One of the most frequent lesions in congenital heart disease is hypoplasia of the aortic arch and the co-existence o×f aortic coarctation in older children, which is very difficult to treat. A new technique for treating this condition was recently described and it requires extensive suture lines and an accurate management of coagulation disorders associated with the use of extracorporeal circulation. We present a case of an 8 years and 9 months old girl with Turner's syndrome, aortic coarctation and aortic arch hypoplasia who was admitted for sliding arch aortoplasty and received thromboelastography guided transfusion therapy.
Una de las lesiones más comunes en el grupo de cardiopatías congénitas es la hipoplasia del arco aórtico y la coexistencia de coartación aórtica en niños mayores, lo cual es de muy difícil manejo. Recientemente se describió una técnica para tratar esta patología que requiere extensas líneas de sutura y un preciso manejo de las alteraciones de coagulación asociadas con el empleo de circulación extracorpórea. Presentamos el caso de una niña de 8 años y 9 meses de edad con síndrome de Turner, coartación aórtica e hipoplasia de arco aórtico, que ingresó para plastia del arco por deslizamiento y que recibió terapia transfusional guiada mediante tromboelastografía.
Aortic arch hypoplasia (AAH) and coarctation of the aorta (CoA) are frequently associated with other congenital pathologies, including Turner's syndrome. This syndrome – also known as monosomy X – is the most frequent genetic disorder in women, with one case per every 2000–2500 live births.1 This is the only life-compatible monosomy and it is characterized by small size, premature ovarian failure and congenital cardiovascular defects in a female phenotype patient. The most usual cardiovascular disorders are coarctation of the aorta, also associated with AAH, bivalve aorta and mitral stenosis.2
Patients with Turner's syndrome exhibit a higher incidence of aortic dissection than patients with non-syndromic coarctation of the aorta, in addition to having a higher morbidity–mortality risk related to postoperative bleeding and perioperative rupture of the aorta.3–5
The surgical treatment of patients with concomitant CoA and HHA is Aortic Advancement; nevertheless, older children are not illegible for this procedure due to the decreased age-related elasticity of the aorta.6 Other surgical interventions to correct these defects (patch-plasty and graft interposition) have limitations regarding the child's growth because the prosthetic materials do not experience progressive growth, and hence result in recurrent obstructions that can be linked to a high morbidity (enteric and bronchial fistulae, pseudo aneurysms, endocarditis, etc.).6,7
The so-called “sliding technique” of the ascending aorta was recently described as an alternative for managing these patients, on the grounds of a potential circumferential growth as the primary benefit, preserving the normal contour and elasticity of the aortic tissue, as well as other advantages regarding the non-utilization of prosthetic material. However, this technique involves very long suture lines and thus a considerable risk of trans-operative bleeding.6
Some of the required trans-operative surveillance factors required in highly complex procedures include the usefulness of thromboelastography to guide the transfusion therapy in heart surgery, with a subsequent reduction in the transfusion requirements and the total transfused volume, mainly during the postoperative period, probably reflecting the early correction of coagulation disorders.8 The advantages of thromboelastography include the possibility to dynamically identify hemostatic disorders, even prior to reverting the required anti-coagulation for extracorporeal circulation, allowing for early harvesting of blood by-products.
Case presentationThis is a female patient, 8 years and 9 months old, with a history of coarctation of the aorta and hypoplasia of the Aortic Arch diagnosed at 3 years of age. The patient was admitted for a sliding aortic arch aortoplasty (“sliding technique”). She was diagnosed Turner's syndrome (45×) and was being followed by Endocrinology and Medical Genetics. At the time of admission, she was not receiving any medication, was never hospitalized previously and exhibited a normal psychomotor development, with no other relevant history.
The pre-anesthesia evaluation reported that patient did not experience any recent respiratory infections, her dental hygiene was good; her weight was 21.5kg and she was 114cm tall. Vital signs were within the normal range for her age: BP (blood pressure measured in the right arm) 94/60mmHg, BP (lower right limb) 82/43mmHg, HR (heart rate) 80beats/min, RR (respiratory rate) 21breaths/min.
