Careful placenta examination and injection studies are crucial to understand the differences between the various complications in monochorionic (MC) pregnancies. In this review, we will first describe an accurate and simple method of placental injection and then discuss the placental characteristics of normal MC, twin–twin transfusion syndrome (TTTS), twin anemia-polycythemia sequence (TAPS), selective intrauterine growth restriction (sIUGR), monoamniotic (MA) and other special cases.
El examen cuidadoso de la placenta y los estudios de inyección son cruciales para comprender las diferencias existentes entre las diversas complicaciones de los embarazos MC. En esta revisión, vamos a describir primero un método preciso y simple de inyección placentaria y, posteriormente, abordaremos las características de una placenta normal en MC, el síndrome de transfusión fetal-fetal (STFF), la secuencia anemia-policitemia (SAP), la restricción del crecimiento intrauterino selectivo (CIRs), la placenta monoamniótica (MA) y otros casos especiales.
Twin pregnancies can be classified into two different groups: monochorionic (MC) and dichorionic. MC twins have a 3–6-fold increased risk of adverse perinatal outcome.1,2 Adverse outcome in MC twinning is due to complications associated with the presence of placental vascular anastomoses. Vascular anastomoses connecting the circulation of the twins are ubiquitous in MC placentas but are extremely rare in dichorionic placentas. These placental vascular anastomoses may lead to several complications including twin–twin transfusion syndrome (TTTS), spontaneous twin anemia-polycythemia sequence (TAPS), selective intrauterine growth restriction (sIUGR) and twin reversed arterial perfusion (TRAP).2–6 Imbalance of volume of blood flow through the vascular anastomoses may cause hypovolemia and/or anemia in one twin (donor) and hypervolemia and/or polycythemia in the co-twin (recipient). In addition, MC twins may also be monoamniotic (MA) which may lead to complications such as cord entanglement and double fetal demise.7
In this review, the methodology for injecting placentas is extensively described.
Dye-colored injection of MC placentasAll MC placentas should be routinely examined and injected after birth in order to understand the pathogenesis of the various complications. In addition, in TTTS placentas treated with fetoscopic laser coagulation, injection studies are of paramount importance to evaluate the accuracy and completeness of laser surgery, an important tool for laser therapy specialists. A detailed protocol for placental injection used at our center is reported here below and can be viewed using the following links: http://www.youtube.com/watch?v=Qm4bdLkl9BE.8
Preparation of the placenta after deliveryUse clamps to label the umbilical cords of the twins with one for the first-born or two for the second-born. Then, inspect the maternal and fetal surface of the placenta for completeness or disruption and record the following data: type of cord insertion (central, eccentric, marginal or velamentous), number of blood vessels in the umbilical cord (usually one vein and two arteries, sometimes only one artery) and color difference between both placental shares. A section of the dividing membranes can be sent to Pathology to confirm the type of chorionicity. The placenta can then be placed in a plastic bowl and refrigerated until the final examination (best within one week) and color dye injection. The placenta must not be frozen or fixed (do not use formalin).
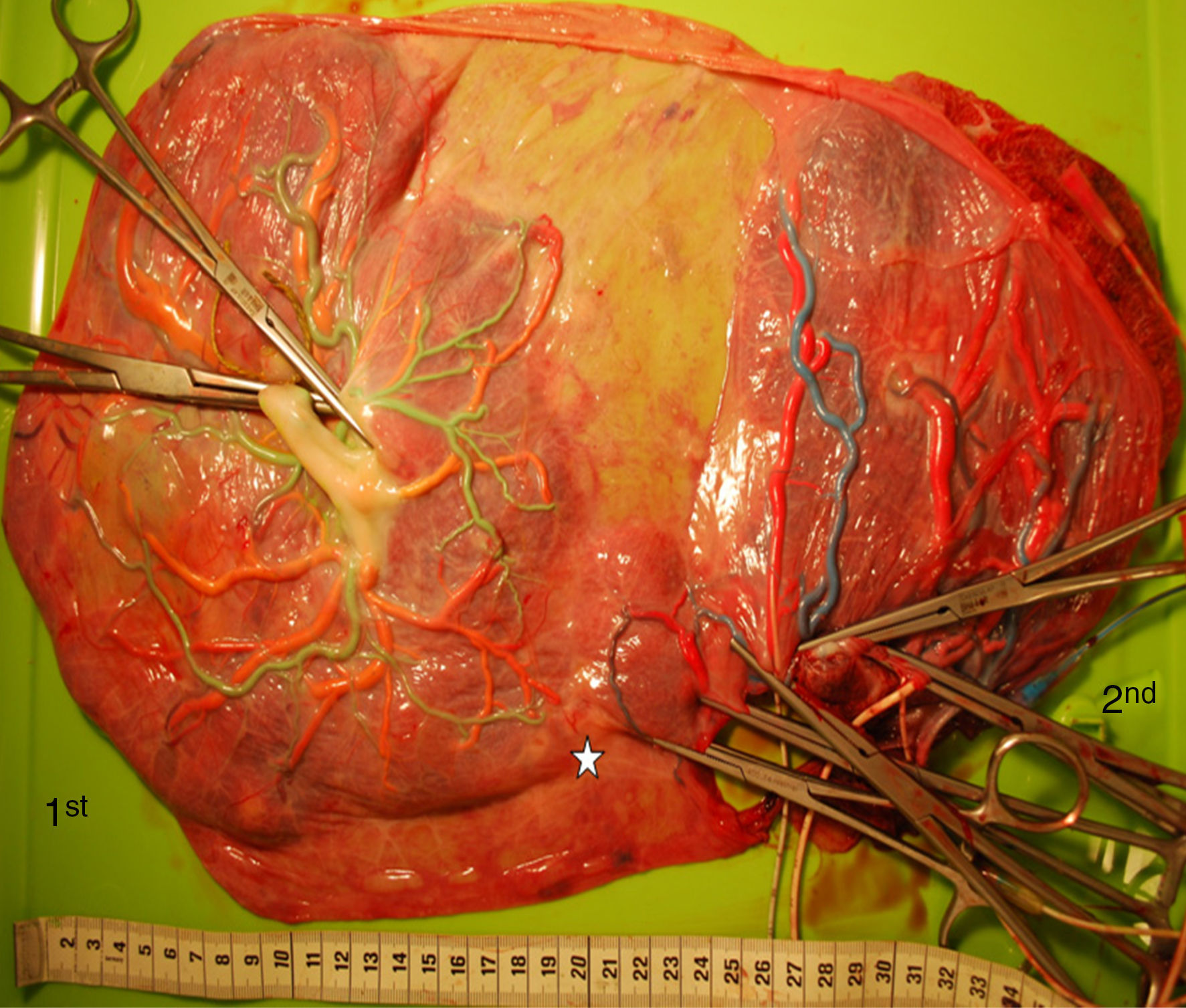
