Phantom limb pain may be present in up to 80% of patients subjected to amputation because of trauma or peripheral vascular disease. Several factors have been associated with its occurrence, including pre-amputation pain, the etiology, and the amputation level.
ObjectiveTo review the current status of the pathophysiological mechanisms, treatment options and their efficacy for the management of phantom limb pain.
MethodNon-systematic review of the literature in PubMed and Cochrane, of articles describing the pathophysiology and treatment of phantom limb pain.
Results and conclusionsThe proposed pathophysiological mechanisms are still in research and include peripheral, central and psychological factors. Treatment options are still limited, and less than 10% of patients report long-term improvement.
El dolor de miembro fantasma puede ocurrir hasta en el 80% de los sujetos con amputación por trauma o enfermedad vascular periférica. Varios factores se han asociado a su generación, como el dolor preamputación, la etiología y el nivel de la amputación.
ObjetivoRevisar el estado actual de los mecanismos fisiopatológicos, las opciones de trata-miento y su eficacia en el dolor de miembro fantasma.
MétodosSe realizó una revisión de la literatura no sistemática en las bases de datos PubMedy Cochrane sobre artículos que describieran la fisiopatología y el tratamiento del dolor de miembro fantasma.
Resultados y conclusionesLos mecanismos fisiopatológicos propuestos aún se encuentran en investigación e incluyen factores periféricos, centrales y psicológicos. Las opciones de tratamiento continúan siendo limitadas, y menos del 10% reportan mejoría a largo plazo.
Phantom limb pain (PLP) refers to the presence of painful sensations in an absent limb and is classified as pain of neuropathic origin.1,2 The French surgeon Ambroise Paré was the first to notice in 1552 that patients complained of severe pain after the amputation of a limb, and proposed peripheral and central factors to explain that sensation. Centuries later, Silas Weir Mitchell (1872) coined the term PLP to characterize this entity.3–6 The incidence varies from 2% to 80%, regardless of the etiology.2–4 Such differences in the incidence reported by different studies are due to the absence of a unified definition of PLP, or to the fact that many patients do not report their pain for fear of being stigmatized as mentally ill.1,3,7 The incidence of phantom pain appears to be independent of gender, the level of the amputation, and age in adults. Despite the above, phantom pain continues to be less frequent in children and young adults, and it is practically non-existent in individuals born without a limb.1,2,8
MethodA non-systematic review of the literature was conducted in Pubmed and Cochrane, introducing the following key words in English: Pathophysiology, Phantom limb pain, Pain, Neuropathic pain. All the articles were read and referenced articles related to the topic were also reviewed. Overall, 51 references were selected using this methodology.
ResultsPathophysiology of PLPPLP may be of short duration, with the presence of painful cramps, or it may be constant, associated with intense perception of the lost limb. Characteristically, it is more intense in the distal portions and it is shooting, throbbing, burning or cramp-like pain. It may be of immediate onset or appear many years after the amputation.2,9 Prospective studies have reported that 50% of subjects may experience pain within the first 24h after amputation, and 60–70% may do so one year later.3,7,8 Although it is more common after limb amputation, it may also occur following surgical removal of any part of the body like the eyes, breasts, face, among others.1,2,10
The onset and nature of PLP may differ depending on the cause of the amputation, although there are no clear data to reach definitive conclusions. In western countries, the main causes of amputation are diabetes mellitus and chronic vascular disease, with tumors being a less frequent cause. In other parts of the world, civil wars and land mines are causes of traumatic amputations in otherwise healthy individuals.3,11
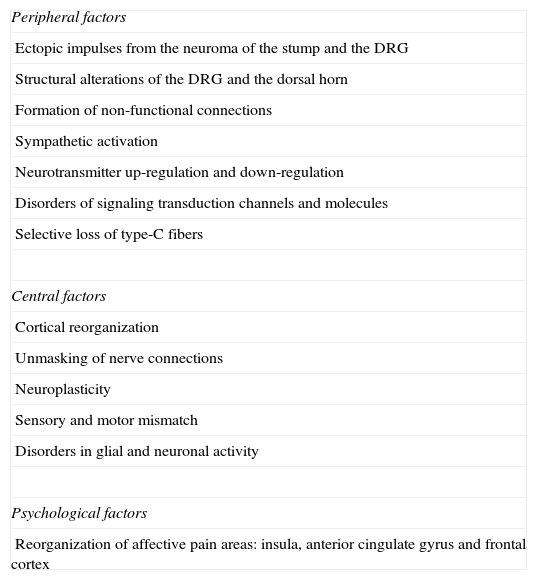
There are some factors associated with the onset of phantom pain (Table 1)4,8,12:
Factors associated with PLP generation.
