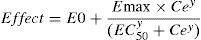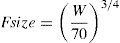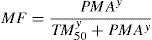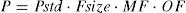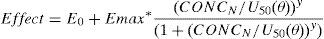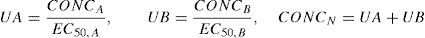Adult parameter estimates for pharmacokinetic models over-predict plasma concentrations in children. Parameter sets that describe 3-compartment pharmacokinetic models for propofol have been published and some are now incorporated into commercially available paediatric infusion pumps. Pharmacokinetics in children is influenced by size and age. Other covariates remain poorly understood. There are no integrated pharmacokinetic-pharmacodynamic analyses available. However, methodology exists that enables us to link pharmacokinetic parameter sets with pharmacodynamic data in order to calculate a rate constant linking serum and effect site concentrations. Literature concerning paediatric total intravenous anaesthesia was reviewed to appraise these parameter sets (‘models’) and to outline the pharmacology behind them, their limitations and pitfalls.
Los parámetros farmacocinéticos estimados en los modelos de adultos sobreestiman las concentraciones plasmáticas en niños. Se han publicado diversos conjuntos de parámetros que describen modelos de tres compartimentos para propofol y algunos están disponibles para bombas de infusión de uso pediátrico. La farmacocinética en niños está influenciada por el tamaño y la edad. Otras variables siguen siendo pobremente entendidas. No existen análisis integrados farmacocinéticos-farmacodinámicos. Sin embargo, existe la metodología que permite establecer un nexo entre los parámetros farmacocinéticos con datos farmacodinámicos y poder así calcular la constante de velocidad que vincula las concentraciones plasmáticas con las del sitio de efecto. En este estudio se hizo una revisión de la literatura en relación con el tema de anestesia intravenosa en pediatría para evaluar estos parámetros (modelos) y para destacar la farmacología detrás de ellos, sus limitaciones y problemas.
The goal of pharmacologic treatment is a desired response, known as the target effect. An understanding of the concentration–response relationship (i.e. pharmacodynamics, PD) can be used to predict the target concentration required to achieve this target effect in a typical child.1 Pharmacokinetic (PK) knowledge (e.g. clearance, volume) then determines the dose that will achieve the target concentration. Each child, however, is somewhat different and there is variability associated with all parameters used in PK and PD equations (known as models). Covariate information (e.g. weight, age, pathology, drug interactions, pharmacogenomics) can be used to help predict the typical dose in a specific patient. The Holy Grail of clinical pharmacology is prediction of drug PK and PD in the individual patient2 and this requires knowledge of the covariates that contribute to variability.
Anaesthesiologists are practicing pharmacologists and the use of total intravenous anaesthesia (TIVA) using propofol is a good example of ‘pharmacology in action’. There is a defined target effect (e.g. bisprectral index (BIS) 50), there is a target concentration of propofol known to achieve this (e.g. 3mg/L) and the PK of propofol in children have been described. Advanced concepts in pharmacokinetic modelling and computer technology have led to sophisticated delivery systems that facilitate anaesthesia given by the intravenous route. There remain some limitations to the current use of target controlled infusion in children. Hardware limitations, PK data lack in infants, and target monitoring issues restrict use. Drug dose is less than that in adults and care is required with dead volume, flow rates and delivery fluid and concomitant infusions.3,4 Although computerised pumps are a considerable advance over earlier manual techniques for children5,6 that targeted plasma alone, they require input of both pharmacokinetic and pharmacodynamic parameters and a lack of robust PK–PD estimates limits the use of TCI in children.
A literature review was undertaken using PubMed with search words and phrases related to children, total intravenous anaesthesia, PKPD modelling, maturation and size models. This review appraises PK parameter sets (‘models’) currently available for children and reviews the pharmacology behind them, their limitations and pitfalls.