Catheterization of the umbilical vesselsWash the placenta with warm water or saline, trim the peripheral membrane, remove the inter-twin dividing membrane and peel off the amnions (for better visualization of the vascular anastomoses and better quality of the placental pictures). Transect each umbilical cord at approximately 5cm distance from the cord insertion and gently squeeze out blood clots from the umbilical vessels and placental vessels. Then, cannulate the umbilical cord vessels. Cannulate the umbilical vein with an appropriately sized catheter, avoiding false passages. Cannulate one of the two umbilical arteries with a smaller catheter using tweezers to widen the lumen of the artery. Only one of the 2 umbilical arteries needs to be catheterized since an anastomosis (of Hyrtl) connects the 2 arteries near the cord insertion. Cannulation of the vessels of the other cord is same. Placement of the catheter can be facilitated by gentle back and forth massaging of the umbilical vessels. Any type of catheter can be used for this procedure. We choose to use (and recycle) the catheters used at our neonatology ward for umbilical catheterization in neonates. Tie a piece of tape around both cords to avoid back flow of the colored dye during dye injection.
Injection with colored dyeConnect a 20ml syringe filled with colored dye to each catheter. Any viscous colored dye can be used to visualize the placental angio-architecture. Use contrasting colors to allow good visualization of the anastomoses (dark colors for the arteries, bright colors for the veins). Gently inject (with low pressure) the colored dye in the vein while an assistant gently pushes the dye to allow the colored dye to fill all placental vessels, also the smallest ones. Pay particular attention to the small vessels near the vascular equator (the vascular equator is the place where the anastomoses from either twin connect with each other). Repeat the previous steps to inject colored dye into the artery. Of note: arteries may be more difficult to inject and require more patience. Repeat above steps for the other umbilical cord.
Evaluation and documentation of the placenta after colored dye injectionCarefully examine the vascular equator and record the number and types of anastomoses. Place a measuring tape on the placenta to measure the diameters and placental shares on the digital picture. Use a high-resolution digital camera and take pictures of the injected placenta. Make sure that the pictures are taken perpendicular to the placenta.
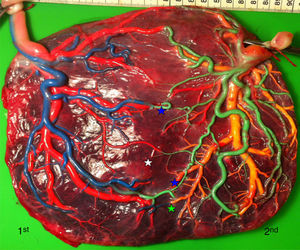
Vascular anastomoses include 3 types: arterioarterial (AA), venovenous (VV) and arteriovenous (AV) or venoarterial (VA) anastomoses. The first two types are superficial with bidirectional blood flow and directly linking the arteries and veins of two umbilical cords, while AV anastomoses form at a deep capillary level within shared cotyledons and allow only unidirectional blood flow. Of note, color dye injected in AA and VV anastomoses mixes and crosses the vascular equator, whereas color dye in AV or VA anastomoses does not mix and does not cross over the vascular equator.
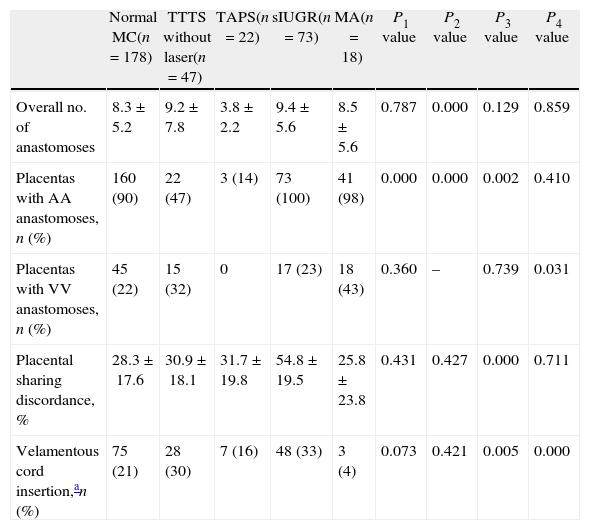
Differences between the various types of MC placentasBetween June 2002 and January 2013 a total of 654 MC placentas were examined at our center. We were not able to inject 46 placentas due to damage caused by maceration or destruction (n=41) or formaline (n=5). The results of the 608 injected placentas are summarized in Table 1 shows the differences in angio-architecture between the various subtypes. A detailed description of the differences between each subtype of MC placentas is reported here below.
Placental characteristics of MC placentas of various types.
| Normal MC(n=178) | TTTS without laser(n=47) | TAPS(n=22) | sIUGR(n=73) | MA(n=18) | P1 value | P2 value | P3 value | P4 value | |
| Overall no. of anastomoses | 8.3±5.2 | 9.2±7.8 | 3.8±2.2 | 9.4±5.6 | 8.5±5.6 | 0.787 | 0.000 | 0.129 | 0.859 |
| Placentas with AA anastomoses, n (%) | 160 (90) | 22 (47) | 3 (14) | 73 (100) | 41 (98) | 0.000 | 0.000 | 0.002 | 0.410 |
| Placentas with VV anastomoses, n (%) | 45 (22) | 15 (32) | 0 | 17 (23) | 18 (43) | 0.360 | – | 0.739 | 0.031 |
| Placental sharing discordance, % | 28.3±17.6 | 30.9±18.1 | 31.7±19.8 | 54.8±19.5 | 25.8±23.8 | 0.431 | 0.427 | 0.000 | 0.711 |
| Velamentous cord insertion,an (%) | 75 (21) | 28 (30) | 7 (16) | 48 (33) | 3 (4) | 0.073 | 0.421 | 0.005 | 0.000 |
Data are shown as mean±SD.