No difficult airway predictors were identified during the physical examination and the cardiac auscultation presented enhanced second heart sound and a systolic murmur grade II/IV in crescendo – decrescendo of higher intensity at the left parasternal level, and no fremitus. The femoral and peripheral pulses in the lower limbs were decreased and no focal neurological deficit was identified.
The paraclinical, pre-surgical exams showed an Hb (hemoglobin) 13mg/dl, HCT (hematocrit) 41%, white blood cells count 9100 with normal differential count; platelets 218,000, PT 12s, PPT 30s, INR 1.0. The chest X-ray showed no cardiomegaly or pulmonary infiltrates. Electrocardiogram showed sinus rhythm, left axis deviation, heart rate 106beats/min and signs of left ventricular hypertrophy. The Echocardiogram showed situs solitus, left aortic arch, coarctation of the aorta with a maximum gradient of 73mm Hg and a mean gradient of 24mm Hg; aortic arch hypoplasia with multilevel strictures, the most significant with a minimum 2mm diameter. It also showed left ventricular hypertrophy with mild dilation and adequate left ventricular systolic function
The physical condition classification was ASA 3 and the RACHS-1 surgical risk was category 4.
The patient was brought to the OR, basic monitoring was performed and the patent venous access in the upper left limb was confirmed. The patient was pre-medicated with midazolam 2mg IV. IV induction was performed with fentanyl 150mcg, propofol 50mg and cisatracurium 6mg followed with the administration of a dose of cephalotin and ¿-aminocaproic acid (75mg/kg/dose); successful orotracheal intubation was then accomplished and adequate positioning was verified with auscultation, capnography and radiographic control.
Invasive monitoring was possible through the placement of a three lumens (5.5F) central venous catheter in the right internal jugular artery and the right radial artery was catheterized (22G). Additionally, brain oximetry was performed (NIRS: near infrared spectroscopy) showing a 65% baseline. Maintenance was achieved with a 10mcg/kg/h infusion of sevoflurane 1 CAM and fentanyl, in addition to an infusion of ¿-aminocaproic acid (75mg/kg/h). The innominate artery was cannulated for selective cerebral perfusion, before the start of perfusion.