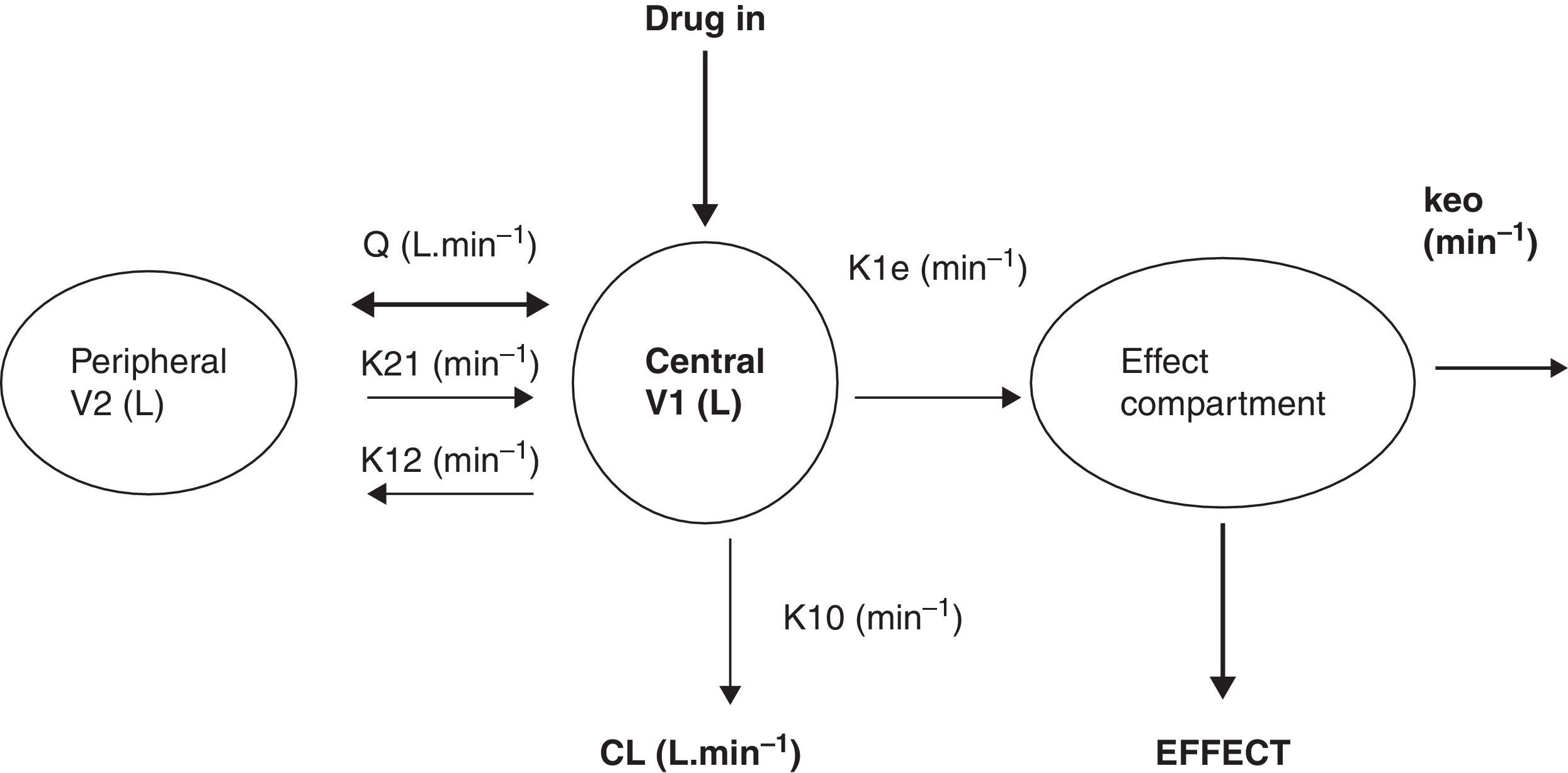
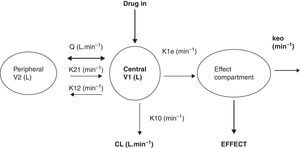
What is a PK–PD model?PK–PD models are equations used to describe both plasma concentration changes with time (PK) and concentration–effect relationships (PD). The two main pharmacokinetic properties of a drug are clearance (CL) and volume of distribution (V). The two main pharmacodynamic properties of a drug are the maximum effect (Emax) and the concentration producing 50% of the maximum effect (EC50). There is invariably a time delay between the concentration in the plasma and that presumed directly responsible for response at the effect site and this delay is commonly described by a first order rate constant. Fig. 1 shows a schematic for a drug described using a 2-compartment model with an additional compartment for the effect site.
The 2-compartment PK model is often described using two volumes (V1, V2) and two clearances (CL, Q), where Q is the inter-compartment clearance and volume of distribution at steady state (Vss) is the sum of V1 and V2. Another system of parameterisation is to use a central volume and three rate constants (k10, k12, k21) that describe drug distribution between compartments.
The relation between drug concentration and effect may be described by the Hill equation7:
where E0 is the baseline response, Emax is the maximum effect change, Ce is the concentration in the effect compartment, EC50 is the concentration producing 50% Emax and y is the Hill coefficient defining the steepness of the concentration–response curve. Jeleazcov et al.,8 have described propofol pharmacodynamics in children 1–16 years using BIS where E0 was estimated as 93.2, Emax −83.4, EC50 5.2mgL−1 and y 1.4. The rate constant (keo) describing for the effect compartment was 0.6min−1 (T1/2keo 1.15min).When both PK and PD data are collected simultaneously and parameters for both models are estimated together, then the model is described as ‘integrated’. PK estimates cannot be applied with PD estimates taken from a different data set without a few ‘fudge factors’. There are currently no integrated PK–PD analyses for children that span the paediatric age range.