P1: normal MC vs TTTS without laser; P2: normal MC vs TAPS; P3: normal MC vs sIUGR; P4: normal MC vs MA.
Although vascular anastomoses are always present in MC placentas, most MC pregnancies proceed well without complications, suggesting a balance in inter-twin blood exchange.
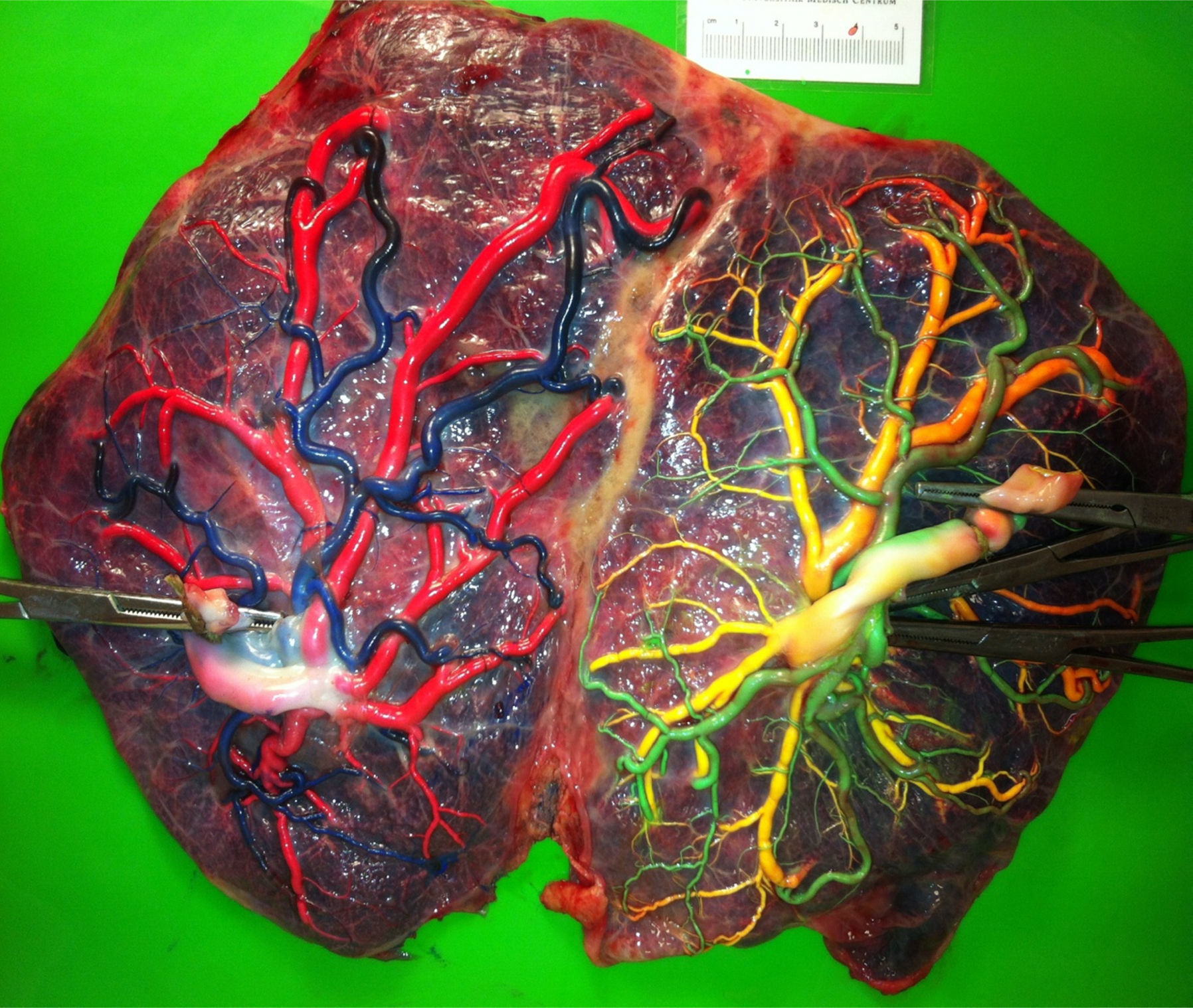
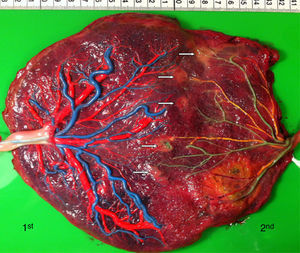
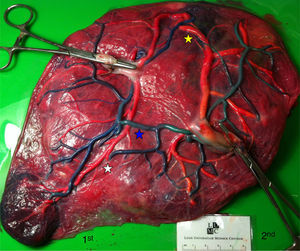
The mean number of vascular anastomoses in normal MC placentas varies in different studies from 2 to 7.9–12 This may be attributed to different techniques of placenta examination: from detection with the naked eye to colored dye injection with milk, water or color dye. We routinely inject MC placentas with color dye and injected to date a total of 178 normal MC placentas (Fig. 1). The mean number of anastomoses per placenta was 8.3±5.2. The prevalence of AV (and VA) anastomoses, AA anastomoses and VV anastomoses was 99% (176/178), 90% (160/178) and 25% (45/178), respectively. The high rate of AA anastomoses is typical of normal MC placentas. AA anastomoses are thought to prevent the development of various complications due to compensation through bidirectional blood flow.13 The role of VV anastomoses is not clear and remains to be elucidated.9
In normal MC twins, placental sharing discordance is usually small.14 The rate of velamentous cord insertion is approximately 21%, which is higher than in singleton placentas (2%) or dichorionic placentas (7%).15 The results of sharing discordance and type of cord insertion in normal MC placentas in our cohort are shown in Table 1.