The cardiopulmonary bypass was initiated with no events reported, maintaining perfusion pressures between 30 and 43mmHg. The heart was protected with an antegrade crystalloid cardioplegia with appropriate electrical activity suppression. The ascending sliding arch aortoplasty was performed. A pH-stat strategy was used for gas control during cooling and maintenance and alfa-stat during rewarming.
The cardiopulmonary bypass lasted for 2h and 29min, while the ischemia lasted for 1h and 22min, with a selective perfusion period of time of 1h and 8min; the minimum temperature was 18°C. The levels of brain oximetry (NIRS) were kept at 70–95%. Milrinone was started with a loading dose of 50mcg/kg in 30min and the infusion was continued between 0.5 and 0.75mcg/kg/min.
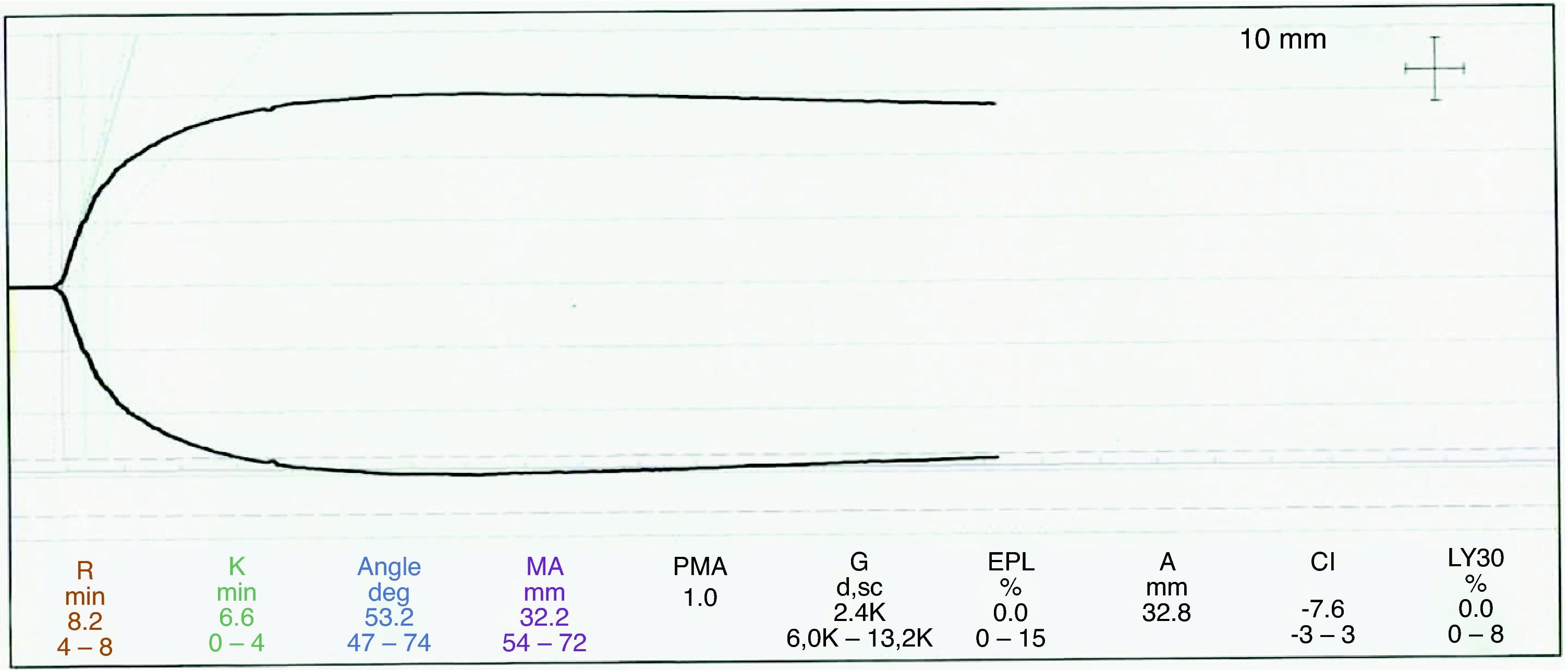
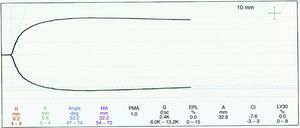
The thromboelastography protocol included an initial baseline sample, then a sample for thromboelastography (TEG) with heparinase during rewarming and another heparinase-free sample following the correction, based on the previous sample, in order to ascertain adequate reversal of the coagulation disorders. The sample corresponding to the rewarming period (with heparinase) showed time R at the upper normal limit, suggestive of deficient coagulation factors, prolonged K time and decreased maximum amplitude, indicating platelet dysfunction/thrombocytopenia. There were no findings suggestive of primary or secondary fibrinolysis (see Fig. 1).
The thromboelastography protocol included an initial baseline sample, then a sample for thromboelastography (TEG) with heparinase during rewarming and another heparinase-free sample following the correction, based on the previous sample, in order to ascertain adequate reversal of the coagulation disorders.
Exit from the cardiopulmonary bypass was uneventful and no vasopressor support was needed. Following the anticoagulation reversal using protamine sulfate (62mg) an ACT control of 109s was achieved, transfusion of two platelet apheresis (300ml) and fresh frozen plasma (10ml/kg) was initiated, in addition to the administration of 0.3mcg/kg IV of desmopresin. Persistent layered bleeding was observed following the transfusion that led to the transfusion of 3 (30ml) units of cryoprecipitate for improved hemostasis. Later on, a 120ml packed red cells transfusion was administered (5.5ml/kg) due to a decline in the level of hemoglobin down to 9.9mg/dl, which improved according to a lab control of 12.9mg/dl with 36% HCT.