| Peripheral factors |
| Ectopic impulses from the neuroma of the stump and the DRG |
| Structural alterations of the DRG and the dorsal horn |
| Formation of non-functional connections |
| Sympathetic activation |
| Neurotransmitter up-regulation and down-regulation |
| Disorders of signaling transduction channels and molecules |
| Selective loss of type-C fibers |
| Central factors |
| Cortical reorganization |
| Unmasking of nerve connections |
| Neuroplasticity |
| Sensory and motor mismatch |
| Disorders in glial and neuronal activity |
| Psychological factors |
| Reorganization of affective pain areas: insula, anterior cingulate gyrus and frontal cortex |
Source: Authors’.
After nerve sectioning, there is retrograde degeneration and shortening of afferent neurons as a result of the injury, edema and axon regeneration. This phenomenon is known as sprouting and gives rise to neuroma formation, i.e., expanded and disorganized A and C fiber endings with ectopic firing that increases during mechanical and chemical stimulus. Type C fibers are characterized by the expression of an ectopic discharge showing a slow irregular pattern, associated with an up-regulation or “de novo” expression of sodium channels, and down-regulation of the potassium channels; additionally, there is an alteration of the transduction molecules for mechano-sensitivity signals.2,4,13 An example of the above is the fact that local anesthesia of the stump does not eliminate pain in all cases, while the injection of gallamine, a substance that increases sodium conductance, creates phantom pain.3,4 The non-functional connection between axons may also contribute to abnormal spontaneous activity.2 However, PLP manifests in some patients immediately after amputation and before neuroma formation, meaning that this latter mechanism does not fully explain the pathophysiology of PLP.1,4
An additional site of ectopic discharge is the dorsal root ganglion (DRG), which joins the ectopic activity originating in the stump neuroma and amplifies it, or produces crossed excitation, leading to the depolarization of the neighboring neurons.2,4 It has been shown that pain is reduced with the use of beta-adrenergic blockers or during the surgical blockade of sympathetic activity, while it increases with epinephrine injections. Several external factors such as temperature, oxygenation, and local inflammation over the neuromas or the DRG also play a critical role on the pathophysiology of this disease.2,9
Central factorsSpinal plasticityAfter peripheral nerve injury, there is evidence of central sensitization of the neurons of the posterior horn of the spinal cord. This process is characterized by long-term potentiation, where short-lasting nociceptive stimuli generate increased post-synaptic potentials during a long period of time.14,15 There is also hyperexcitability, down-regulation of inhibitory processes, structural changes in the primary central sensory nerve endings, interneurons and neuronal projections.16 Gabaergic and glycinergic interneurons in the spinal cord may be destroyed by rapid firing from the injured tissues and other effects of axotomy, or they may switch from inhibitory to excitatory due to the influence of the brain-derived neurotrophic factor (BDNF), thus contributing to hyperexcitability.2,4 Additionally, there is down-regulation of the opioid receptors on primary afferent endings and intrinsic spinal neurons. For this reason, cholecystokinin, and endogenous opioid receptor inhibitor, is up-regulated, exacerbating the disinhibitory effect.2,17,18 Another mechanism to explain sensitization is the inflammation-induced glutamate NMDA (N-methyl-d-aspartate) receptor facilitation.2,11
Nerve injury also produces a functional connection of low threshold inputs with ascending spinal projection neurons that transmit nociceptive information to supraspinal centers. An additional mechanism triggered by the injury is substance P release (normally produced by type Aδ and type C fibers) by Aβ mechanoreceptor fibers that behave as nociceptive. This may allow ectopic or normal activity in the Aβ fibers to trigger or maintain central sensitization. When this happens a normal harmless input from the Aβ fibers, an ectopic afference, and residual low threshold afferences may contribute to phantom pain sensation.2,15 Likewise, there is degeneration of C fibers in lamina II, making room for growth of Aβ fibers over this area (ending normally in laminae III and IV). This causes second order neurons in lamina II, which normally receive high threshold sensory signals, to begin to receive low threshold signals, giving rise to the perception of tactile stimuli as nociceptive, and inducing allodynia.2,9,15 On the other hand, altered sensory and motor responses have also been proposed, considering that the abnormal painful sensation might be related to a disconnect between motor intention and the sensory response associated with the activation of frontal and parietal areas in the brain.2