TTTS placentas (with and without laser treatment)TTTS is the most severe complication in MC twin pregnancies and develops in about 10% of MC pregnancies.4 The diagnosis of TTTS is based on ultrasound signs: oligohydramnios (deepest vertical pocket ≤2cm) present in the sac of one twin with a collapsed bladder (the donor) and polyhydramnios (deepest vertical pocket ≥8cm) present in the sac of the other twin with a distended bladder (the recipient).16
The development of TTTS is mainly attributed to imbalanced blood flow between donor and recipient and hormonal imbalance leading to twin oligo-polyhydramnios sequence (TOPS).17–19 TTTS may be treated with serial amnioreduction or fetoscopic laser coagulation of the vascular anastomoses. Randomized controlled trials and systematic reviews of the literature have shown that laser surgery is the optimal treatment for TTTS.20,21 Laser surgery was introduced as the treatment of choice at our center in August 2000.
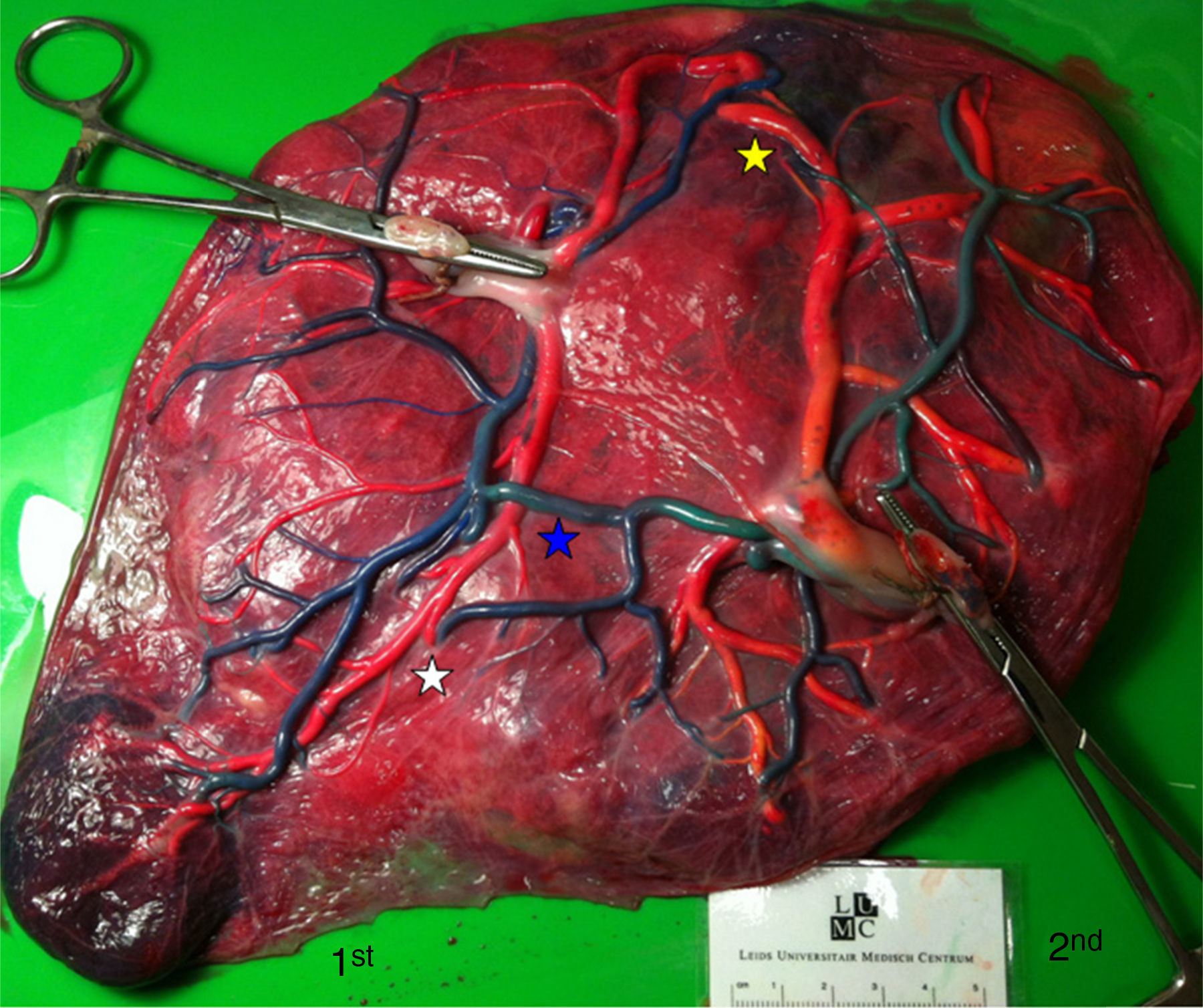
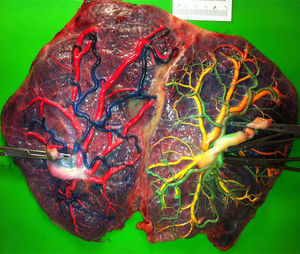
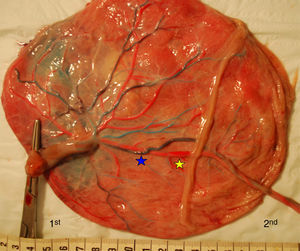
TTTS placentas treated without laserWe injected a total of 41 TTTS placentas not treated with laser (Fig. 2). The mean number of anastomoses per placentas was 9.2±7.8. The prevalence of AV (and VA) anastomoses, AA anastomoses and VV anastomoses was 96% (45/47), 47% (22/47) and 32% (15/47). The mean number of anastomoses in TTTS is similar to that of normal MC placentas. In TTTS placentas, however, the net blood flow and/or number of AV anastomoses are not balanced, which causes the transfusion from donor to recipient. The low rate of AA anastomoses is typical of TTTS placentas.9 The absence of AA anastomoses is thought to lead to insufficient compensation of blood loss of the donor twin and promote the chronic inter-twin polyhydramnios-oligohydramnios sequence.