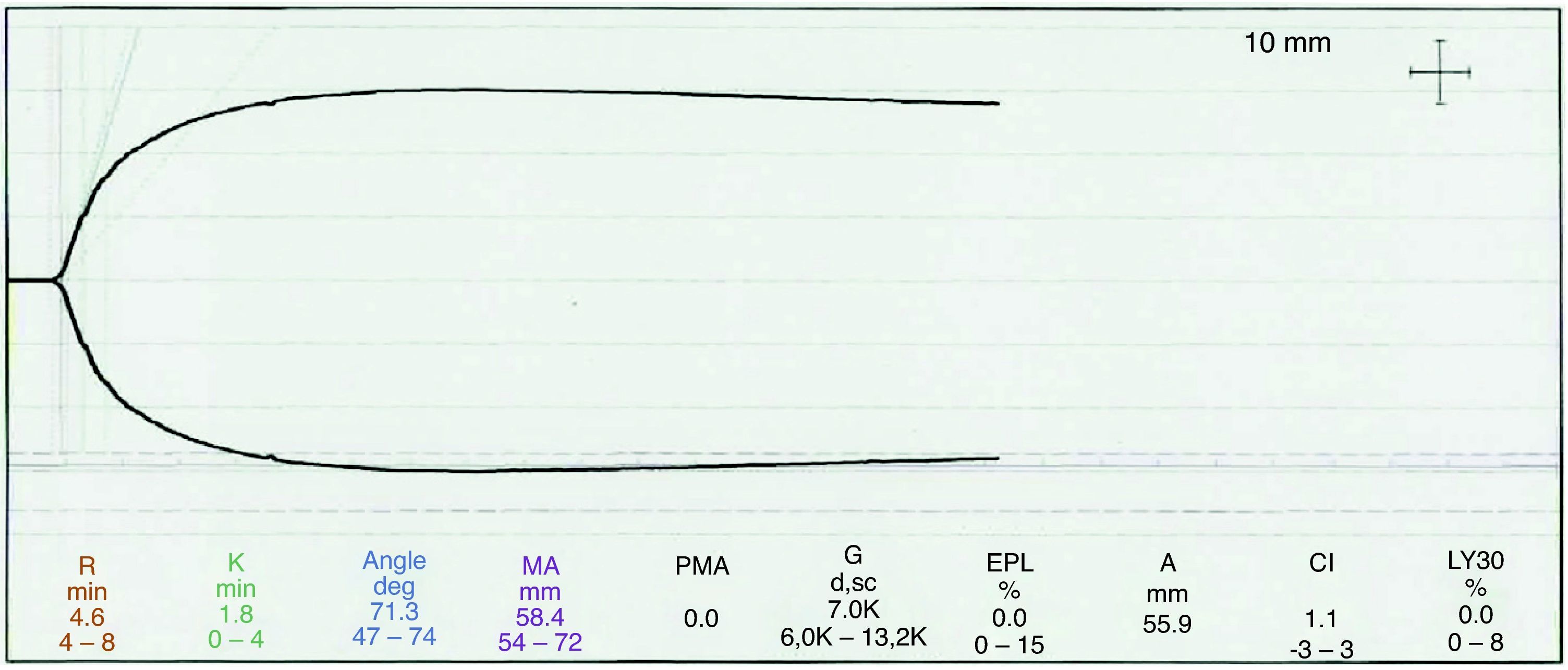
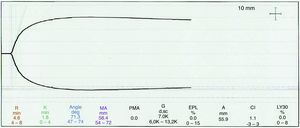
The second TGE sample following the transfusion showed values within the normal limits (see Fig. 2).
The controls of arterial gasses showed acid-base and hydro-electrolytic balance with serum lactate<2mmol/L. Then followed a tendency to high blood pressure and nitroglycerin 0.5–1.5mcg/kg/min was initiated to maintain the systolic blood pressure (SBP) at approximately 80mmHg and followed by preparation for transferring the patient to the cardiovascular ICU. Sedation and analgesia were maintained with fentanyl 10mcg/kg/h and the patient was started on dexmedetomidin 0.5–0.7mcg/kg/h, no loading dose. The patient was transferred with assisted ventilation for programmed extubation, and her vital signs were BP 82/49mmHg, MBP 58mmHg, HR 132lpm, RR 23rpm, SPO2 100%, NIRS 73%. The intraoperative bleeding was estimated at 600ml.
Nitroglycerin requirements persisted during the post-operative period for controlling high blood pressure and the patient developed hypophosfatemia that required corrective action. There was no need for transfusion of blood byproducts in the postoperative period. Orotracheal extubation was successful on the second day post-op and the patient was discharged from the cardiovascular ICU upon weaning from the inodilator (Milrinone) and adequate blood pressure control with Captopril and Amlodipine. The patient was discharged from hospital on the sixth day after surgery.
