Introduction: Prior studies have shown that the conventional management of post-partum pain (acetaminophen, NSAIDS) is insufficient. In our setting, no advantage is taken of the epidural catheter placed as part of the analgesic management of pregnant women during labor.
Objective: To determine the efficacy of 2 and 3 mg doses of epidural morphine used for analgesia during vaginal post-partum in patients receiving epidural analgesia for labor, compared to placebo.
Patients and methods: Double-blind randomized clinical trial with 114 patients, in which 38 patients received 10 ml of anesthetic solution with 2 mg of epidural morphine, 39 received 3 mg of epidural morphine, and another 37 received 10 ml of 0.9% epidural saline solution (control group), 1 hour after labor. The analgesic efficacy and the side effects occurring within the first 24 hours after administration were evaluated.
Results: The dose of morphine was effective at controlling pain after delivery, making it possible to reduce the need for additional analgesia in the group receiving 2 mg (NNT=4.56), as well as in the group receiving 3 mg of morphine (NNT=3.66). The outcome was more marked in patients who needed perineorrhaphy (NNT =1.6) and in primiparity cases (NNT 2.4). In the experimental group, the analgesic effect extended over the 24-hour follow-up period.
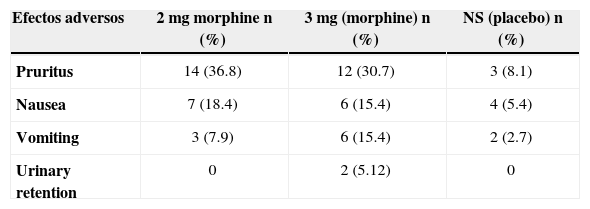
The incidence of side-effects in each of the treatment groups was as follows: pruritus, 30% and 36%; nausea, 18.4% and 15.4%; vomiting, 7.9% and 15.4%.
Conclusions: The use of epidural morphine during the immediate post-partum period is effective for managing pain ensuing after labor. Although there was evidence of adverse side effects at the doses used, they were tolerable and did not require treatment. The present management of post-partum analgesia is insufficient..
© 2012 Sociedad Colombiana de Anestesiología y Reanimación. Published by Elsevier. All rights reserved.
Introducción: Estudios previos han demostrado que el manejo convencional de dolor posparto (acetaminofén, AINE) es insuficiente. En nuestro medio se desaprovecha el uso del catéter epidural que es colocado como parte del manejo analgésico de las gestantes en el trabajo de parto.
Objetivo: Determinar la eficacia de dosis de 2 mg y de 3 mg de morfina epidurales versus placebo, empleadas para analgesia durante el posparto vaginal en pacientes que recibieron analgesia epidural para su trabajo de parto.
Pacientes y métodos: Ensayo clínico, doble ciego y aleatorizado realizado con 114 pacientes. 38 pacientes recibieron 10 ml de solución anestésica con 2 mg de morfina epidural, 39 pacientes recibieron 3 mg de morfina epidural, y 37 otras 10 ml de solución salina al 0,9% por vía epidural (grupo control), 1 hora después de la atención del trabajo de parto. Se evaluó la eficacia analgésica así como los efectos secundarios presentados durante las 24 horas posteriores a la administración.
Resultados: La dosis de morfina fue efectiva para el control del dolor consecutivo al parto y se logró disminuir la necesidad de analgésico adicional tanto en el grupo de 2 mg (NNT 4,56) como en el de morfina 3 mg (NNT = 3,66). El resultado fue más marcado en el grupo de pacientes con necesidad de perineorrafia (NNT = 1,6) y en las madres primíparas (NNT 2,4). En el grupo experimental, el efecto analgésico se extendió a las 24 horas de seguimiento. En nuestro estudio, la incidencia de efectos secundarios fue la siguiente: prurito, 30% y 36%; náuseas, 18,4% y 15,4%; y vómito, 7,9% y 15,4% para cada uno de los grupos experimentales. Conclusiones: El uso de morfina epidural en el posparto inmediato es eficaz para el manejo del dolor que sobreviene al trabajo de parto. Si bien se evidenció una importante presencia de efectos adversos a las dosis usadas, estos fueron tolerables y no requirieron de tratamiento. El manejo actual de la analgesia posparto es insuficiente.