The placental sharing discordance and incidence of velamentous cord insertion is similar between TTTS and normal MC placentas.22 The results of sharing discordance and type of cord insertion in TTTS placentas in our cohort are shown in Table 1.
The velamentous insertion in TTTS usually belongs to the donor twin.22 The exact role of placental sharing discordance and velamentous cord insertion in the development of TTTS is controversial and requires further study.
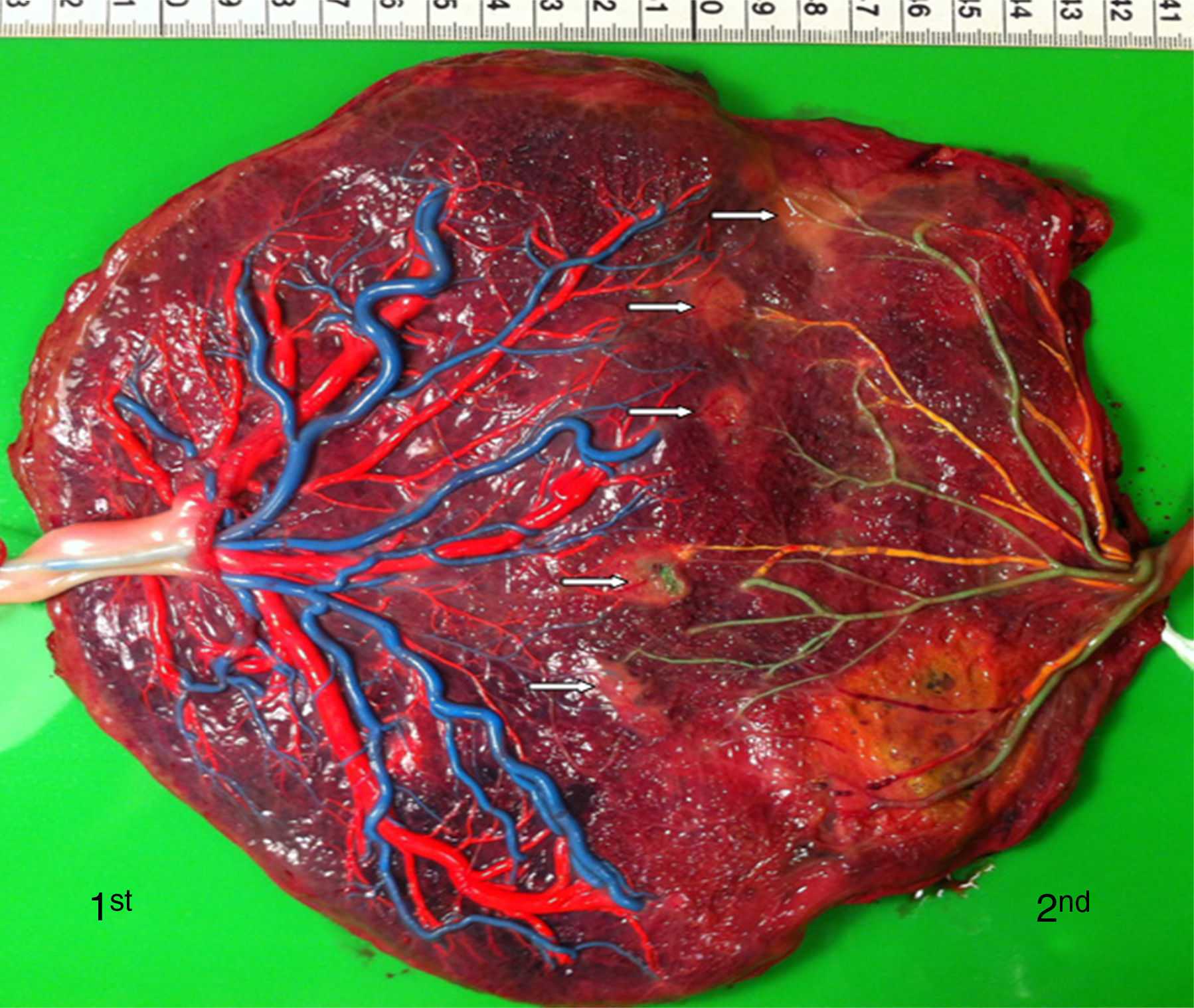
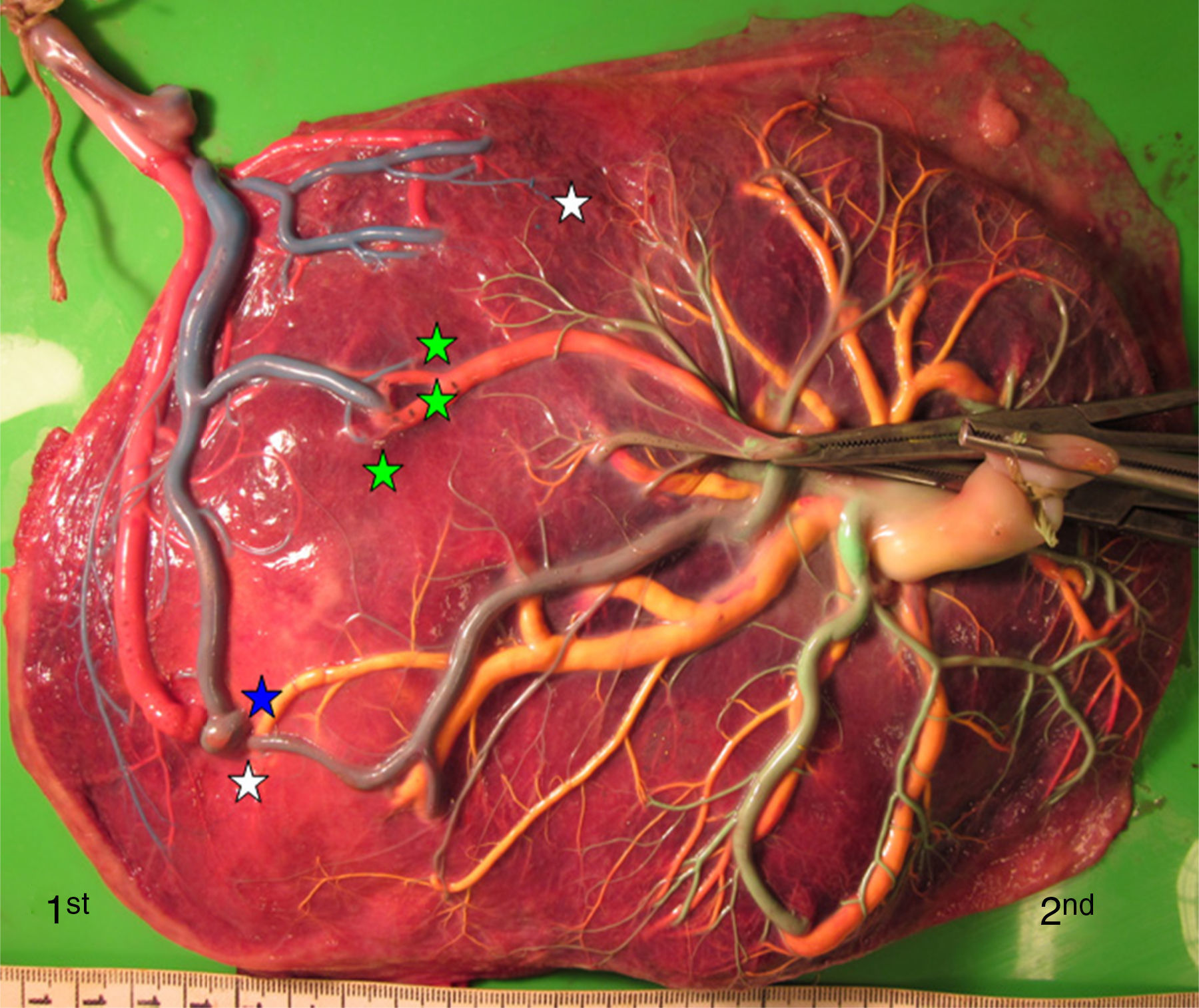
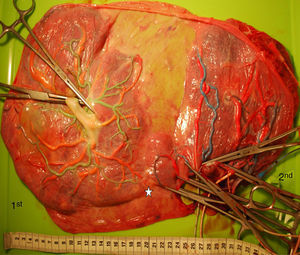
TTTS treated with laserWe injected a total of 270 TTTS placentas treated with laser (Fig. 3). In TTTS placentas treated with fetoscopic laser surgery, injection studies are useful in determining the presence of residual anastomoses, which can be associated with recurrent TTTS and post-laser TAPS.23,24 Residual anastomoses may thus be difficult to visualize with the naked eye and can only be evaluated using careful injection with color dye. Most residual anastomoses are extremely small and localized near the placental margin.24 The reported rate of residual anastomoses in several studies varies from 4 to 33%.24–28 Discordances in rates of residual anastomoses may be due to different injection technique with varying accuracy as well as differences in laser technique. Recently, we conducted a randomized