Why adult PK parameters do not work in childrenThe use of adult parameter sets in TCI pumps for children result in concentrations lower than those observed in adults. A simple manual regimen for propofol infusion in adults9 is of a bolus of 1mgkg−1 followed by an infusion of 10mgkg−1h−1 (0–10min), 8mgkg−1h−1 (10–20min) and 6mgkg−1h−1 thereafter. Requirements for children however, are greater. A loading dose of 2.5mgkg−1 followed by an infusion rate of 15mgkg−1h−1 for the first 15min, 13mgkg−1h−1 from 15 to 30min, 11mgkg−1h−1 from 30 to 60min, 10mgkg−1h−1 from 1 to 2h and 9mgkg−1h−1 from 2 to 4h resulted in a steady state target concentration of 3mgL−1 in children 3–11 years.5 Increased requirements in children can be attributed to size factors. Decreased requirements in neonates are due to immature enzyme clearance systems. Organ dysfunction will also result in reduced requirements.
SizeClearance in children 1–2 years of age, expressed as Lh−1kg−1, is greater than that observed in older children and adolescents. This is a size effect and is not due to bigger livers or increased hepatic blood flow in that subpopulation. This ‘artifact of size’ disappears when allometric scaling is used. Allometry is a term used to describe the nonlinear relationship between size and function. A great many physiological, structural and time related variables scale predictably within and between species with weight (W) exponents (PWR) of 3/4, 1 and 1/4 respectively.10 These exponents have applicability to pharmacokinetic parameters such as clearance (CL exponent of 3/4), volume (V exponent of 1) and half-time (T1/2 exponent of 1/4).11 The factor for size (Fsize) for total drug clearance may be expressed:
Remifentanil clearance in children 1 month to 9 years is similar to adult rates when scaled using an allometric exponent of 3/4.12 A standardised clearance of 2790mlmin−170kg−1 is similar to that reported by others in children13,14 and adults.15,16 Nonspecific blood esterases that metabolise remifentanil are mature at birth.17