Cortical reorganizationExperiments have shown that after amputation of a digit in an adult monkey there is invasion of neuronal areas adjacent to the cortical area where the amputated digit was represented, consistent with neuroplasticity changes in the primary motor cortex (M1) and the primary somatosensory cortex (S1).1 Likewise, Ramachandran et al. observed reorganization of Penfield's humunculus by approximately 2–3cm in four upper limb amputees, with correspondence between stimulus sites in the face and phantom sensations in the amputated limb, suggesting that cortical reorganization may be the origin of this phantom phenomenon. However, it has been proposed that other areas of the brain may also be involved, since the phantom sensation in amputated arms may be evoked from distal regions of the arm representation in S1, such as the toe.1,2 It has also been reported that the more displaced the mouth representation is toward the anterior area of the arm, the more intense the PLP, indicating that topographic reassignment changes with time.4
Psychological factors, although they appear not to be part of the etiology, may affect the course and severity of pain. It may be that reorganization occurs not only in sensory pain areas but also in affective pain areas such as the insula, the anterior cingulate gyrus and the frontal cortex.4,8,12
Treatment of PLPAt present, there is no clear consensus about the efficacy of PLP treatment, since less than 10% of patients receiving medical treatment obtain long-term pain relief.19–21 Treatment may be pharmacological and non-pharmacological.1,3
Pharmacological treatmentOpioidsOpioids bind to opioid receptors centrally and in the periphery, creating analgesia without loss of touch, proprioception or consciousness.1 Huse et al.17 conducted a cross-over double-blind study in 12 patients with chronic PLP refractory to medical management and with an intensity of more than 3/10 on the Visual Analog Scale (VAS), randomly assigned to receive oral morphine (maximum dose of 300mg/day) or placebo. Their results showed a significant pain reduction during the treatment with oral morphine, in relation to the baseline level (t=3.51, p<0.01) compared with placebo (t=−1.99, p=0.036), and no significant reduction in this latter group in relation to the baseline level (t=2.18, p=0.026). In 42% of the patients treated with oral morphine, there was more than 50% pain reduction (p<0.05). In the same fashion, Wu et al.22 determined the effectiveness of intravenous (IV) morphine in a cross-over, randomized, double-blind, placebo-controlled study in 32 subjects with chronic PLP and stump pain. They showed significant pain reduction after 30min of completing the infusion, compared with placebo (p<0.01) (VAS score of 48 before the infusion, and of 30 after the infusion). The NNT to reduce pain by 30% with IV morphine was 2. Later, Wu et al.23 randomized 60 subjects with post-amputation chronic pain (stump pain and PLP) greater than 3/10 on the VAS to administration of oral morphine, mexiletine or placebo, and they found a mean change in pain intensity in relation to the baseline level (−1.4 for placebo, −1.5 for mexiletine and −2.8 for morphine, p<0.0001). A greater significant pain reduction was shown with oral morphine when compared with placebo and mexiletine (p=0.0003). The estimated NNT required to obtain 50% and 33% pain reduction with oral morphine was 5.6 and 4.5, respectively. In a cross-over, double-blind study, Ben Abraham et al.24 assessed the effectiveness of oral dextromethorphan (120 or 180mg/day) versus placebo during three weeks in three patients with PLP secondary to amputation due to neoplasm. They determined that the mean pain score on the VAS before treatment in all three patients was 8.5 to 10, and dropped to 2.8–7.1 (p<0.05) after completing three weeks of treatment with 120mg of dextromethorphan.