trial (Solomon trial; www.trialregister.nl, trial ID: NTR1245) aimed at reducing the rate of residual anastomoses. In this trial, a coagulation line is drawn with the laser beam across the entire vascular equator from one placental margin to the other, instead of coagulating only the visible anastomoses (Fig. 4). Hypothetically, this technique may be more effective in coagulating all vascular anastomoses, in particular the very small anastomoses which may be difficult to identify during fetoscopy. The results of this study are currently being evaluated.
Twin anemia-polycythemia sequence (TAPS) is also a form of chronic inter-twin transfusion, characterized by a large inter-twin difference in hemoglobin level without oligohydramnios-polyhydramnios sequence. TAPS may occur spontaneously or after laser surgery for TTTS due to small residual anastomoses. The incidence of spontaneous TAPS is approximately 3–5% and the incidence of post-laser TAPS ranges from 2% to 14%.5,23,24 TAPS can be diagnosed antenatally or postnatally. The antenatal diagnosis criteria are based on the Doppler ultrasound abnormalities showing an increased peak systolic velocity in the middle cerebral artery (MCA-PSV>1.5MoM) in the donor twin (indication of fetal anemia) and a decrease (MCA-PSV<1.0MoM) in the recipient twin (indication of polycythemia); the postnatal diagnostic criteria for TAPS require an inter-twin hemoglobin difference>8.0g/dL, reticulocyte count ratio>1.7 and/or placenta with only small (diameter<1mm) vascular anastomoses.5
