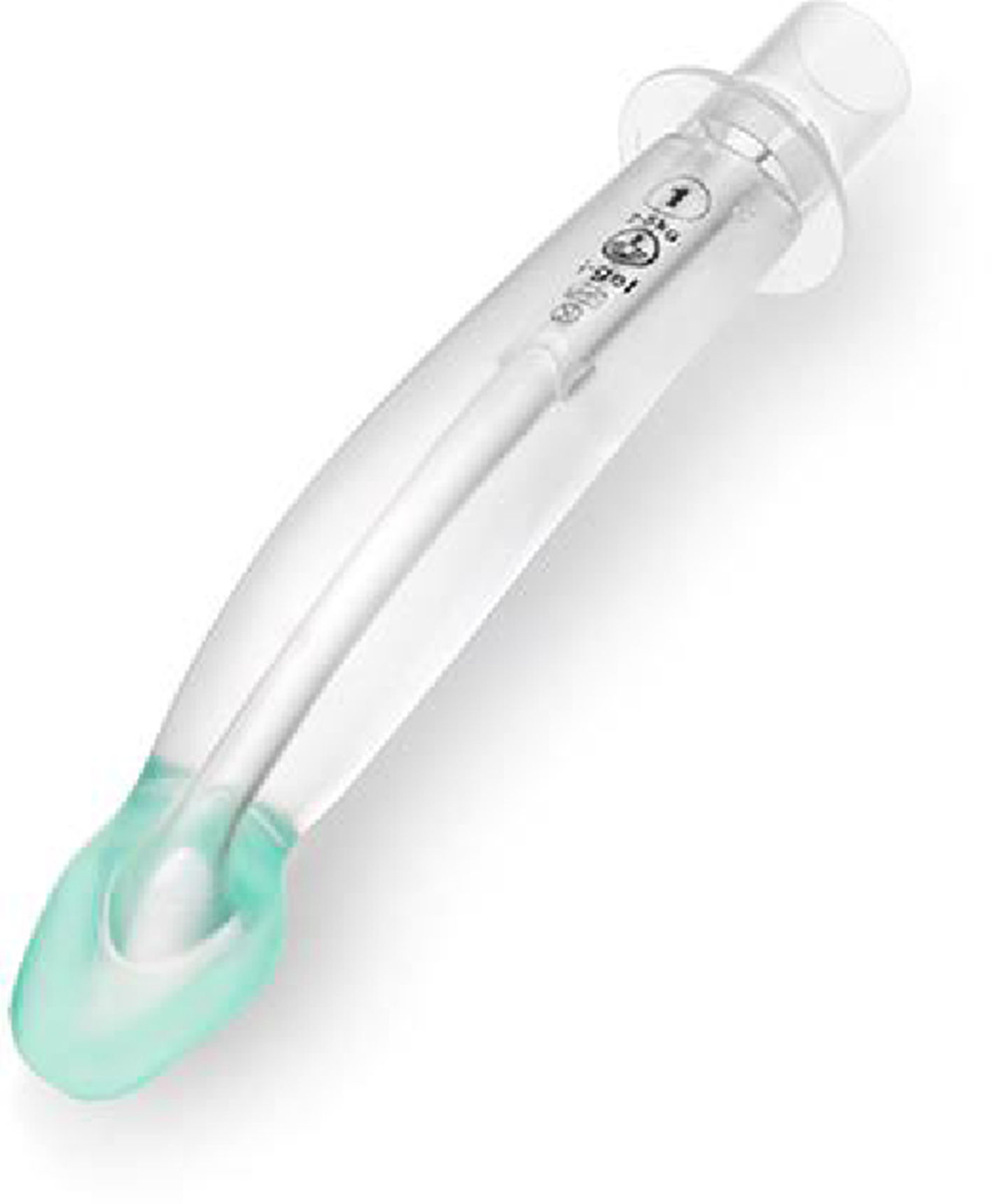
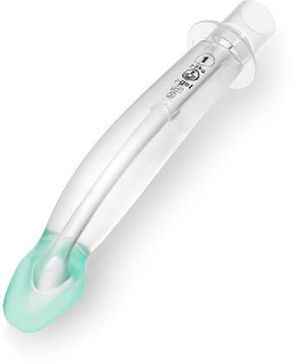
i-GEL™The i-gel (Fig. 7) is a single use, cuffless SGAD, made of an elastomer gel (styrene ethylene butadiene styrene). Its shape resembles the inflated Proseal LMA. It has a short wide-bore airway tube with no grilles, an elliptical shaped stem, an ‘anatomically’ shaped bowl, an integral bite block and a gastric access channel in all sizes except size one. These features provide low resistance to gas flow, stability, improved pharyngeal seal and adequate conduit for tracheal intubation. The elastomeric gel warms up to body temperature improving the seal of the mask around the airway. The i-gel was introduced to paediatric anaesthesia practice in 2009.19
There are multiple published accounts of the use of the i-gel in paediatric patients with observational studies, randomized control trials and meta-analyses, evaluating its performance.46–50
Two studies51,52 compared the cLMA with the i-gel, they concluded that, specially in the infant population, the time required for insertion was shorter for the igel than for the classic LMA. This group, in particular, has been reported to be at risk of movement and dislodgement of SGAD.
