Transfusion therapy is probably one of the most widely used therapies with poor supporting evidence, despite the long years of practical clinical use.
ObjectiveTo adapt the evidence-based recommendations on the use of blood products to the Colombian setting: red blood cells, platelets, cryoprecipitates and irradiated blood products in cancer patients under 18 years of age.
MethodsStandard methodologies were followed in the development of recommendations. First, the clinical questions were addressed, and the evidence-based clinical practice guidelines were identified, graded and selected to answer the clinical questions. A systematic methodology was used to qualify, obtain and describe the relevant information to generate recommendations based on the SIGN system. The results were then presented and discussed in a group of experts to establish the practical value of the evidence and to adapt the recommendations to the Colombian environment.
ResultsOut of 107,441 preliminary titles, 56 studies were analyzed, and from them 3 clinical practice guidelines and 4 Cochrane systematic reviews were selected. This evidence was evaluated using AGREE II and AMSTAR. Red blood cells transfusion support is recommended using the restrictive strategy. Prophylactic platelet transfusion is the recommended indication. Cryoprecipitate is recommended when fibrinogen levels fall below 100mg/dL, and indications on irradiated blood products were established.
ConclusionsThis paper is an evidence-base approach on the recommendations for transfusion therapy in children with cancer.
La terapia transfusional es quizá una de los tratamientos de mayor uso sin buen respaldo de evidencia, a pesar de muchos años de uso en la práctica clínica.
ObjetivoAdaptar recomendaciones basadas en evidencia al contexto colombiano sobre el uso de hemocomponentes: glóbulos rojos, plaquetas, crioprecipitados y hemocomponentes irradiados en el paciente oncológico menor de 18 años.
MétodosSe utilizaron metodologías estándares para el desarrollo de las recomendaciones. Primero se formularon las preguntas clínicas, se identificaron, calificaron y seleccionaron las guías de práctica clínica basadas en la evidencia que respondían las preguntas clínicas, utilizando una metodología sistemática se realizó la calificación, extracción y descripción de los aspectos relevantes para generar recomendaciones usando el sistema SIGN, luego se realizaron exposición y discusión de los resultados obtenidos con un grupo de expertos para seleccionar la utilidad de la evidencia y adaptar las recomendaciones al contexto colombiano.
ResultadosDe 107.441 títulos preliminares, se analizaron 56 estudios, y de estos se escogieron 3 guías de práctica clínica y 4 revisiones sistemáticas Cochrane. Se evaluó esta evidencia con AGREE II y AMSTAR. Se recomienda soporte transfusional de glóbulos rojos usando la estrategia restrictiva, la estrategia transfusional profiláctica de plaquetas es la indicación recomendada. El valor de fibrinógeno menor de 100mg/dl es el recomendado para utilizar crioprecipitados y se determinaron las indicaciones sobre hemocomponentes irradiados.
ConclusionesEste trabajo representa un enfoque basado en la evidencia sobre las recomendaciones de terapia transfusional para niños con cáncer.
Notwithstanding the current knowledge on the principles of immunology that govern allogeneic transplants from a temporary tissue and the strict biological principles including molecular techniques aimed at minimizing any potential transfusion therapy risks; transfusions are not totally safe. This is why additional evidence is needed to determine the most appropriate instance for using transfusion therapy in pediatric cancer patients. Key documents such as the “European Cancer Anemia Survey” that evaluated 15,367 patients to establish the incidence and the prevalence of this condition, reported 39% prevalence and an incidence of 53.7% in six months. Moreover, 38.9% of the anemic patients were treated, of which 14.9% were transfused; hence the importance of establishing specific indications for transfusion therapy.1
Moreover, since 2009, the World Health Organization (WHO) reports indicate that the lowest blood donation rates are seen in developing countries (2.3 per 1000 inhabitants).2 Although, specifically in Colombia these rates have grown consistently as compared to the other countries of the region, the availability of blood products is inadequate (12 units per 1000 inhabitants).3 When considering the transfusion-associated adverse events, as listed in the clinical practice guidelines of the 2012 American Association of Blood Banks (AABB), the risk of Human Immunodeficiency Virus (HIV) transmission was 6,8/10 million transfused blood products from 2007 to 2008 and the residual risk for Hepatitis B virus transmission was 1/282,000 transfused blood components. Additionally, the incidence reported in 2009 for non-infectious transfusion-related adverse events of acute pulmonary injury was 0.81 (95% C.I., 0.44–1.49) per 10,000 transfuse blood products.4 So we may conclude that currently there is a big need to collect the best evidence regarding the most common practices in transfusion therapy of the pediatric cancer patient.
To accomplish the objectives herein established, a set of evidenced-based recommendations was adapted to the Colombian environment to assist in decision-making.
MethodologyEvidence-based recommendations for transfusion indications in pediatric perioperative cancer patients were developed, according to the standard international methodologies. The systematic reviews in the literature were identified, using the SIGN methodology for making recommendations,5 aimed at answering four research questions of clinical value in pediatric oncology. These questions were discussed and approved by the research team and a group of pediatric cancer hematologists.
Clinical questions to be answered- 1.
What are the indications for leukocyte-depleted red blood cell transfusions in pediatric cancer patients?
- 2.
What are the platelet transfusion indications in pediatric cancer patients?
- 3.
What are the indications for cryoprecipitate transfusion in pediatric cancer patients?
- 4.