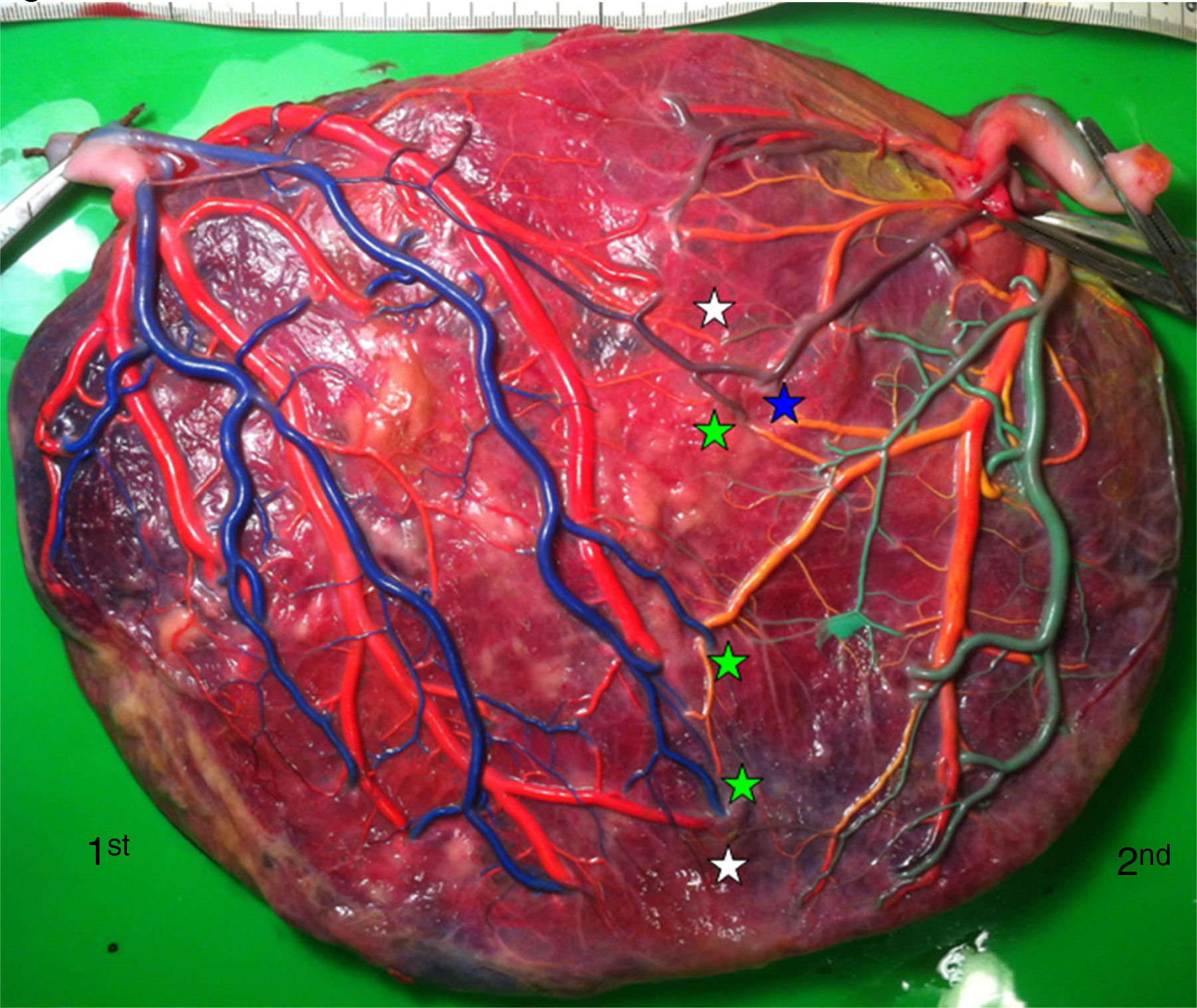
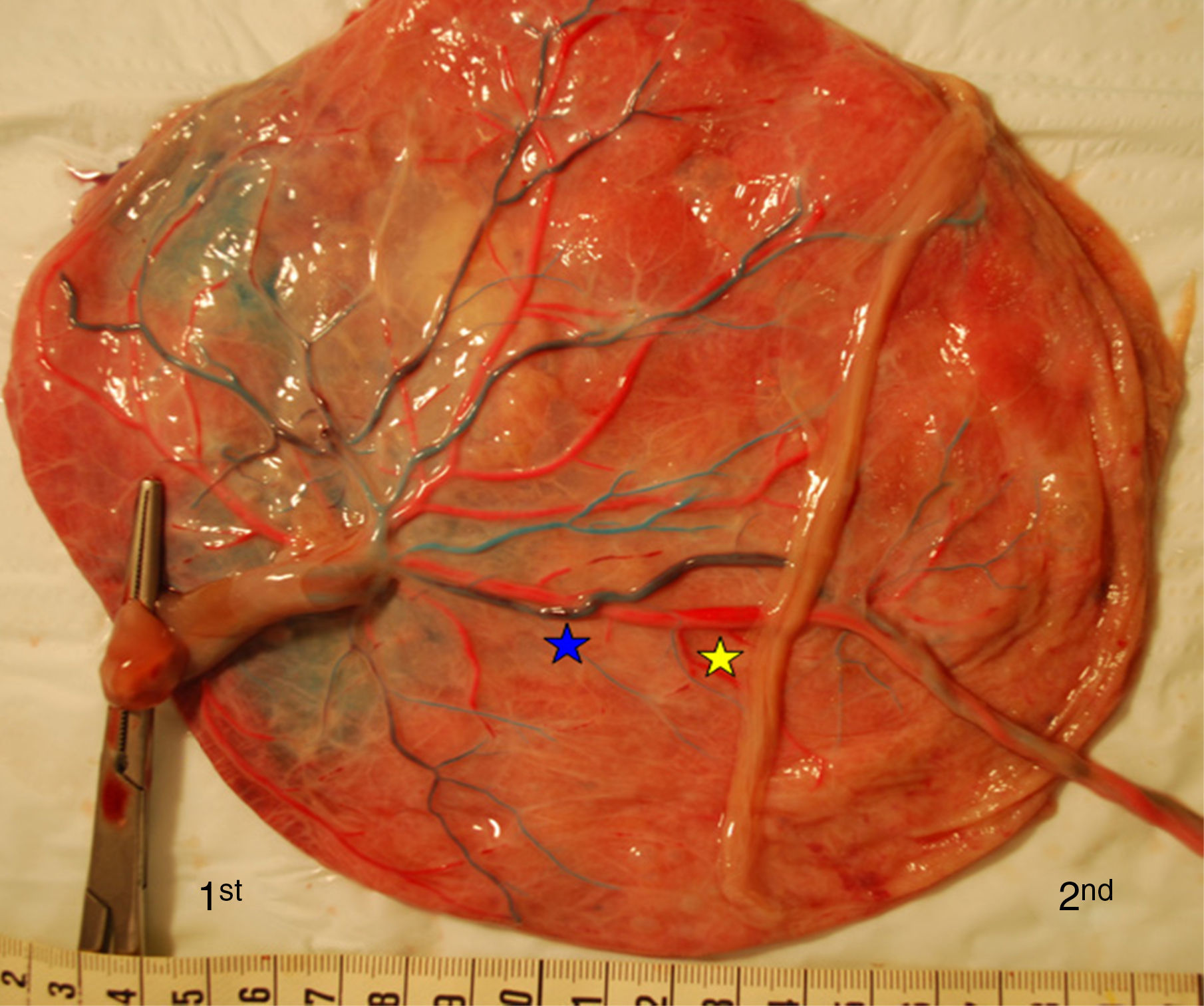
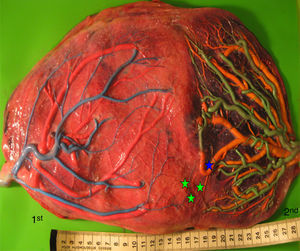
TAPS placentas are characterized by the large difference in color between the plethoric share of the recipient and the pale share of the donor. In addition, TAPS placentas have typically only a few minuscule anastomoses.29 We injected a total of 22 spontaneous TAPS placentas (Fig. 5). The mean number of anastomoses per placentas was 3.8±2.2. The prevalence of AV (and VA) anastomoses, AA anastomoses and VV anastomoses was 100% (22/22), 14% (3/22) and 0% (0/22). The mean number of anastomoses in TAPS placentas is significantly lower than in normal MC placentas. In addition, the diameter of AV and AA anastomoses is extremely small (mean 0.16±0.01mm). The small diameter of anastomoses and low rate of AA anastomoses in TAPS placentas probably leads to chronic inter-twin transfusion and insufficient compensation, but without the large volume imbalance as seen in TTTS. The incidence of velamentous cord insertion and placental territory discordance of TAPS placentas are similar to that of normal MC placentas. The results of sharing discordance and type of cord insertion in TAPS placentas in our cohort are shown in Table 1.