© 2012 Sociedad Colombiana de Anestesiología y Reanimación. Publicado por Elsevier. Todos los derechos resevados.
Opioids are the fundamental pillar in the treatment of post-operative pain. The use of opium dates back 4000 years, and its side effects, in particular respiratory depression, were noted approximately 600 years ago.1
The use of opioids in obstetric patients started in 1907 in Austria with Richard Von Steimbuchel,2 who described the use of morphine and subcutaneous scopolamine for the analgesic management of women in labor. The technique was also used by Carl Gauss and Bernard Kronig in Germany.
In 1947 in Havana (Cuba), Manuel Martínez Curbelo reported the first cases where an epidural catheter was used for continuous analgesic management.
The finding in 1971 of opioid receptors in cellular fragments of mouse brains,3 in mammal brains in 1973,4 and in primate bone marrow in 1976,5 led authors Yaksh and Rudy to study intrathecal opioid effectiveness for pain relief in animals in 19766 and, finally, Behar7 and Wang8 and their collaborators to study their use in humans in 1979. Pharmacokinetic and pharmacodynamic studies were also undertaken, although these characteristics are still not very well understood.9-11
In gynecological and obstetric anesthesia, attention has been focused on labor analgesia and management, with less attention paid to post-partum analgesia (which has a ceiling effect); no use is made of the epidural access already established as part of the initial management during labor.12, 13
Many studies have shown the incidence of post-partum pain, in particular perineal pain caused by many factors, including primigravity, vaginal tears, instrumentation, etc. Rates of perineal pain of up to 75% have been found after vaginal delivery, triggered by labor.14, 15
Post-partum epidural administration of morphine is a simple, inexpensive procedure, particularly when the catheter for analgesic management of pain during labor is already in place (and is usually removed after delivery).
Spinal and epidural morphine has shown effectiveness because of rostral migration, providing analgesic effect for more than 24 hours after a single dose.
Patients and methodsA double-blind controlled clinical trial was conducted with 114 patients in the Mother and Child Institute in Bogotá, Colombia. The women were aged 14 to 45, and were in vaginal post-partum period after receiving conventional analgesic management through an epidural catheter during labor. Patients with an absolute contraindication for epidural analgesia, or who were unable to provide an informed consent were excluded, as well as those with, systemic infection, coagulation disorder, spinal disease, mental disorders, history of drug addiction, sleep apnea syndrome, and patients with a history of any degree of reaction to opioids.
Definition of the interventionThe intervention consisted of the epidural delivery of morphine in two different doses of 2 mg and 3 mg vs. placebo (saline solution) one hour after vaginal delivery in patients who had received epidural analgesia during labor. The objective was to assess the post-partum analgesic efficacy of the two doses vs. placebo.
In order to determine whether the proposed doses were effective at controlling pain, the analysis consisted of determining the differences between the experimental groups and the control group, as well as examining the statistical significance of the differences encountered. This was done by applying the corresponding tests, depending on whether or not each variable distributed normally.
The outcome variables were the following: pain intensity rating, number of analgesics required, analgesic dose required to achieve adequate pain control, number of pain episodes, percentage of patients with no pain episodes, and length of action of the dose used. Confounding variables analyzed were parity and the need for perineorrhaphy, either due to episiotomy or because of the presence of perineal wall tears warranting suture, the need for instrumentation during delivery, or permanence with the newborn.
Safety was evaluated by determining the incidence of side effects, in particular those described in the literature, such as pruritus, nausea, vomiting, urinary retention and respiratory depression.
Anesthetic techniqueThe subjects included in the study were ASA 2 patients in labor receiving analgesia through an epidural catheter. A conventional 18 Touhy catheter was introduced using the technique of loss of resistance, ideally at the L3-L4 level.
Patients were started on a conventional dose of analgesia with 19 mg of 0.5% bupivacaine + fentanyl 100 mcg in a 10 cc bolus. Additional boluses of bupivacaine 10 mg + fentanyl 100 mcg were administered depending on the need for a boost in a 10 cc bolus followed by 8cc -16cc every hour.