What are the indications for irradiated product transfusion in pediatric cancer patients?
Upon identifying the clinical questions and establishing the corresponding outcomes, the PICO/PECO process was followed with the appropriate adaptations for each individual question5 (Annex 1).
Searching for evidenceThe first step was searching for evidence-based clinical practice guidelines in PubMed, Cochrane, library, MEDLINE, Embase(OvidSP), LILACS, current controlled trials, CRD – centre for reviews and dissemination database, PAHO – library directory, WHOLIS – World Health Organization Library Information System, biomed central, research records: PdQ clinical trials database, NHMRc clinical trials center, Google scholar, tripdatabase, specialized agencies, National Cancer Institute Journal, grey literature (technical reports, congress minutes, thesis and unpublished assays), manual search. The search strategy was adapted to each search engine and finally validated with the field expert in accordance with the type of clinical issue (Annex 2).
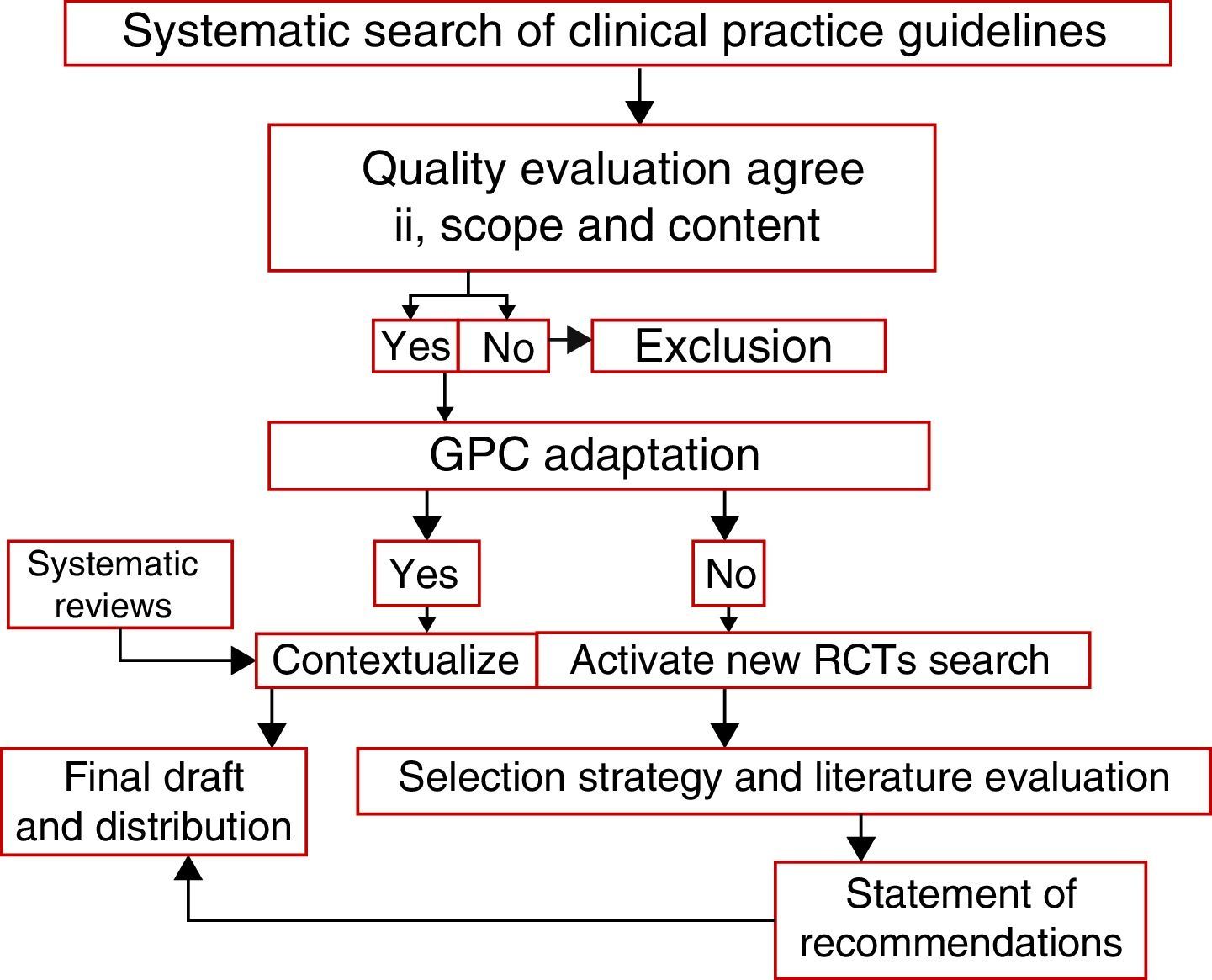
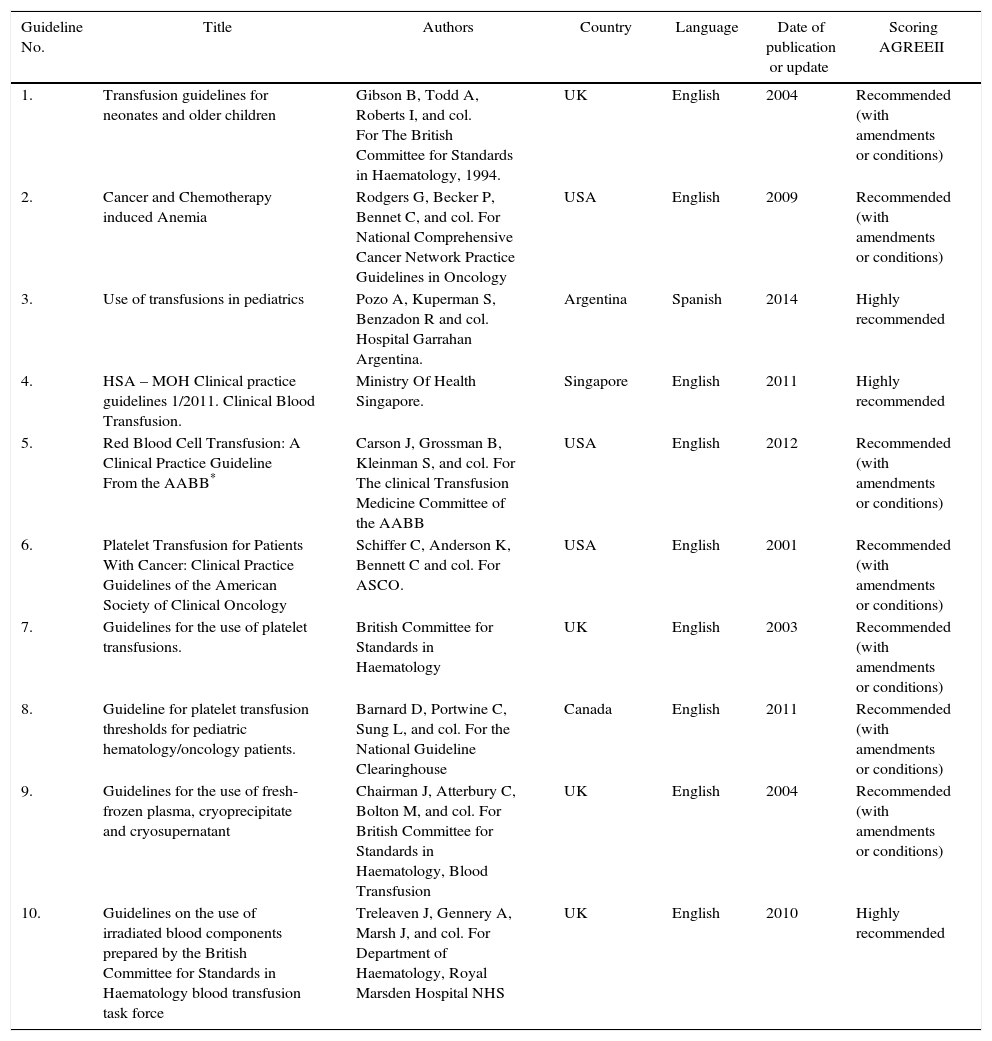
Selection of evidence and quality of the clinical practice guidelinesThe English and Spanish guidelines search comprised from January 1950 through December 2015, that yielded 107,441 PubMed titles for review (see Annex 2). All the probable titles for a primary analysis were selected, resulting in 56 articles for analysis, to then filter out 10 clinical practice guidelines relevant for the study.5 In order to evaluate the guidelines selected, the Appraisal of Guidelines Research and Evaluation II (AGREE II) was used.6 Each selected guideline was evaluated using the items in the various tool domains and total score was established. Upon evaluation of the domains, a statement was made about the overall quality of the guideline reviewed that led to three clinical practice guidelines based on quality ratings and the potential of implementation of those guidelines based on the criteria of the adaptation matrix decision and the relevancy of the clinical setting (Table 1).
Overall characteristics of the guidelines reviewed for making recommendations.
| Guideline No. | Title | Authors | Country | Language | Date of publication or update | Scoring AGREEII |
|---|---|---|---|---|---|---|