MaturationAllometry alone is insufficient to predict clearance in neonates and infants from adult estimates for most drugs (remifentanil excluded). The addition of a model describing maturation is required. The Hill model7 has been found useful for describing this maturation process (MF).
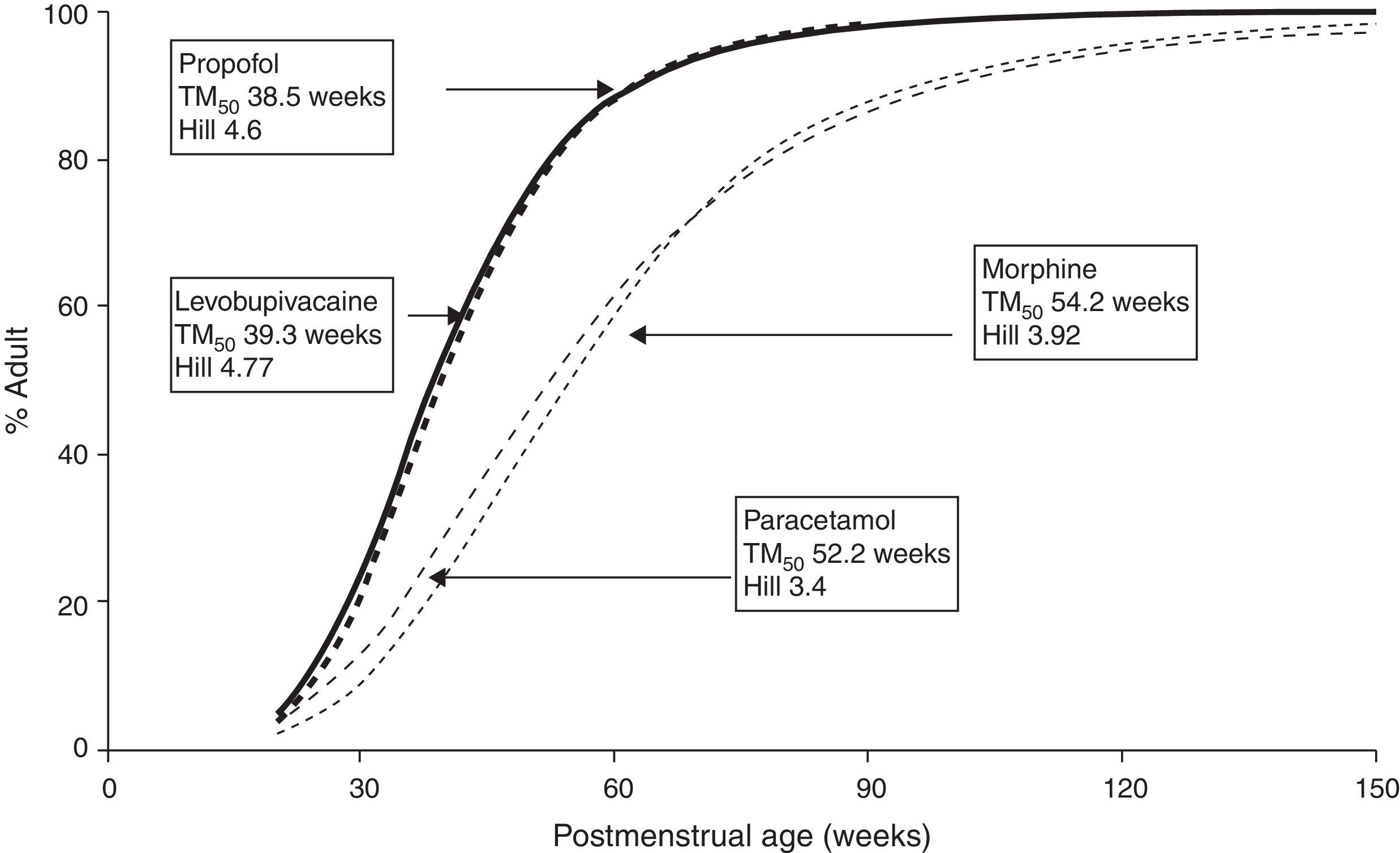
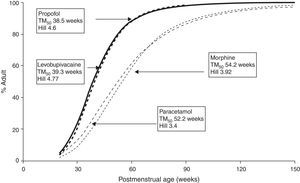
The TM50 describes the maturation half-time, while the Hill coefficient (y) relates to the slope of this maturation profile. Maturation of clearance begins before birth, suggesting that post-menstrual age (PMA) would be a better predictor of drug elimination than postnatal age (PNA). Fig. 2 shows the maturation profile for propofol using allometry and a maturation model. Clearance is immature in infancy and matures rapidly within the first 6 months of life.
Infusion rate of a drug at steady state is directly related to clearance. A failure to appreciate that bupivacaine clearance (CYP3A4) was immature at birth resulted in seizures in neonates given epidural infusions delivered at rates used in adults.18
Organ functionCompromised hepatic or renal function will reduce clearance. Pharmacokinetic parameters (P) can be described in an individual as the product of size (Fsize), maturation (MF) and organ function (OF) influences where Pstd is the parameter value in a standard size adult without pathological changes in organ function19:
Consequently, the programming of PK parameters for children over all ages (0–16 years) in TCI pumps may be slightly more complex than those in adults.
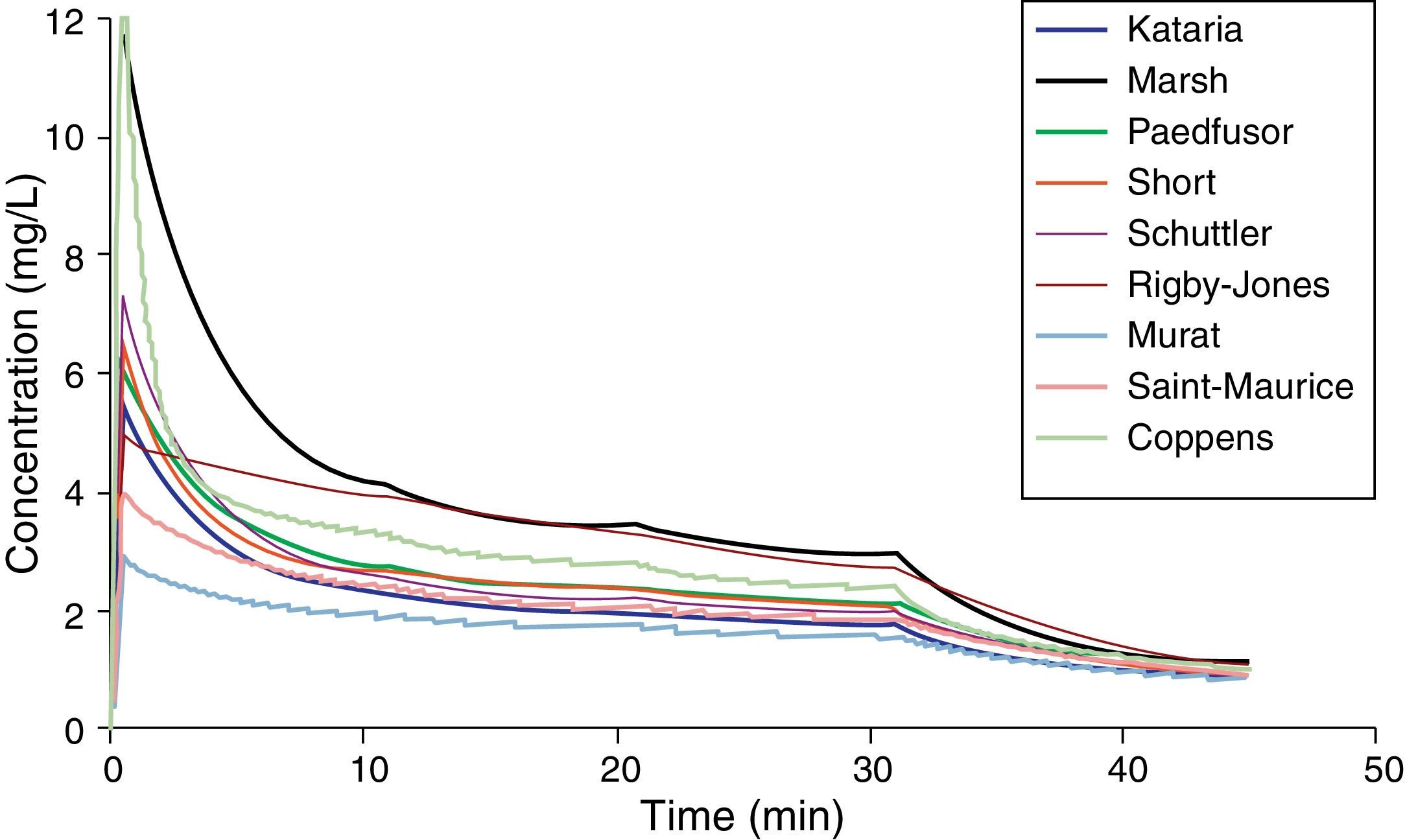
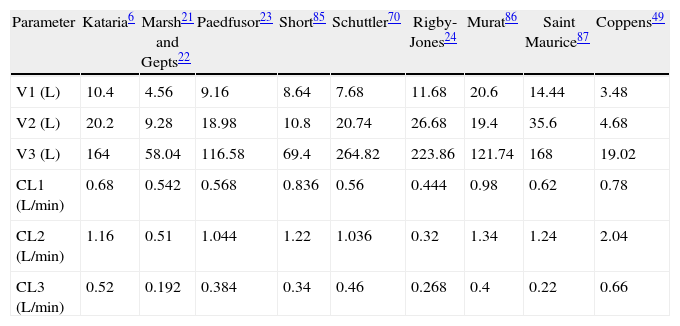
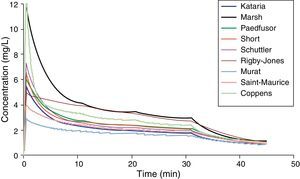