NMDA receptor antagonistsIn a double-blind, placebo-controlled study, Maier et al.25 randomized 36 subjects with chronic PLP to receive 30mg daily of memantine or placebo. At the end of 4 weeks, both groups showed pain improvement as measured by the Numerical Classification Scale (NCS). In the memantine group, the pain score dropped from 5.1 (±2.13) to 3.8 (±2.3), and in the placebo group, it dropped from 5.2 (±2.02) to 3.2 (±1.46) (p<0.05). The mean improvement of pain was also similar in both groups (47% and 40%, respectively) and the NNT was 4.5. Likewise, Schwenkreis et al.26 conducted a double-blind, placebo-controlled study in 16 subjects with chronic PLP randomized to receive up to a maximum dose of 30mg of memantine per day, or placebo, during a three-week period. Intra-cortical inhibition (ICI) and intra-cortical facilitation (ICF) were determined using transcranial magnetic stimulation (TMS) on days 1 and 21 of treatment. The average baseline pain score on the NCS was 4.1 (1.7–6.3) for the memantine group, and 6.8 (0.3–7.7) for the placebo group. In the memantine group, there was a significant increase in ICI (p<0.05) after 3 weeks of treatment (average of −0.3%, −13%, −22.0% in the placebo group versus −25.5%, −42% to +7.0% in the memantine group). Likewise, the IFC dropped significantly (p<0.05) in the memantine group (average of −1.5%, −57.0 to +51.0% in the placebo group versus −37.7%, −131.0 to +19.0% in the memantine group). A mean reduction of phantom pain was observed in both groups (−0.9, −3.2 to +1.2 in the placebo group; −2.5, −6.3 to +0.3 in the memantine group, p<0.05), with no significant differences between the two groups. Memantine-mediated mechanisms may significantly influence the increase of ICI and the reduction of IFC over the area of the brain contralateral to the amputation. However, results suggest that these changes in cortical excitability and in PLP are independent. Likewise, Weich et al.27 conducted a randomized cross-over, double-blind, placebo-controlled study in 8 patients with chronic PLP and found no significant changes in mean pain intensity measured on the VAS between the baseline level and after four weeks of treatment with 30mg daily of memantine or placebo, or between the two treatments (mean baseline pain between the two groups was 40, and after 4 weeks it was 42 with memantine and 43 with placebo, p=0.16). During memantine treatment, 5 patients reported a slight increase in pain (46.98±20.38 mean baseline pain, 51.51±20.61 with memantine). In a randomized, double-blind, cross-over study, Eichenberger et al.28 compared ketamine i.v. with calcitonin, ketamine plus calcitonin, and placebo in 20 patients with chronic PLP greater than 3/11 on the VAS. The percent change in pain immediately after completing the treatments showed that calcitonin was no different than placebo, while ketamine alone and in combination resulted in a significant reduction of pain on the VAS, compared to placebo and calcitonin (p<0.05).
AnticonvulsantsGabapentin exerts its analgesic effect as it binds to the δ2α subunits of voltage-dependent calcium channels of posterior horn neurons9. Bone et al.29 assessed gabapentin in a randomized, double-blind, cross-over, placebo-controlled study in 19 patients (mean age: 55) with chronic PLP of more than 4/10 on the VAS. A maximum dose of 2.4g of gabapentin per day was administered for six weeks, followed by one week with no treatment, with a finding that the mean difference in pain intensity on the VAS in the gabapentin group was significantly greater than in the placebo group at the end of treatment (3.2±2.1 versus 1.6±0.7, p=0.03). Smith et al.30 conducted a randomized, double blind, placebo-controlled, cross-over study in 24 subjects with chronic PLP of an intensity greater than 3/10 on the NCS who received 3.6g of gabapentin per day. At the end of treatment, no significant differences were found in pain score changes before and after treatment in either group (0.94±1.98 versus 0.49±2.20 for placebo, p=0.70). The joint analysis of the results of the two latter studies for the change in pain intensity with respect to placebo showed a mean difference of −1.16 (95% CI, −1.94 to −0.38, p=0.004) in favor of gabapentin.31 On the other hand, despite the fact that carbamazepine has been used in the management of neuropathic pain, there is only one case reported in PLP, with negative results.19,32 Although pregabalin is recommended as first-line option for the management of neuropathic pain, only a few cases of PLP have been reported. There is no sound evidence to date supporting the use of other anticonvulsants such as topiramate, lamotrigine or oxcarbazepine, although they have been shown to be successful in a few case reports.33 Additionally, phenytoin, the first anticonvulsant used as antinociceptive has not shown consistent evidence of reducing neuropathic pain.34