DiscussionThis case illustrates the anesthetic management of a Turner's syndrome patient, with coarctation and hypoplasia of the arch of the aorta, for a surgical intervention as recently described in the literature, associated with extensive vascular suture lines and with the use of profound hypothermia, that leads to significant disorders in hemostasis. Thromboelastography was used to guide the transfusion therapy with evidence of adequate recovery of hemostasis and a satisfactory postoperative evolution that enabled rapid withdrawal of mechanical ventilation and hospital discharge (<1 week).
Although patients with aortic arch hypoplasia and Turner's syndrome usually do not exhibit any acquired coagulopathies, as is frequently the case in patients with cyanogenic heart disease and polycythemia, undergoing complex repair procedures associated with the use of extracorporeal circulation and profound hypothermia promotes the development of hemostatic disorders that demand rapid and timely intervention. Likewise, blood transfusion-related adverse events have been described in the literature, such as the development of infections (HIV, HBV, HCV, nvCJD), acute transfusion reactions and transfusion-related acute lung injury (TRALI).9 All these factors require the development of transfusion guidelines to prevent the morbidity and mortality associated with coagulopathy and also with massive transfusion.
The use of thromboelastography in this particular patient enabled early correction of the coagulation disorders following extracorporeal circulation with profound hypothermia, as demonstrated by the low requirement of packed red blood cells (5.5ml/kg) and by the fact that no transfusion was needed during the postoperative period. During the intraoperative period and thanks to the use of TEG, only 14ml/kg of apheresis platelets were used, 10ml/kg of frozen fresh plasma and 1.5ml/kg of cryoprecipitates, which is a relatively small transfused volume considering the complexity of the surgical procedure, the extended use of extracorporeal circulation and the presence of profound hypothermia.
A description has been made of the thromboelastography parameters and the level of postoperative bleeding in pediatric patients.10–12 It has also been observed that the TEG variables that are more closely related to bleeding following extracorporeal circulation are the alpha angle (α) and the maximum amplitude (MA).13,14
In addition to the inclusion of kaolin to promote clot formation and heparinase to neutralize heparin in anticoagulated patients, some changes have been introduced to enable a quick rendering of TEG curves under critical circumstances. Examples of these changes are the addition of Celite and tissue factor for faster results and hence rapid administration of treatment to the patient.15 The use of each activator may result in specific alterations in the TGE values. For this reason, there have been some studies in pediatric populations to assess these changes. These studies have indicated that the kaolin-activated TGE reference values do not differ significantly in children between 1 month and 16 years of age and healthy adults.16 The main changes seen following tissue factor activation in patients less than 2 years old are shortening of the R & K times and increased alpha angle (α) and maximum amplitude, enabling an even faster interpretation of the results since the alpha angle value can be obtained in 4–6min.17
In this particular case, despite the use of an institutional algorithm to correct any TGE changes, a decision was made to transfuse cryoprecipitates because of the persistent layered bleeding even after the appropriate corrections were made; the TGE curve was reassessed and the presence of hypofibrinogenemia was suspected due to the prolongation of time k and the reduced maximum amplitude, despite a normal alpha angle. Then, the second thromboelastography showed a normal curve and thus, no additional blood products were administered, except for packed red blood cells.
It must be said, however, that TEG is not widely available as are the standard laboratory tests and this is a limitation for its routine use as a guide for transfusion protocols. Nevertheless, research in this area will help in assessing the impact of using thromboelastography over the morbidity–mortality associated with transfusions and bleeding of pediatric patients undergoing heart surgery, for improved decision-making.
Currently there are no literature reports on the specific use of TEG-guided transfusion protocols in patients in whom this surgical technique is used.
Conflict of interestNone.
FundingNone.
Please cite this article as: Pérez Pradilla AC, et al. Tromboelastografía como guía para terapia transfusional en paciente con síndrome de Turner, hipoplasia de arco aórtico y coartación aórtica sometido a aortoplastia con «técnica de deslizamiento»: reporte de un caso. Rev Colomb Anestesiol. 2012;40:318–22.