Paediatric PK parameter setsMost TCI techniques use propofol and remifentanil as the principle drugs for induction and maintenance of anaesthesia.20 Popular paediatric programmes used for propofol infusion targeting a plasma concentration are based on data from Marsh21 and Gepts22 (Diprifusor), Kataria6 or Absalom23 (Paedfusor). Parameter estimates are different for each author (Table 1). Covariate influences such as severity of illness are unaccounted for; the volume in the central compartment, for example, is increased in children after cardiac surgery.24 Both the administration method (intravenous bolus or infusion)25 and the collection of venous blood for assay rather than arterial blood will have influence on PK parameter estimates in the early phase when movement of drug into the effect compartment is occurring. Time–concentration profiles (Fig. 3) and context-sensitive half-lives will differ depending on which ‘model’ is used.26
Propofol parameter estimates for a 20kg child. Performance of these models differed markedly during the different stages of propofol administration. Most models underestimated propofol concentration 1min after the bolus dose, suggesting an overestimation of the initial volume of distribution. Not all models tested were within the accepted limits of performance (MDPE<20% and MDAPE<30%). The model derived by Short and colleagues performed best26 in children 3–26 months.
| Parameter | Kataria6 | Marsh21 and Gepts22 | Paedfusor23 | Short85 | Schuttler70 | Rigby-Jones24 | Murat86 | Saint Maurice87 | Coppens49 |
| V1 (L) | 10.4 | 4.56 | 9.16 | 8.64 | 7.68 | 11.68 | 20.6 | 14.44 | 3.48 |
| V2 (L) | 20.2 | 9.28 | 18.98 | 10.8 | 20.74 | 26.68 | 19.4 | 35.6 | 4.68 |
| V3 (L) | 164 | 58.04 | 116.58 | 69.4 | 264.82 | 223.86 | 121.74 | 168 | 19.02 |
| CL1 (L/min) | 0.68 | 0.542 | 0.568 | 0.836 | 0.56 | 0.444 | 0.98 | 0.62 | 0.78 |
| CL2 (L/min) | 1.16 | 0.51 | 1.044 | 1.22 | 1.036 | 0.32 | 1.34 | 1.24 | 2.04 |
| CL3 (L/min) | 0.52 | 0.192 | 0.384 | 0.34 | 0.46 | 0.268 | 0.4 | 0.22 | 0.66 |
Source: author from several references.