| 1. | Transfusion guidelines for neonates and older children | Gibson B, Todd A, Roberts I, and col. For The British Committee for Standards in Haematology, 1994. | UK | English | 2004 | Recommended (with amendments or conditions) |
| 2. | Cancer and Chemotherapy induced Anemia | Rodgers G, Becker P, Bennet C, and col. For National Comprehensive Cancer Network Practice Guidelines in Oncology | USA | English | 2009 | Recommended (with amendments or conditions) |
| 3. | Use of transfusions in pediatrics | Pozo A, Kuperman S, Benzadon R and col. Hospital Garrahan Argentina. | Argentina | Spanish | 2014 | Highly recommended |
| 4. | HSA – MOH Clinical practice guidelines 1/2011. Clinical Blood Transfusion. | Ministry Of Health Singapore. | Singapore | English | 2011 | Highly recommended |
| 5. | Red Blood Cell Transfusion: A Clinical Practice Guideline From the AABB* | Carson J, Grossman B, Kleinman S, and col. For The clinical Transfusion Medicine Committee of the AABB | USA | English | 2012 | Recommended (with amendments or conditions) |
| 6. | Platelet Transfusion for Patients With Cancer: Clinical Practice Guidelines of the American Society of Clinical Oncology | Schiffer C, Anderson K, Bennett C and col. For ASCO. | USA | English | 2001 | Recommended (with amendments or conditions) |
| 7. | Guidelines for the use of platelet transfusions. | British Committee for Standards in Haematology | UK | English | 2003 | Recommended (with amendments or conditions) |
| 8. | Guideline for platelet transfusion thresholds for pediatric hematology/oncology patients. | Barnard D, Portwine C, Sung L, and col. For the National Guideline Clearinghouse | Canada | English | 2011 | Recommended (with amendments or conditions) |
| 9. | Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant | Chairman J, Atterbury C, Bolton M, and col. For British Committee for Standards in Haematology, Blood Transfusion | UK | English | 2004 | Recommended (with amendments or conditions) |
| 10. | Guidelines on the use of irradiated blood components prepared by the British Committee for Standards in Haematology blood transfusion task force | Treleaven J, Gennery A, Marsh J, and col. For Department of Haematology, Royal Marsden Hospital NHS | UK | English | 2010 | Highly recommended |
A systematic review search of the various databases was undertaken, using the AMSTAR tool for evaluation. 4 additional systematic Cochrane reviews that met the study objectives were identified.