AntidepressantsTricyclic antidepressants are used most commonly. They modulate pain by blocking calcium and sodium channels and the NMDA receptor, and they also block monoamine-reuptake inhibition.21 Robinson et al.35 compared amitriptyline 125mg daily versus benztropine mesylate in 39 subjects with chronic phantom pain, and found no significant differences between the two groups on the NCS (3.1±2.7 for amitriptyline versus 3.1±2.9 for benztropine, p<0.05) after 6 weeks of treatment. Likewise, a systematic review showed that amitriptyline is not effective for the treatment of PLP.36 Other antidepressants such as duloxetine, venlafaxine, chlorimipramine and nortriptyline have been studied only in case reports.33
CalcitoninThe mechanism of action of calcitonin in PLP is still unknown.9 Jaeger et al.37 compared calcitonin versus placebo in 21 subjects with severe PLP occurring with 0–7 days after amputation. The first infusion of calcitonin or placebo was administered when the pain score was higher than 3 on the Numerical Analog Scale (NAS), and if it persisted, the infusion was repeated (cross-over). After 24h of 200IU of calcitonin, the average pain score dropped from 7 to 4 in both groups (p<0.001), independently of whether the first infusion was calcitonin or placebo. No changes were found in the pain score (mean of 7 on the NAS, p>0.1). Eichenberger et al.28 assessed pain intensity on the VAS at 48h of infusion of a similar dose, and found no improvement. The number of individuals who had more than a 50% improvement was no different from the placebo group in these two studies (2 out of 20 versus 1 out of 19). These conflicting results may be explained by the fact that calcitonin might have no effect on central sensitization in chronic PLP.30,37
AnestheticsCasale et al.38 in a double blind, placebo-controlled, cross-over study, assessed myofascial injections contralateral to the area of pain with a single administration of 2.5mg of bupivacaine in 8 patients with chronic PLP and found no significant differences in either group after the first (7.6±1 versus 7.7±0.6 on the VAS, p=0.9) or the second injection (8±1 versus 7.6±0.3 on the VAS, p=0.45). In the bupivacaine group there was a significant reduction in the pain score versus placebo 1h after its administration (−5.3±1.4 versus −1.5±1.3, p=0.003). Lidocaine was ineffective after an infusion of 4mg/kg (p>0.05) during 30minutes in 31 subjects with chronic PLP, perhaps due to the peripheral action of lidocaine and its minor central effect, reducing ectopic discharge. The exact mechanisms by which local anesthetic injections reduce pain are not known.31
On the other hand, Lambert et al.39 randomized 30 subjects to receive epidural bupivacaine (0.166%, 2–8mL/h) and diamorphine (0.2–0.8mg/h) 24h before, during and 3 days after surgery (14 patients) or to receive perineural bupivacaine (0.25%, 10mL/h) during and after surgery (16 patients). They found that at 3 days, 6 and 12 months of follow-up, 29%, 63% and 38% in the epidural group versus 44%, 88% and 50% in the perineural group had phantom pain (p=0.32; p=0.25; p=0.61, respectively) and concluded that epidural blockade within 24h of the amputation is not better than local perineural anesthetic in preventing PLP. Likewise, Borghi et al.40 conducted a prospective study in 62 patients with PLP who received 0.5% ropivacaine intra-operatively (5ml/h) continued for an average of 30 post-operative days. The ropivacaine infusion was restored if the score on the Verbal Scoring Scale (VSS) was greater than 1, and it was interrupted if the score remained between 0 and 1. After the first post-operative day, 73% of patients had a score greater than 2 on the VAS, but at 12 months of follow-up the incidence of severe-to-intolerable pain was 3%, while 84% reported absence of pain. They concluded that prolonged post-operative perineural infusion of ropivacaine was effective in the treatment of PLP, probably because it prevents transmission of nociceptive inputs from A and C fibers during a prolonged period of time. This prevents spontaneous firing and increases the number of central nerve endings that are able to maintain central sensitization and the permanent structural changes in the synaptic region of the posterior horn of the spinal cord.