Morphine, previously packaged in the laboratory and masked for the anesthetist at the time of applying the dose of 2 mg, 3 mg, or the saline solution, was administered one hour after delivery. This time period was selected in order to avoid the residual analgesic effect of the local anesthetic and the opioid given for epidural analgesia during labor.
All patients were managed with the conventional analgesic regime used at the Institution (acetaminophen 1g PO every 6 hours). In those cases where additional analgesics were required, the first option was diclofenac 75 mg IM or a dose of tramadol 100 mg IV, and titrated doses of morphine only if additional analgesic was required.
The patients were under conventional monitoring for 4–6 hours during the initial post-partum period in the post-anesthetic care unit. They were then checked 4, 8, 16 and 24 hours afterwards in order to determine the level of sedation, heart rate, the presence of pruritus, nausea or vomiting, and the pain scale.
The primary objective during monitoring was to determine the need for additional analgesia based on the description of the numerical pain scale.
Variables such as parity, the need for perineorrhaphy, either due to episiotomy or due to perineal wall tears requiring suture were defined.
This was a single-center, parallel group double blind study, and the subjects were randomly assigned in the same proportion to the active drug groups or the placebo group.
Sample size was calculated on the basis of the two-tail hypothesis, alpha = 0.025 and beta =0.05, and taking into consideration the report that post-partum use of conventional analgesia with acetaminophen analgesia is effective in 25% of patients.15