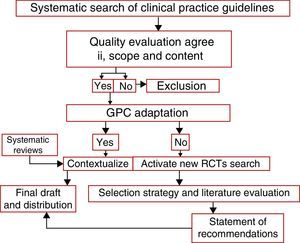
Statement of the recommendationsFor the clinical practice guidelines: Use of pediatric transfusions, Pozo et al., Clinical practice Guidelines from the Ministry of Health of Singapore and Guidelines on the use of irradiated blood components prepared by the British Committee for Standards in Haematology Blood Transfusion Task Force, in addition to the 4 systematic Cochrane reviews. All the relevant aspects applicable to the regional context were obtained and adapted, using some specific elements of the ADAPTE epidemiological tool as well (see Fig. 1).
Finally, the recommendations were listed with grades and levels of evidence (see Annex 3). Based on the results of the evaluation, the final document was produced and discussed with the panel of expert oncology hematologists to decide on the best adaptation for the indications. The paper was presented at the National Congress of Pediatric Hematology-Oncology for discussion of the recommendations with Colombian pediatric hemato-oncologists and an international expert.
The authorizations to translate and publish these results were requested and obtained and the material was used to answer each of the research questions.
RecommendationsIndications for leukocyte-depleted red blood cell transfusions in pediatric cancer patientsThe pathophysiology of cancer anemia is classified into three groups: decreased red blood cell (RBC) production, increased RBC destruction, and blood losses.7,8 Although this syndrome may present as a multifactorial effect, there are controlled clinical trials describing the myelosuppressive effect of cytotoxic agents that further show its additive effect; for example, Wilson et al.,8 reports a 19.5% rise in anemia (hemoglobin <12g/dL) during the first cycle up to 46.7% following the fifth chemotherapy cycle. Anemia may also be result of bleeding, renal failure, nutritional deficiencies, and we most not forget that cancer may directly suppress hematopoiesis from bone marrow invasion or the production of iron-sequestering cytokines that impairs RBC production.7–9
EvidenceA key principle in transfusion therapy states that the cause of anemia may be identified prior to RBC transfusion (Grade D, level 4).10 Furthermore, the decision to transfuse this blood component shall always be governed by the symptoms rather than by the hemoglobin levels and keep in mind that RBC transfusion is only recommended in patients requiring immediate anemia management and not for reaching a “normal” concentration of hemoglobin (Grade D, level 4).10
There are two therapeutic strategies described in the literature on this topic. The restrictive strategy that indicates a transfusion based on two principles: hemoglobin levels below 8g/dL and/or evidence of hemodynamic decompensation. The second strategy is liberal and establishes higher levels of hemoglobin neglecting the hemodynamic impact.11–13 Consequently, analyzing the evidence identified, the restrictive strategy for the stable perioperative patient has the strongest evidence10,11,13 with a 39% risk reduction of receiving a transfusion with a RR 0.61 (95% CI, 0.52–0.72), preventing the transfusion of 1.19 units per patient (95% CI, 1.85–0.53 units), and 23% less intraoperative mortality with RR of 0.77 (95% CI, 0.62–0.95). Moreover, this strategy did not increase the number of cardiac events (MI, cardiac arrhythmias, pulmonary edema or angina) with a RR 0.96 (95% CI, 0.70–1.32) and lowered the infections rate by 19% with RR 0.81, 95% CI, 0.66–1.00. No significant differences were found in terms of pulmonary edema, pneumonia and CVA with the approaches evaluated (Grade A, level 1a).12 The evidence regarding the levels of hemoglobin shows that when hemoglobin ranges from 7 to 10g/dL, the decision to transfuse shall be based on signs and symptoms, or concomitant medical or surgical comorbidities (i.e., cardiovascular pathology, respiratory disease, active blood loss, or coagulopathy). RBC transfusion is not indicated at levels above 10g/dL, unless justified on the basis of a specific underlying cause that should be explicitly defined and documented (Grade, level 1b).10,11 In asymptomatic patients or when a different treatment option is available, transfusion shall not be required (Grade C, level 2b).10,11
In patients that do not require immediate management, their overall status permitting, a baseline serum iron analysis should be entertained, including total iron and ferritin to evaluate the supplementation requirement of these patients, with additional regular evaluations (Level 4).4,11
The clinical evidence from trials in adult cervical, head and neck, and lung cancer patients shows the impact of anemia with respect to the tumor radiosensitivity. The recommendation for patients undergoing radiotherapy is to maintain Hb levels between 10 and 12g/dL. As far as the impact of chemotherapy, there is some scientific literature on anemia associated with the administration of carboplatin, cyclophosphamide, and doxorubicin; hence, for patients undergoing chemotherapy, the recommendation is to maintain Hb levels between 8 and 10g/dL (Level 4).11,14
The evidence also suggests that a restrictive strategy is at least equally effective and probably superior to the liberal strategy in critical patients. Likewise, a Hb between 7 and 9g/dL is the most appropriate level in the absence of signs, symptoms or any other evidence of hematological disability to meet the tissue oxygen (O2) demand (Grade A, level 1a).10,13,15,16