Validation studies are few. The Paedfusor has been examined23 and reported to have a MDPE (median performance error, bias) of 4.1% and a MDAPE (median absolute performance error, precision) of 9.7% over the age range investigated (1–15 years). A later study suggested that all except Marsh performed acceptably in children 3–26 months.26 Others have described a poor fit for Kataria, the most widely used model.27 However, clearance (Lh−1kg−1) decreases with age and MDPE is minimised at low CL and exaggerated at higher values. Evaluating models outside of the age range that they were determined from will increase bias and worsen precision.
Adult remifentanil PK parameters15 continue to be used in TCI devices for all ages, despite an increasing knowledge about this drug in children.28 There is an element of safety with this approach because both volume of distribution13 and clearance (expressed as mLmin−1kg−1)12 decrease with age throughout childhood and because the elimination half-life is small with a constant context sensitive half-life. The larger volume of distribution results in lower peak concentrations after bolus; the higher clearance in children results in lower plasma concentration when infused at adult rates expressed as mgmin−1kg−1. Owing to these enhanced clearance rates, smaller (younger) children will require higher remifentanil infusion rates than larger (older) children and adults to achieve equivalent blood concentrations.
The very youngUnfortunately, commonly used propofol data sets6,21–23 have only investigated PK in children out of infancy. Allegaert has attempted to link neonatal data with those from children,29 suggesting that clearance is only 10% that of the mature value at 28 weeks gestation and 38% at term (Fig. 2). A term neonate will achieve 90% that of an adult clearance (1.83Lmin−170kg−1) by 30 weeks after birth.30 Postnatal age (PNA) may also have an additional effect on maturation of propofol clearance above that predicted by PMA.31
Propofol infusion rates for infants have been suggested.32 Those regimens were determined by adapting an adult dosage scheme to the requirements of the younger population. Total number and time of administration of boluses and time to awakening were registered and used as criteria to adjust the dosage scheme. Predicted infusion rates are high (e.g. 24mgkg−1h−1 for the first 10min in neonates) and should be used cautiously. Delayed awakening, hypotension and an increased incidence of bradycardia were reported in neonates and infants.32 Propofol can cause profound hypotension in neonates and PK–PD relationships in this age group remain elusive.33
Pharmacodynamic endpointsThe common effect measure used to assess depth of anaesthesia is the electroencephalogram or a modification of detected EEG signals (spectral edge frequency, Bispectral index, entropy, cerebral state index).34 The BIS showed a close relationship with the modelled effect-site propofol concentration, and serves as a measure of anaesthetic drug effect in children older than 1 year.8 In infants their use cannot yet be supported in theory or in practice.35,36 During anaesthesia, the EEG in infants is fundamentally different from that in older children; there remains a need for specific neonate-derived algorithms if EEG-derived anaesthesia depth monitors are to be used for neonates and infants.