The elliptical shaped tube was designed to increase stability and prevent axial rotation, however Jagannathan et al. reported that, if the i-gel required adjustment to ensure airway patency, especially in smaller children, bimaxilary taping was useful and recommended in this age group to prevent further device movement.46
The i-gel is potentially easier to use as insertion is rapid without the need for cuff inflation, the ventilation and safety profile is similar to the cLMA but in a disposable SAD. Its design and performance characteristics make it an alternative to the cLMA.
Airway rescue with i-gel facilitated, fibreoptic-guided intubation has been reported.53
There have been 2 recent meta-analyses42,54 which included 9 randomized controlled trials comparing the i-gel and other supraglottic airways in paediatrics. These meta-analyses concluded that the i-gel, not only had higher airway leak pressure when compared to other SGAD, but also better fibreoptic views. However, no difference was found in regards to success rate on insertion and complication rate.
AIR Q™The air Q (Fig. 8) was introduced in the UK in 2012. This SGAD has a mask that is oval in shape and a curved, short airway tube. It also has an orifice designed to prevent epiglottic folding.19
The Air-Q has 3 versions, produced for either multiple or single-use:
- 1.
Standard – Cuffed.
- 2.
Self-pressurized (Air Q; no inflatable cuff).
- 3.
Air Q with oesophageal blocker. This device has a gastric channel but is not available in paediatric sizes.
One of the main features of the air-Q is its wider and shorter airway tube (Fig. 8). These characteristics may facilitate the passage of cuffed endotracheal tubes via this device both in infants and children. The AirQ can be safely removed after intubation.46,55–57
Jagannathan et al. concluded, after a paediatric case series of children with anticipated difficult airway due to craniofacial abnormalities and a reduced mouth opening, that the ventilation with the air-Q was satisfactory even when using a smaller air-Q size and that the device was also adequate when attempting endotracheal intubation with the correct size tube.57
The air Q has been used to facilitate oxygenation whilst facilitating fibreoptic intubation in the known or unpredicted difficult airway.46
A recent study by Jagannathan that compared the air-Q to the i-gel58 concluded that both SGAD could be safely and effectively used as conduits for endotracheal intubation guided by a fibreoptic scope. He also noted that the i-gel size 1.5 was the SGAD that experience more movement and dislodgement during the study.
In a systematic reviewed59 performed evaluating the efficacy of the Air Q, Jin Ahn et al. concluded that the Air Q was more difficult to insert that any other device but the fibreoptic view was better than with any other supraglottic device improving the success rate for intubation via SGAD.
DiscussionA great number of SADs have been developed over the 35 years since the classic LMA was first designed and used. Although there are many devices currently available for paediatric practice the decision as to which device should be used, in a particular situation, can be difficult. There is no perfect device that fulfils all requirements.
The cLMA has been studied the most and probably has the strongest available evidence base, supporting its provision of excellent conditions in most situations. Until a large study is conducted, we think that, in healthy children undergoing procedures where spontaneous ventilation is appropriate, the cLMA or its disposable version, may be the device of choice.
In ENT, dental, ophthalmology and some head and neck surgery, the flexible LMA maybe be advantageous, given its flexible reinforced breathing tube.
LMA's that have a gastric port and higher airway leak pressures, for example the ProSeal or i-gel, may be the most appropriate to use in intermittent positive pressure ventilation and for laparoscopic surgery, however the evidence base for this is limited.
Where an anticipated difficult airway is encountered, or in the setting of a rescue device for failed intubation, the i-gel appears to give a good fibreoptic view. The air-Q was also designed for this purpose and will accommodate an appropriate size ETT even with a smaller size air-Q. It can also be easier to remove once the airway has been secured with an endo-tracheal tube.
In pre-hospital medicine and emergency situations, such as cardiac arrest, both the cLMA and newer devices have been used. There remains insufficient evidence to support the use of any particular device over another. Patient characteristics, surgical requirements and personal preference will determine the choice of a specific SGAD.
As new devices continue to be developed with a clear indication of clinical relevance and genuine advantages, so high quality research will also continue to be required. Only after this rigorous evaluation of safety and efficacy can any new SAD's be recommended for widespread use in paediatric anaesthesia.
FundingNo funding used.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Stendall C, Glaisyer H, Liversedge T. Actualización en dispositivos supraglóticos para la vía aérea pediátrica. Rev Colomb Anestesiol. 2017;45:39–50.