Preston et al.7 systematic review showed that top quality studies are needed to establish the effectiveness of RBC transfusions in palliative care cancer patients. The use of objective quality of life scales showed that 31–70% of these patients require transfusions and only a transient benefit is obtained as of the second day and only until day fourteen following the transfusion procedure. Therefore, this study suggests that close to one third of the patients may not benefit from transfusion therapy and the duration of the response to this approach in these patients is short-lived and insufficient (Grade A, level 2c).7
Additional concepts: Simancas et al.,17 recommend in their paper leukocyte-depleted products to lower the risk of non-hemolytic transfusion events, including pruritus, rash, and erythema that usually develop a few minutes following the transfusion. No additional severe transfusion-related events were found (Grade A, Level 2c).17 It should then be concluded that patients should undergo RBC transfusions according to the compatibility guidelines with ABO and Rh-D groups always compatible (Good practice).10,11 Any child undergoing transfusion therapy must be vaccinated against Hepatitis B, if possible before to the transfusion, and should have and extended RBC phenotype to avoid any transfusion-associated adverse events (Good Practice).11 Lastly, the RBC transfusion rate shall not exceed 5ml/kg/h (Good Practice).11
RecommendationsThe result of the systematic review recommends the restrictive strategy (Hemoglobin <7g/dL in the hemodynamic stable patient), to reduce the proportion of transfused patients and the amount of RBC transfused, with no impact on morbidity or length of hospital stay. The conclusion is therefore that the benefits of minimizing transfusions are greater that the risk of RBC transfusion (Grade A, Level 1a).10,11,13,15
The most important clinical factor when a transfusion is required and the levels of Hb are 7–10g/dL, is hemodynamic involvement. Other factors to keep in mind are: underlying disease, fever, systemic infection, or active bleeding (Grade A, level 1a).10,11,13
Platelet transfusion indications in pediatric cancer patientsEvidenceClinical trials have shown that the cause of thrombocytopenia must always be established prior to considering platelet transfusion, except for a life-threatening hemorrhagic event (Good Practice).10,11
Platelet transfusion support is determined on the basis of two strategies: a prophylactic strategy characterized by maintaining a minimum platelet count to prevent the risk of a life-threatening hemorrhage10,11,18,19; and the therapeutic strategy that recommends platelet transfusion only when bleeding is present.10,11,18,19
According to these guidelines, the prophylactic platelet transfusion is indicated of pediatric cancer patients under the following circumstances: active bleeding-associated thrombocytopenia (GI, pulmonary, and CNS) where the recommendation is to start the platelet transfusion to maintain a platelet count >50×109/L (Grade C, Level 2a).10 Furthermore, in patients with bone marrow aplasia secondary to cancer pathology and/or chemotherapy with a platelet count below 10×109/L in the absence of other bleeding-related factors (Grade A, Level 1b)10,11,18; in this same group of patients when the platelet count is below 20×109/L in the presence of associated bleeding risk factors including Sepsis, severe mucositis WHO grade IV, high probability of platelet count <10×109/L prior to the following evaluation, rapid platelet count drop, or in those patients undergoing invasive procedures that should be individually evaluated (Grade C, Level 2b).11,19 In patients with certain types of solid tumors (particularly CNS, gynecologic, melanoma, bladder or colon with large tumor necrosis sites), the suggested platelet threshold to prescribe a transfusion is 20×109/L (Grade B, Level 2a).11 The recommendation is to maintain a platelet count of 20–40×109/L in the following cases: disseminated intravascular coagulation (DIC), hyperleukocytosis or coagulation disorders (i.e., promyelocytic leukemia) or with limited access to a healthcare institution that presumably increases the risk of bleeding (Grade C, Level 2b).10,19
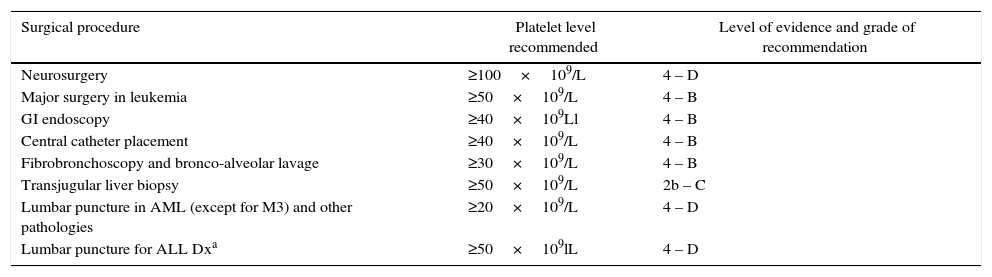
Surgical procedures in thrombocytopenic patientsPatients receiving chemotherapy often require invasive diagnostic and therapeutic procedures. According to the literature review, a count of 40×109/L and 50×109/L is considered to be safe to undergo surgery (Grade C, Level 2a).10,11,19 There are specific levels for each surgical scenario (see Table 2). In the absence of coagulation disorders, some procedures including bone marrow biopsy and aspiration may be done safely with counts over 20×109/L (Grade D, Level 4).11 In patients undergoing surgery or invasive procedures (kidney or liver biopsy) the platelet count should be above 50×109/L, in the absence of additional risk of bleeding associated conditions (Grade C, Level 2b).10,11 For neurosurgical and ophthalmology procedures, levels over 100×109/L may proof beneficial (Grade D, Level 4).11,19 Patients with active post-surgical bleeding and prothrombin time >1.5 over the control, have an indication for platelet transfusion in the presence of severe bleeding (having ruled-out other causes for hemostatic imbalance and bleeding due to poor suturing) or when the platelet count falls below 50×109/L (Grade A, Level 1a).11 Furthermore, a platelet count above 50×109/L is enough for dental extractions and transbronchial biopsies in patients with normal coagulation (Grade C, Level 3).11
Platelet count for invasive procedures.