Randomization for each of the three groups was done independently, and the sterile syringes were prepared by a pharmacist according to the assignment.
The syringes were marked with numbers so that the investigator would not we aware of their content at the time of application. The samples were unmasked at the end of the trial.
The patients included accepted and signed the informed consent. This study was approved by the Ethical and Research Committee of the Hospital La Victoria- Instituto Materno Infantil de Bogotá. The protocol of this study was registered at Clinicaltrial.gov with the number 7245408".
Statistical analysisThe analysis was performed using the SPSS 18 and Epidat statistical packages. A descriptive analysis was conducted for the independent variables, the analgesic effectiveness, and the side effects. Proportion differentiations were established between the two groups by calculating the relative risk (RR) for the two treatment groups compared with the placebo group.
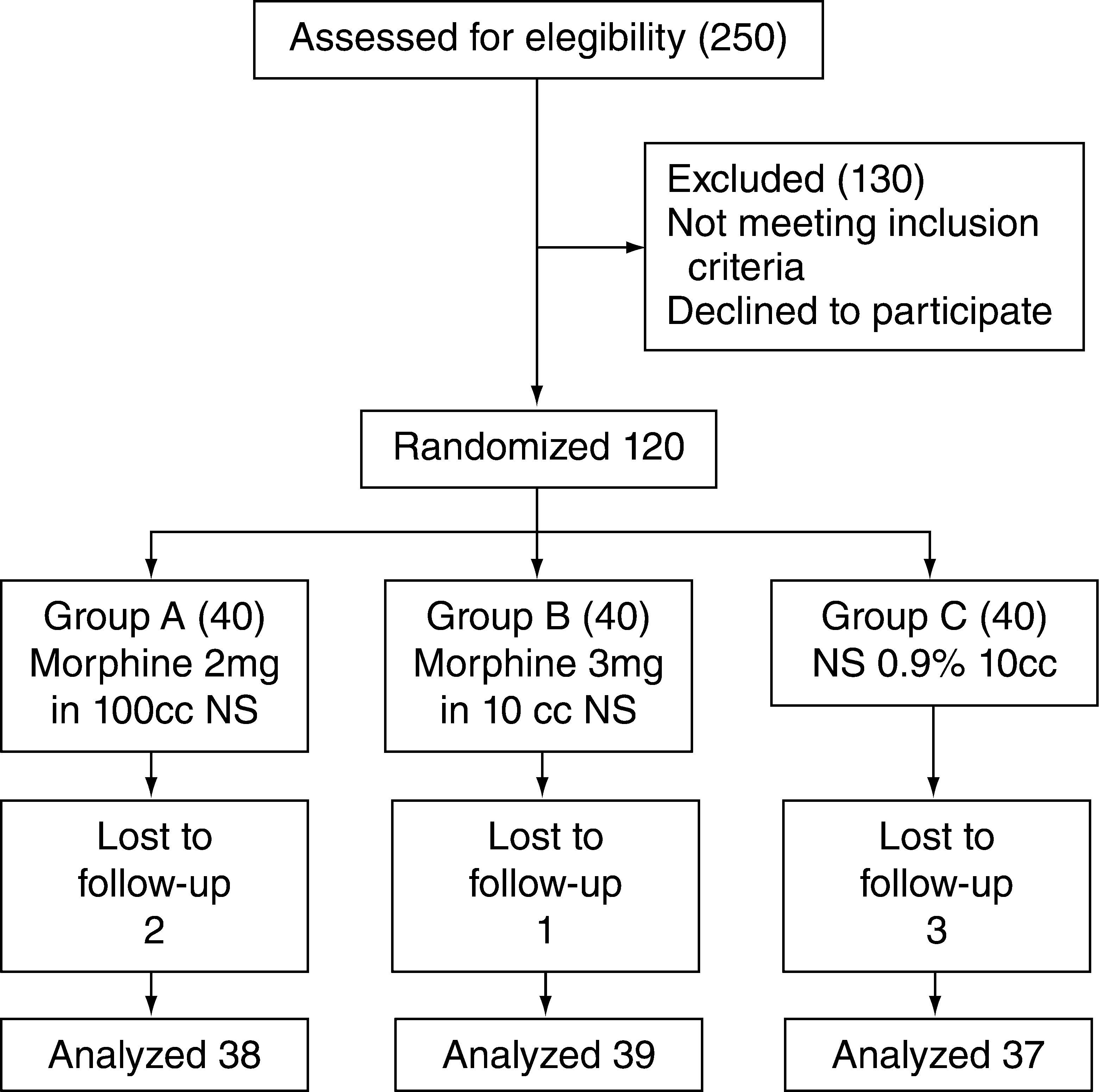
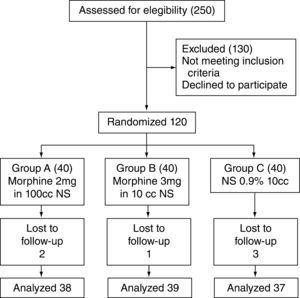
ResultsOne hundred and thirty-six patients were recruited between March 16, 2009 and April 12, 2010. Of these, only 114 received the dose of the medication and the remaining subjects were excluded due to catheter dislodgement or because follow up could not be assured.
Patient recruitment and follow-up are shown in accordance with the CONSORT parameters (fig. 1).16
Description of the recruitment and assignment process.14
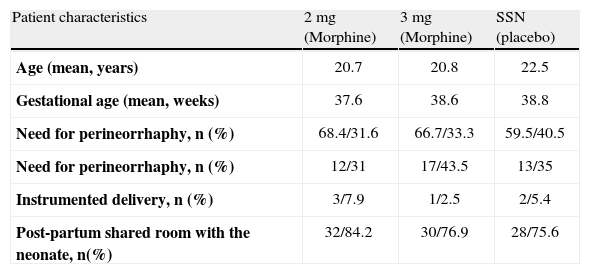
The cases that met the necessary requirements were studied (morphine 2 mg, n=38; morphine 3 mg, n=39 and placebo group, n=37) and no differences were found in terms of the demographic variables when the three groups were analyzed (table 1).