The target concentration desired in the effect site will vary with the effect sought. A propofol concentration of 2–3mgL−1 is commonly used for sedation, while 4–6mgL−1 is sought for anaesthesia. Both the loss and return of consciousness occur at similar target effect-site propofol concentrations (2.0 SD 0.9mgL−1 vs. 1.8 SD 0.7mgL−1) in adults37 and a “wake-up” concentration of 1.8mgL−1 is described in children.38
Target effect-site remifentanil concentrations commonly used for TIVA also vary. A target of 2–3μgL−1 is adequate for laryngoscopy, 6–8μgL−1 for laparotomy and 10–12μgL−1 might be sought to ablate the stress response associated with cardiac surgery.39 An ED50 of 3–3.5μg/kg for intubation when used in conjunction with propofol 5mgkg−1 has been suggested, although dose may change with age.40 Older children had a longer duration of apnea than infants, reflective of increased clearance (per kilo) in infants. Target concentration may also vary with pathology. For example opioid dosing is commonly reduced in children with cognitive impairment41; a practice supported by lower BIS scores in this cohort.42 Determination of bolus dose to achieve a target concentration is dependent on volume of distribution and this increases with decreasing age.13
The link parameterOpen-loop rather than closed-loop TCI is the routine in paediatric anaesthesia.43,44 A single first order parameter (T1/2keo) describes the equilibration half-time between the central and effect compartments. An accurate estimate of this delay is therefore a key issue for controlling depth of anaesthesia during induction, recovery or drug titration during the maintenance phase.45 It allows TCI systems to directly target the effect site concentration and optimise drug delivery by achieving a chosen level of effect as fast as possible without overshoot.45
Neither the T1/2keo for propofol nor for remifentanil in children has been described from an integrated PK–PD model. A commonly used estimate for T1/2keo in the adult Marsh model is 2.6min, although derivation of this value is vague; the estimate of 3.4min from Billard46 is longer, but it is a lot less than 0.58min of Struys47 or 0.43min of Schnider.25 The latter estimate was determined in a novel way to get around the common difficulty in clinical pharmacology simulation, where there is a wide choice of pharmacokinetic models but only one or two published linked PK–PD models.45 The time course of propofol concentrations in the effect site as predicted by a linked PK–PD model25,48 were used to simulate a graph showing the time of peak concentration (Tpeak) in this effect site. Paediatric parameters from the Marsh model21 were then used to estimate a T1/2keo (1.93min) that would achieve the same Tpeak. The estimated T1/2keo is specific to the PK parameters used and cannot be indiscriminately applied to a different PK parameter set.49 Parameter estimates vary between investigators and predict different time–concentration profiles. The T1/2keo will also be different for other effect measures. A T1/2keo of 0.3min was estimated for the Paedfusor using state entropy in children 6–15 years.50 In addition, Tpeak will be dependent on the rate at which drug is infused along with other hypnotic drug interactions and the effect desired.51,52 Anxiety and catecholamine release may further complicate this measure.52
The use of processed EEG signals may be associated with delay in signal processing. Clinical endpoints such as the blink reflex or arm movement are crude. The use of incorrect or poorly understood models can result in adverse effects, e.g. awareness or hypotension. For example, we might expect a shorter T1/2keo with decreasing age, based on allometric theory, and this is exactly what has been described by Jeleazcov.53 The model dependence of the T1/2keo was demonstrated by an estimate of 1.7min with the Kataria6 data set and 0.8min with the Paedfusor model.23 The effect measure used to calculate delay between plasma and effect will also have impact. BIS recordings in children produced shorter times to Tpeak (65 SD 14s) than those produced by an auditory evoked potential monitor (201 SD 74s).54 An alternative to an integrated PK–PD model is to relate dose directly to clinical effect (K-PD model).55 This is an effective clinical tool, but PK variability does not go away; it is simply shifted onto pharmacodynamic parameters.
An incorrect T1/2keo will result in excessive dose in a young child if the effect site is targeted and Tpeak is anticipated to be later than it actually is because it was determined in a teenager or adult. Practices such as targeting the plasma concentration rather than the effect site concentration may avoid hypotension, but the sophistication of current TCI pumps could allow better control if they were programmed with improved information.
PKPD parameter variabilityThere is considerable variability in any measured plasma concentration when identical doses are given to individuals. Typical values for population PK parameter variability are 50% for compliance with medication regimens, 30% for absorption, 10% for tissue distribution, 50% for metabolic elimination and 20% for renal elimination.56 Similar variability exists for PD parameters.
A number of factors other than size and age contribute to this variability; temperature, pathology, type of surgery, circadian rhythms, pharmacogenomics will all have impact.57 One factor that is becoming increasingly important is obesity.
A scaler for dosing in obese childrenPharmacokinetic properties of some drugs are known to be changed in obesity.58 Although body fat has minimal metabolic activity, fat mass contributes to overall body size and may have an indirect influence on both metabolic and renal clearance. On the other hand, the volume of distribution of a drug depends on its physicochemical properties.59 There are drugs whose apparent volume of distribution may be independent of fat mass (e.g. digoxin) or be extensively determined by it (e.g. diazepam). A number of size descriptors have been put forward for use in the obese patient, e.g., total body weight (TBW), lean body weight (LBW), ideal body weight (IBW), body mass index (BMI), fat free mass (FFM), normal fat mass (NFM).