| Surgical procedure | Platelet level recommended | Level of evidence and grade of recommendation |
|---|---|---|
| Neurosurgery | ≥100×109/L | 4 – D |
| Major surgery in leukemia | ≥50×109/L | 4 – B |
| GI endoscopy | ≥40×109Ll | 4 – B |
| Central catheter placement | ≥40×109/L | 4 – B |
| Fibrobronchoscopy and bronco-alveolar lavage | ≥30×109/L | 4 – B |
| Transjugular liver biopsy | ≥50×109/L | 2b – C |
| Lumbar puncture in AML (except for M3) and other pathologies | ≥20×109/L | 4 – D |
| Lumbar puncture for ALL Dxa | ≥50×109lL | 4 – D |
Another surgical procedure frequently practiced in oncology is lumbar puncture; the recommendation for this procedure is a platelet count above 20×109/L, although patients with newly diagnosed lymphoid leukemia the requirement is >50×109/L; and in patients with promyelocytic leukemia, platelet transfusion is required when the platelet count is below 40×109/L (Good Practice).10,11,19
Additional considerationsIn the case of platelet transfusions (in patients less than 45kg), alloimmunization shall be avoided, respecting the ABO platelet group (Grade D, Level 4).10,11 Non-identical ABO platelet administration is an acceptable transfusion practice when platelet concentrate shortages are an issue, or when the patient requires HLA compatible platelets that are not ABO matched (Grade D, Level 4).10,11 As far as possible, in RH D negative patients, concentrates should be negative, particularly in women (Grade D, Level 4).10 The platelet recommended volume is 10–20ml/kg for children under 15kg and one unit for apheresis for patients over 15kg (Good Practice),11 at an infusion rate of 20–30cc/kg/h (Good Practice).11
Recommendations- -
Prophylactic platelet transfusion is indicated for pediatric cancer patients (Grade A, Level 2b).10,11,19
- -
Pediatric patients with cancer pathologies should receive platelet transfusions when the platelet count is below 10×109/L in the absence of additional bleeding risks (Grade A, Level 2b).11 Patients with platelet counts below 20×109/L, and associated bleeding risk factors (sepsis, severe mucositis, high probability of platelet count <10×109/L prior to the next evaluation) should be transfused (Grade C, Level 2b).10,11
- -
In cancer patients receiving chemotherapy and that require invasive diagnostic or therapeutic procedures, the recommendation is a platelet count between 40×109/L to 50×109/L (Grade C, Level 2a).10,11 Specific levels are set for each particular procedure (see Table 2).
The indications to transfuse cryoprecipitate products are very limited and there is minimal evidence. Though it is clear that the risk of allergic reactions and anaphylaxis is higher versus other blood products.20–22
EvidenceTinegate et al.21 in a UK study found that three most common indications for cryoprecipitate transfusion in pediatrics are: disseminated intravascular coagulation, massive transfusion, and hematologic oncology patients. The major indication is for prophylaxis of hemorrhagic events, using fibrinogen levels below 1g/L measured with Clauss method. This study had no statistically significant findings in terms of fibrinogen replacement.10,11,20–22
RecommendationsFibrinogen levels below 1g/L or 100mg/dL should be corrected with cryoprecipitate transfusion under the following circumstances: massive transfusion requirement, DIC, congenital dysfibrinogenemia or secondary to medications (Grade C, Level 2b).10,11 For dysfibrinogenemia management, the dose should be 1 unit per every 5kg of BW, maximum 10 units (adult equivalent) (Grade D, Level 4).11,20,21
Indications for irradiated products transfusion in pediatric cancer patientsA major complication of transfusion therapy is the transfusion associated graft-versus-host disease (TA-GVHD); leukocyte depletion of blood products to ensure less than 5×106 leukocytes has been an acceptable measure, but it is unable to eliminate the risk of TA-GVHD. For this reason, the irradiation of blood products using gamma or X-rays is the validated procedure for the effective prevention of this complication (Grade A, Level 2a).10,11,23
EvidenceBlood components of a first or second-degree relative donor should be irradiated (Grade B, Level 2a).10 Any child under one year old undergoing chemotherapy, should receive irradiated blood products (Grade A, Level 1a).11 The same condition applies to Hodgkin lymphoma patients regardless of the disease status; these patients should receive life-long leukocyte depleted and irradiated platelets and RBC (Grade B, Level 2a),10 as well as patients treated with purine analogs (Fludarabine, Cladribine, and Deoxycoformycin), for at least the next two years until the immune cell function recovers (Grade B, Level 2a).10,11,23 For other purine antagonists and new agents such as Bendamustine and Clofarabine the use of irradiated components is recommended based on their similar action (Grade C, Level 2b).23 For chemotherapy regimens using Alentuzumab (antiCD 52), the irradiated components shall be used after chemotherapy; however, irradiated components are not recommended with Rituximab (antiCD 20) (Grade C, Level 2b).23 In contrast, for patients with aplastic anemia receiving antithymocyte globulin, the use of irradiated blood products is recommended (Grade C, Level 2b).21 Patients requiring irradiated blood components are recommended to wear a badge with their personal information so that they can be easily identified when this therapy is required (Grade C, Level 2b).23
Other considerationsBlood components may be irradiated using gamma or X-rays; both systems are effective for preventing TA-GVHD (Grade B, Level 2a).23 The minimum radiation dose recommended ranges from 15 to 25Gy, never above 25Gy and at any point, doses above 25Gy lead to negative blood products effects and viability (Grade C, Level 2b).11,23,24 Once the RBCs have been irradiated, they should be used in the following 24h and within 5 days of the donation because of the potential risk of hyperkalemia. Platelets may be irradiated through hemolysis at any time upon harvesting, and may stored afterwards for 5 days for viability. There is no need to irradiate blood components for patients undergoing routine surgery with a diagnosis of solid tumors (Grade C, Level 2b).23
RecommendationsTable 3 – Indications for blood component irradiation with level of evidence and grade of recommendation.