Characteristics of the study population
| Patient characteristics | 2 mg (Morphine) | 3 mg (Morphine) | SSN (placebo) |
| Age (mean, years) | 20.7 | 20.8 | 22.5 |
| Gestational age (mean, weeks) | 37.6 | 38.6 | 38.8 |
| Need for perineorrhaphy, n (%) | 68.4/31.6 | 66.7/33.3 | 59.5/40.5 |
| Need for perineorrhaphy, n (%) | 12/31 | 17/43.5 | 13/35 |
| Instrumented delivery, n (%) | 3/7.9 | 1/2.5 | 2/5.4 |
| Post-partum shared room with the neonate, n(%) | 32/84.2 | 30/76.9 | 28/75.6 |
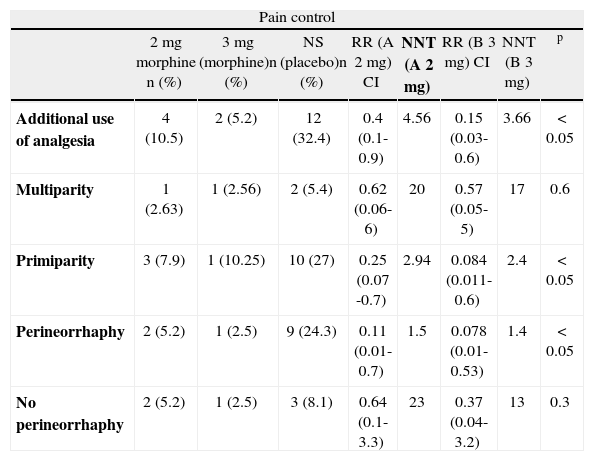
Of the total number of women in the 2 mg group, 4 required additional analgesia (RR 0.4, 95% CI: 0.1-0.9), and of the 39 in the 3 mg group, 2 required additional analgesia (RR 0.15, 95% CI: 0.03-0.6), compared to 12 out of 37 in the control group (table 2).
Need for additional analgesia; subgroup analysis
| Pain control | ||||||||
| 2 mg morphine n (%) | 3 mg (morphine)n (%) | NS (placebo)n (%) | RR (A 2 mg) CI | NNT (A 2 mg) | RR (B 3 mg) CI | NNT (B 3 mg) | p | |
| Additional use of analgesia | 4 (10.5) | 2 (5.2) | 12 (32.4) | 0.4 (0.1-0.9) | 4.56 | 0.15 (0.03-0.6) | 3.66 | < 0.05 |
| Multiparity | 1 (2.63) | 1 (2.56) | 2 (5.4) | 0.62 (0.06-6) | 20 | 0.57 (0.05-5) | 17 | 0.6 |
| Primiparity | 3 (7.9) | 1 (10.25) | 10 (27) | 0.25 (0.07 -0.7) | 2.94 | 0.084 (0.011-0.6) | 2.4 | < 0.05 |
| Perineorrhaphy | 2 (5.2) | 1 (2.5) | 9 (24.3) | 0.11 (0.01-0.7) | 1.5 | 0.078 (0.01-0.53) | 1.4 | < 0.05 |
| No perineorrhaphy | 2 (5.2) | 1 (2.5) | 3 (8.1) | 0.64 (0.1-3.3) | 23 | 0.37 (0.04-3.2) | 13 | 0.3 |
This reduction in the risk of having to give additional analgesia was greater in the subgroup of primigravidas: 3 out of 26 in the 2 mg group (RR 0.25, 95% CI: 0.07-0.7) and 1 out of 39 in the 3 mg group (RR 0.08, 95% CI: 0.01-0.6), compared to 10 out of 22 in the control group. In the two experimental groups, the analysis showed similar results for the patients requiring perineorrhaphy as part of their care during delivery: 2 out of 12 in the 2 mg group (RR 0.11, 95% CI: 0.01-0.7) and 1 out 17 in the 3 mg group (RR 0.078, 95% CI: 0.01-0.6). In the control group, there were 9 out of 12 cases (table 2).
The most frequent adverse effect was pruritus, in particular within the first 4 hours after delivery, with the following incidence: 36.8% (14 patients) in the experimental group receiving 2 mg of morphine, 30.7% (12 patients) in the 3 mg group, and 8.1% (3 patients) in the control group. Nausea and vomiting were present in 15.4% of women in the 3 mg treatment group, compared to 2.7 % in the control group. There were 2 cases of urinary retention in the 3 mg group, requiring the placement of a bladder catheter.
DiscussionThis study shows that the epidural use of morphine in post-partum patients is effective for pain control when compared with conventional oral and intravenous management, given the finding of the reduction in the need for additional analgesic of 22% when 2 mg were used, and of 27% when 3 mg were used. This reduction was even greater when the drug was given to patients undergoing perineorrhaphy or to primigravidas.