Non-pharmacological treatmentThese types of treatments include Transcutaneous Electrical Nerve Stimulation (TENS), electroconvulsive therapy (ECT), mirror therapy, acupuncture, deep brain stimulation, and spinal cord stimulation, among others.1,41
TENSThe technique uses a portable generator of electric current that crosses the intact surface of the skin, activating nerve fibers. Its action depends on the type of fibers that are stimulated. When large diameter Aβ fibers are stimulated, the effect is segmental analgesia (conventional) while if the small diameter Aδ fibers are stimulated, the effect is extra segmental analgesia.41 Mulvey et al.42 published a meta-analysis to assess the analgesic effectiveness of TENS in the treatment of PLP and stump pain in amputees, showing that there is no randomized clinical trial examining the effectiveness of this therapy in phantom pain. Later, Mulvey et al.43 assessed the effect of TENS in 10 subjects with PLP who had a score of more than 3 on the NCS and were prosthesis users. They found that average pain intensity dropped by 1.8±1.6 at rest (p<0.05) and 3.9±1.9 during movement 60min after the therapy (p<0.05), concluding that TENS may reduce PLP at rest and during movement.
ECTRassmussen et al. reported on two patients with severe PLP (9/10 and 10/10 on the VAS) refractory to multiple therapies, with no associated psychiatric disorders, who received 5 ECT sessions. At the end of treatment, pain had improved in one of the patients, who remained in remission after 3.5 years of follow-up. However, the mechanism of action has not been totally elucidated, and there are no randomized trials.44
Mirror therapyThis treatment option was proposed by Ramachandran et al.45 in 1996 and consists of visualizing the movement of the amputated limb and at the same time observing the normal movement of the other limb. This enhances reorganization and integration of mismatch between visual and proprioceptive feedback. It is based on the mirror neuron theory described by Rossi et al.,46 which proposes that a mirror neuron fires when the subject observes and performs the same action with the contralateral limb. Chan et al.47 randomized 18 subjects with PLP to mirror therapy, covered mirror, and mental visualization training. The study consisted of four weeks of 15min of therapy every day, and assessed pain severity. It found that pain diminished in 100% of the subjects in the mirror group, 17% in the covered mirror group, and 33% in the mental visualization group, with worsening of pain in 50% and 67% in the latter two groups, respectively. When changes in the VAS scores were compared after 4 weeks, a significant difference was found in the mirror group in comparison with the other two groups (p=0.04 and p=0.002). Although the mechanisms are not being researched, results suggest that this therapy could be useful in reducing pain in amputees.
Repetitive transcranial magnetic stimulation (rTMS)There is evidence that a single rTMS session may provide transient improvement of pain in patients with chronic neuropathic pain. In the majority of studies to date, stimulation has been applied to the ipsilateral hemisphere.48 Töpper et al.49 did not find any improvement in two patients with PLP who received rTMS (15Hz, 2s) for 3 weeks in the contralateral parietal cortex. In contrast, Di Rollo et al.48 reported one case of PLP treated with rTMS (1Hz for 15min, 600 stimuli/session) on the motor cortex contralateral to the amputation, showing that pain was diminished by 33% over the 3-week period. However, randomized controlled studies are needed in order to determine the effectiveness of this therapy.
AcupunctureBradbrook50 reported three cases of patients with acute and chronic PLP treated with acupuncture on the contralateral limb in order to stimulate normal afferent inputs to the nervous system and produce analgesia. The changes in pain intensity were measured on the VAS, showing pain reduction in two of the three cases after the end of treatment. However, to date there are only case reports describing the effect of acupuncture, and there are now studies with a methodological design that may allow to draw conclusions.
ConclusionAt present, there are no randomized studies with sample sizes that ensure power, or blinded for end-point assessment, to support the evidence on pharmacological and non-pharmacological treatments of PLP. Consequently, more studies with good methodological design are required in order to arrive at clear conclusions about treatment efficacy and to give stronger recommendations for clinical practice.
Note: We declare that both the principal author as well as the co-authors have reviewed and approved the referenced research work and that this work has not been published in other places or medical journals.
FundingSupported by Instituto de Ciencia y Tecnología. COLCIENCIAS. Code Number 6566-49-326169.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Malavera Angarita MA, Carrillo Villa S, Gomezese Ribero OF, García RG, Silva Sieger FA. Fisiopatología y tratamiento del dolor de miembro fantasma. Rev Colomb Anestesiol. 2014;42:40–46.