Indications for irradiation with level of evidence and grade of recommendation.
| Pathology | Grade of recommendation and level of evidence |
|---|---|
| Transfusions with blood components from 1st and 2nd degree relatives | Grade B level 2a |
| Hodgkin lymphoma | Grade B level 2a |
| Patients undergoing treatment with purine analogs | Grade B level 2a |
| Patients with aplastic anemia receiving Thymoglobulin | Grade C level 2b |
Despite the frequent use of transfusion therapy, high-grade evidence is still missing; this is why clinical trials are required, focusing on pediatric cancer patients.
The study did not consider the analysis of the indications for fresh-frozen plasma transfusion, since the research team felt that most of the indications for this blood component are linked to additional comorbidities of the cancer patient, including among others, severe infection or coagulation factor impairment; hence the need to undertake further research addressing this particular blood component.
Finally, this paper represents a specific evidence-based approach on transfusion therapy for children with cancer. Some of the recommendations are similar to those for adults, with some key differences in multiple areas. However, it should be kept in mind that the implementation of these recommendations requires a thorough interpretation of the outcomes for appropriate adaptation to the local environment.
FundingThe authors did not receive sponsorship to undertake this article.
Conflicts of interestThe author has no conflicts of interest to declare.
| Summarized search: PICO/PECO. |
|---|
| P: population (pediatric cancer patients) (aged less than 18) I: Intervention strategy: blood components transfusion (platelets, red blood cells, cryoprecipitate) irradiated products, leukocyte-depleted products, platelet apheresis. Outcomes: indications for transfusions in the specified population. Clinical practice guidelines, systematic literature reviews, and primary trials. Neoplasm: Every cancer diagnosis was considered in the preliminary analysis. Results: 107.441 titles in PUBMED. (Filter by date: 01/01/1950–31/12/2015). |
| (“Infant”[MeSH Terms] OR “Infant*”[Text Word] OR “Child, preschool”[MeSH Terms] OR “Child, preschool”[Text Word] OR “Preschool Child”[Text Word] OR “Preschool Children”[Text Word] OR “Child”[MeSH Terms] OR “Child”[Text Word] OR “Children”[Text Word] OR “Adolescent”[MeSH Terms] OR “Adolescent*”[Text Word] OR “Adolescence”[Text Word] OR “childhood”[tw] OR “Pediatrics”[MeSH] OR “Paediatric$”[tw] OR “Pediatric”[tw] OR “Peadiatric*”[tw] OR Teen * OR Minors[MeSH] OR “Minor*”[tw]) AND “Blood Component Transfusions*” [Text Word] OR “Erythrocyte Transfusions”[Text Word] OR “Red Blood Cell Transfusion”[Text Word] OR “Red Blood Cell Transfusions”[Text Word] OR “Platelet Transfusions”[Text Word] OR “Transfusion, Platelet”[Text Word] OR “Transfusions, Platelet” [Text Word] OR “Blood Platelet Transfusion”[Text Word] OR “Blood Platelet Transfusions”[Text Word] OR “Blood Transfusion” OR “Platelet Transfusion”[Text Word] OR “Transfusion Related”[Text Word] OR “Fresh Frozen Plasma”[Text Word] OR “Platelet Rich Plasma”[Text Word] OR “Platelet Rich Plasma”[Text Word] OR “Plasma Cell”[Text Word] OR “Cryoprecipitate”[Text Word] OR “Transfusion, Blood”[Text Word] OR “Transfusion Blood”[Text Word]OR “Transfusions Blood”[Text Word] OR “Transfusion Therapy”[Text Word] OR “Blood Component Transfusion”[Text Word] OR “Blood Component Transfusions”[Text Word] OR “Blood Transfusions”[Text Word] OR “Erythrocyte Transfusion”[Text Word] OR “Pheresis” [Text Word] OR “Phereses”[Text Word] OR “Apheresis”[Text Word] OR “Aphereses”[Text Word] AND “Tumors*”[Text Word] OR “Neoplasms*”[Text Word] OR “Tumor*”[Text Word] OR “Cancer*”[Text Word] OR “Neoplasms” [MeSH Terms] OR “Neoplasms*”[Text Word] AND ((Randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized controlled trials[mh] OR random