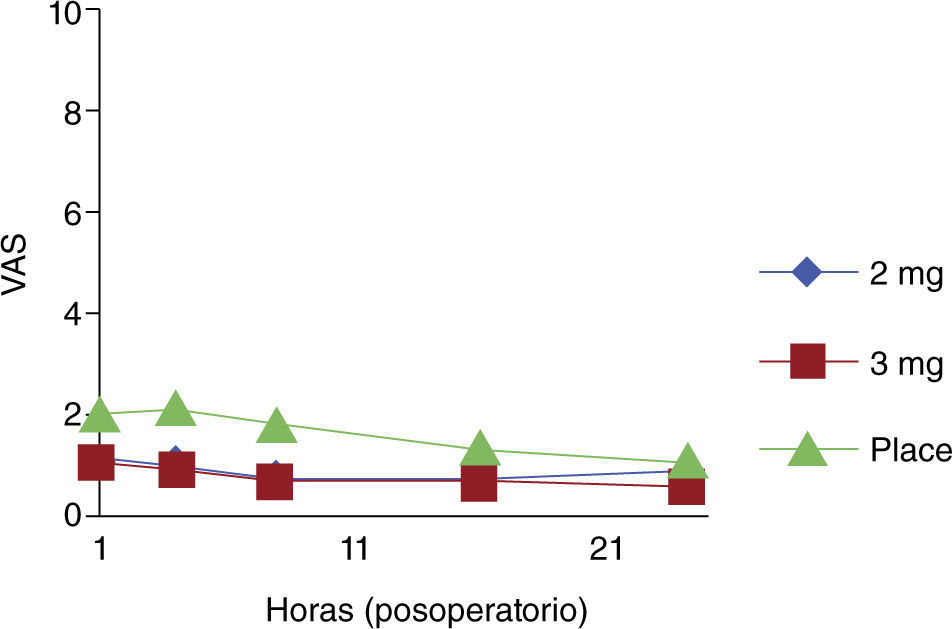
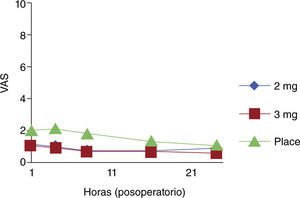
There were no significant differences when the use of morphine was compared with placebo in multigravidas or in patients who did not require perineorrhaphy. Morphine was effective during the first 24 hours post-partum in the two treatment groups (fig. 2).
Adverse effects were more frequent in the experimental groups, in particular in the group receiving 3 mg of morphine.
There are other similar studies assessing the effectiveness of different doses of epidural opioids. In the most recent study with 228 patients,17 Alison Macarthur and Charles Imarengiaye did not find group differences in terms of adverse effects when comparing the use of 2.5 mg with placebo. These authors found rates of pruritus and nausea of 12% and 9%, respectively, in the treatment group, compared with the present study that found a rate of pruritus of up to 36%.
In another study by MacDonald and Bickford in patients with episiotomy who received a dose of epidural morphine, no differences were found regarding the incidence of adverse effects between the treatment groups and the control group.18 Another study showed increased incidences of pruritus and nausea, although the study design was not adequate.19, 20
Considering that the incidence of respiratory depression was low or non-existent at the doses used, in particular in pregnant women, the sample size used is insufficient to draw conclusive evidence regarding this adverse effect.21
The patients who needed perineorrhaphy in the morphine groups showed better pain control compared to the patients in the placebo group; consequently, it may be concluded that primigravidas and patients with perineal tears benefit more from the use of epidural morphine as part of their analgesic management. There was no correlation with greater pain or less need for additional analgesia in patients remaining or not with their newborn after delivery.22
This study shows that, in the control group, analgesic management is deficient, supporting the hypothesis that post-partum patients, particularly with perineorrhaphy, must be considered as cases of moderate to severe pain.
Compared with other studies, the incidence of post-partum pain in this population is lower than that reported in prior studies.17, 18 It is important to bear in mind that the mean age of the patients who participated in this study is less than that reported in other studies (table 1).
There is a need to conduct new studies for validating the efficacy of this technique in specific subgroups (perineorrhaphy), as well as to develop other trials for estimating the probability of adverse events occurring with the use of this technique.
Competing InterestsNone declared.
Funding sources: The author's own resources.