One controversial issue for propofol dose adjustment in the obese has been the selection of an adequate size descriptor to scale pharmacokinetic parameters.60,61 In normal weight subjects, total body weight is a good size descriptor and a good approximation of lean body mass.60 However, in obese patients, total body weight will overestimate lean body mass because the increase in this variable represents only 20–40% of total excess of weight.58,61–63 There remains controversy regarding which size descriptor provides the most accurate information about the relationship between propofol dose and its plasma concentrations.61
The effective dose of propofol for loss of lash reflex was significantly lower in obese paediatric patients (ED95 2.0mgkg−1) in comparison with non-obese patients (3.2mgkg−1). Obese children (BMI>95th percentile for age and gender) require a lower weight-based dose of propofol for induction of anaesthesia than do normal-weight children.64 This is because there is a nonlinear relationship between weight and V1 and CL. Although lean body weight has been put forward as the ideal measure for propofol induction,65,66 allometric scaling using total body weight using an exponent of 0.75 may be more appropriate for maintenance.67,68 An exponent for clearance of 0.75 for propofol in obese adults69 and non-obese adults and children70 is reported. A recent analysis suggests an exponent of 0.8 in obese children,71 an estimate indistinguishable from 0.75 over the human weight range.69
An allometric model using TBW may be appropriate for propofol clearance, but inappropriate for remifentanil where lean body weight is a better size descriptor.72 The size descriptor may differ for each drug. The use of normal fat mass with allometric scaling as a size descriptor may prove versatile.73 Size based on NFM assumes that FFM is the primary determinant of size with an extra factor (which may be positive or negative) that determines how fat mass contributes to size.
Drug interactionsPharmacokinetic interactions between propofol and alfentanil74 and midazolam75 are reported in adults. Both drugs reduce the metabolic and rapid and slow distribution clearances of propofol. Competition for the CYP3A4 clearance pathway between propofol, midazolam and alfentanil may also impact alfentanil clearance. In addition, a reduction in mean arterial blood pressure is associated with propofol pharmacokinetic alterations that increase the blood propofol concentration.75 We might anticipate similar interactions in children.
Phenobarbitone use for seizure control in children induces those enzymes responsible for the clearance of ketamine (CYP3A4, CYP2C9, CYP2B6).76 The concentration–response curve for both ketamine sedation and EEG activity effects are steep.77,78 This means that small serum concentration changes will have dramatic effect on the degree of sedation observed. Consequently children receiving long term phenobarbitone require more ketamine for sedation and rouse earlier than those not on the barbiturate.79
Pharmacodynamic interaction models use a modification of the Emax model
Drug concentrations for the two different drugs are normalised to a relative potency and combined, creating what can be considered a ‘new drug’.80 This is expressed as:
where CONCA is the concentration of drug A, EC50,A is the concentration of drug A associated with 50% of the maximal drug effect, CONCB is the concentration of drug B, EC50,B is the concentration of drug B associated with 50% of the maximal drug effect (or Emax), and CONCN represents the combined normalised concentration of both drugs. This methodology has been used in children. The EC50 Remifentanil, keoRemifentanil, EC50 Fentanyl, and the keoFentanyl were 24.1μgL−1, 0.71min−1, 8.6μgL−1, and 0.28min−1, respectively.8 These data support observations that remifentanil halves the EC50 of propofol for successful insertion of the laryngeal mask airway and laryngeal tube in paediatric patients.81 The additive drug interaction implies that fentanyl and remifentanil may be interchangeable for obtaining a predetermined BIS value.
Adverse effectsThe initial loading dose of remifentanil may cause hypotension. This hypotensive response has been quantified in children undergoing cranioplasty surgery. A steady state remifentanil concentration of 14μgL−1 would typically achieve a 30% decrease in MAP. This concentration is twice that required for laparotomy, but is easily achieved with bolus injection. The T1/2keo of 0.86min for this haemodynamic effect82 is less than remifentanil-induced spectral edge changes described in adults (T1/2keo=1.34min).15
Fentanyl combined with propofol can cause a propranolol-like effect on the sinus node with the potential to enhance cardiac vagal tone.83 Hypotensive effects,33 reversion to neonatal circulation and reduced clearance causing prolonged sedation in neonates remain stumbling blocks to the use of TIVA in this age group.84
ConclusionsTarget controlled pharmacokinetic programmed pumps may both assist clinical anaesthesia and serve as educational tools for demonstrating dynamic pharmacology. Our increasing knowledge of covariates (e.g. size, age, pathology, drug interactions) will assist pump programming in children. However, use in the very young will be limited until pharmacokinetics and adverse effect profiles are clarified. Use in infants will be improved with better effect monitoring. The current multitude of pharmacokinetic parameter sets for children that are poorly validated and the lack integrated PD models confuses novice operators. Despite these limitations, use of paediatric TIVA continues to gain popularity with ‘work around’ (e.g., keo determination by Tpeak methodology) or empirical solutions based on common practice.
Conflict of interestThe author declares no conflict of interest.
Thanks to Dr Ignacio Cortinez (Santiago, Chile) for Spanish translation.
Please cite this article as: Anderson BJ. La farmacología de la anestesia total intravenosa en pediatría. Rev Colomb Anestesiol. 2013. http://dx.doi.org/10.1016/j.rca.2013.05.008.