allocation[mh] OR double-blind method[mh] OR single-blind method[mh] OR clinical trial[pt] OR (“clinical trial”[tw]) OR ((singl*[tw] OR doulb*[tw] OR tripl* OR trebl*[tw]) AND (mask*[tw] OR blind*[tw])) OR (“latin square”[tw]) OR placebos[mh] OR placebo*[tw] OR random*[tw] OR research design[mh:noexp] OR “Evaluation Studies”[Publication Type] OR follow-up studies[mh] OR prospective studies[mh] OR cross-over studies[mh] OR control*[tw] OR prospectiv*[tw] OR volunteer*[tw]) NOT (animal[mh] NOT human[mh])) AND (“evidence-based medicine”[MeSH Terms] OR (“evidence-based”[All Fields] AND “medicine”[All Fields]) OR “evidence-based medicine”[All Fields] OR (“evidence”[All Fields] AND “based”[All Fields] AND “medicine”[All Fields]) OR “evidence based medicine”[All Fields]) OR (evidence-based[All Fields] AND (guideline[tw] OR guidelines[tw] OR recommendations[All Fields])) OR ((evidence[All Fields] AND based[All Fields]) AND (guideline[tw] OR guidelines[tw] OR (recommendation[All Fields] OR recommendation’[All Fields] OR recommendation's[All Fields] OR recommendations[All Fields] OR recommendations’[All Fields] OR recommendations,[All Fields] OR recommendationsa[All Fields] OR recommendationsdagger[All Fields] OR recommendationsfor[All Fields] OR recommendationsimplementation[All Fields] OR recommendationss[All Fields] OR recommendationsthe[All Fields]))) OR consensus development conference[pt] OR (“health planning guidelines”[MeSH Terms] OR (“health”[All Fields] AND “planning”[All Fields] AND “guidelines”[All Fields]) OR “health planning guidelines”[All Fields]) OR guideline[pt] OR (“Cochrane Database Syst Rev”[Journal] OR (“cochrane”[All Fields] AND “database”[All Fields] AND “syst”[All Fields] AND “rev”[All Fields]) OR “cochrane database syst rev”[All Fields]) OR (“ACP J Club”[Journal] OR (“acp”[All Fields] AND “journal”[All Fields] AND “club”[All Fields]) OR “acp journal club”[All Fields]) OR “Health Technol Assess”[Journal] |
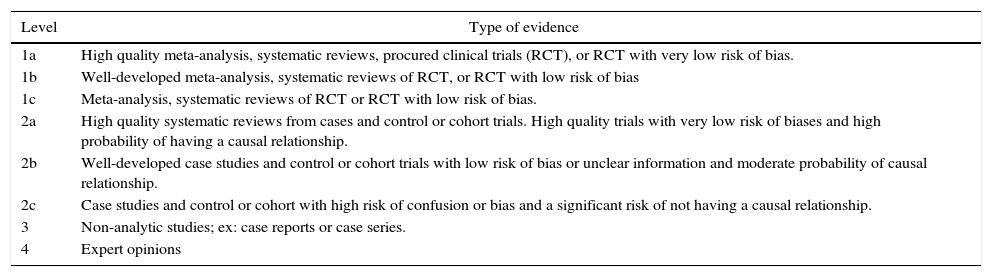
Levels of evidence
| Level | Type of evidence |
|---|---|
| 1a | High quality meta-analysis, systematic reviews, procured clinical trials (RCT), or RCT with very low risk of bias. |
| 1b | Well-developed meta-analysis, systematic reviews of RCT, or RCT with low risk of bias |
| 1c | Meta-analysis, systematic reviews of RCT or RCT with low risk of bias. |
| 2a | High quality systematic reviews from cases and control or cohort trials. High quality trials with very low risk of biases and high probability of having a causal relationship. |
| 2b | Well-developed case studies and control or cohort trials with low risk of bias or unclear information and moderate probability of causal relationship. |
| 2c | Case studies and control or cohort with high risk of confusion or bias and a significant risk of not having a causal relationship. |
| 3 | Non-analytic studies; ex: case reports or case series. |
| 4 | Expert opinions |
Grade of recommendation
| Grade | Recommendation |
|---|---|
| A | At lest one meta-analysis, one systematic review of the literature of a RCT 1a and directly applicable to the target population Mainly 1b trial evidence, directly applicable to the target population and showing consistent results. |
| B | Evidence from 2a trials, directly applicable to the target population, showing consistency with the results or extrapolating evidence of 1a or 1b trials. |
| C | Evidence from 2b trials, directly applicable to the target population, showing consistency with the results or extrapolating 2a trials evidence |
| D | Level 3 or 4 Evidence o extrapolating evidence fro 2b trials. |
| BP (Buena practice) | Best practice recommendations based on clinical experience or guidelines for the developer group |
Please cite this article as: Pardo-González CA, Linares A, Torres M. Recomendaciones basadas en la evidencia de terapia transfusional en el paciente oncológico en pediatría. Rev Colomb Anestesiol. 2016;44:151–160.