sIUGR placentasSelective intrauterine growth restriction (sIUGR) affects approximately 10–20% of MC twin pregnancies compared to 8% in dichorionic twins.30,31 sIUGR results in an increased rate of mortality and morbidity among MC twins.4,31,32 To evaluate the clinical outcome and association with placental anastomoses, sIUGR is classified into 3 types based on the characteristics of umbilical artery (UA) Doppler flow in the smaller twin: Type I (UA Doppler with positive diastolic flow), Type II (persistent absent or reversed end-diastolic flow) and Type III (intermittent absent or reversed end-diastolic flow).33
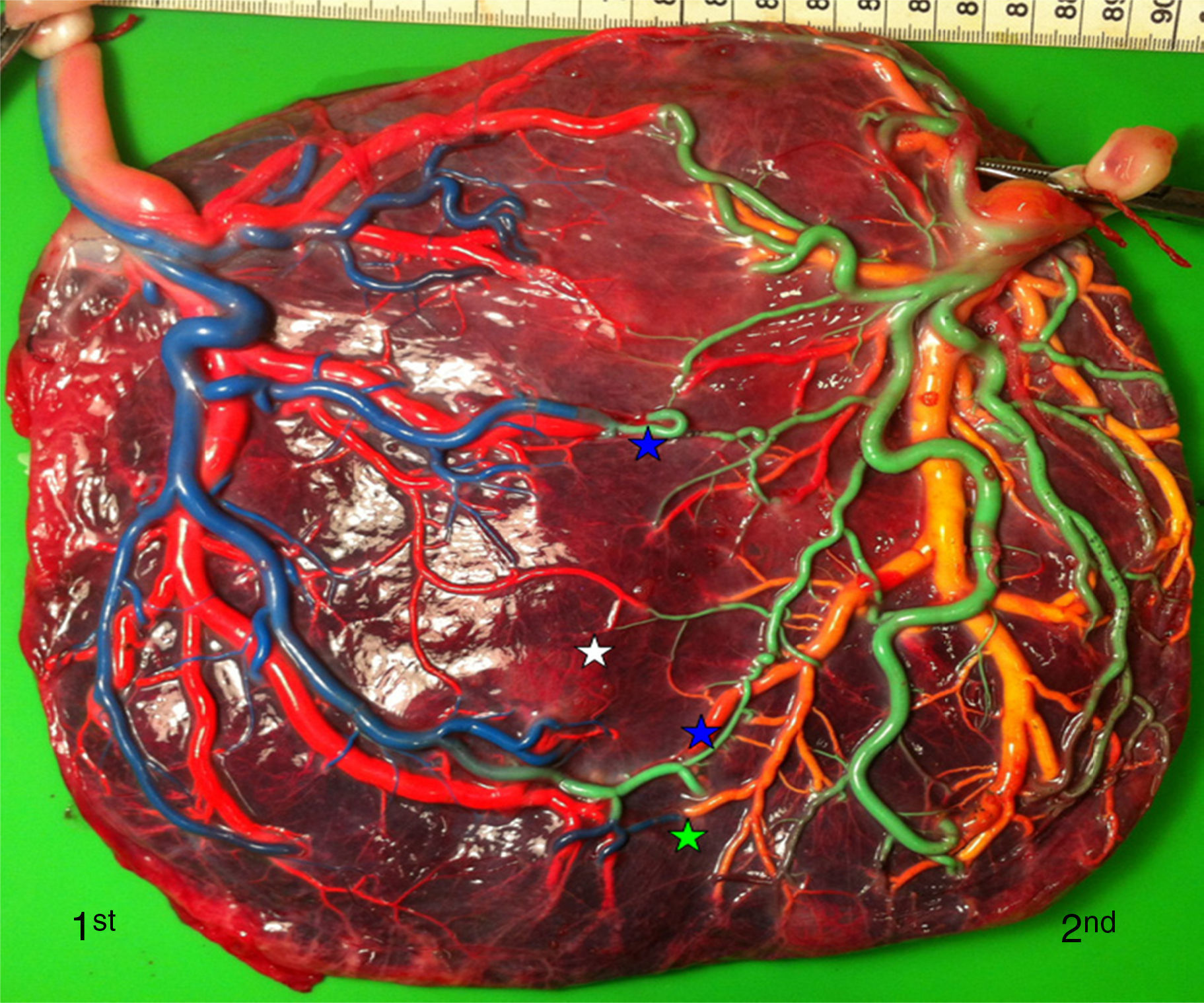
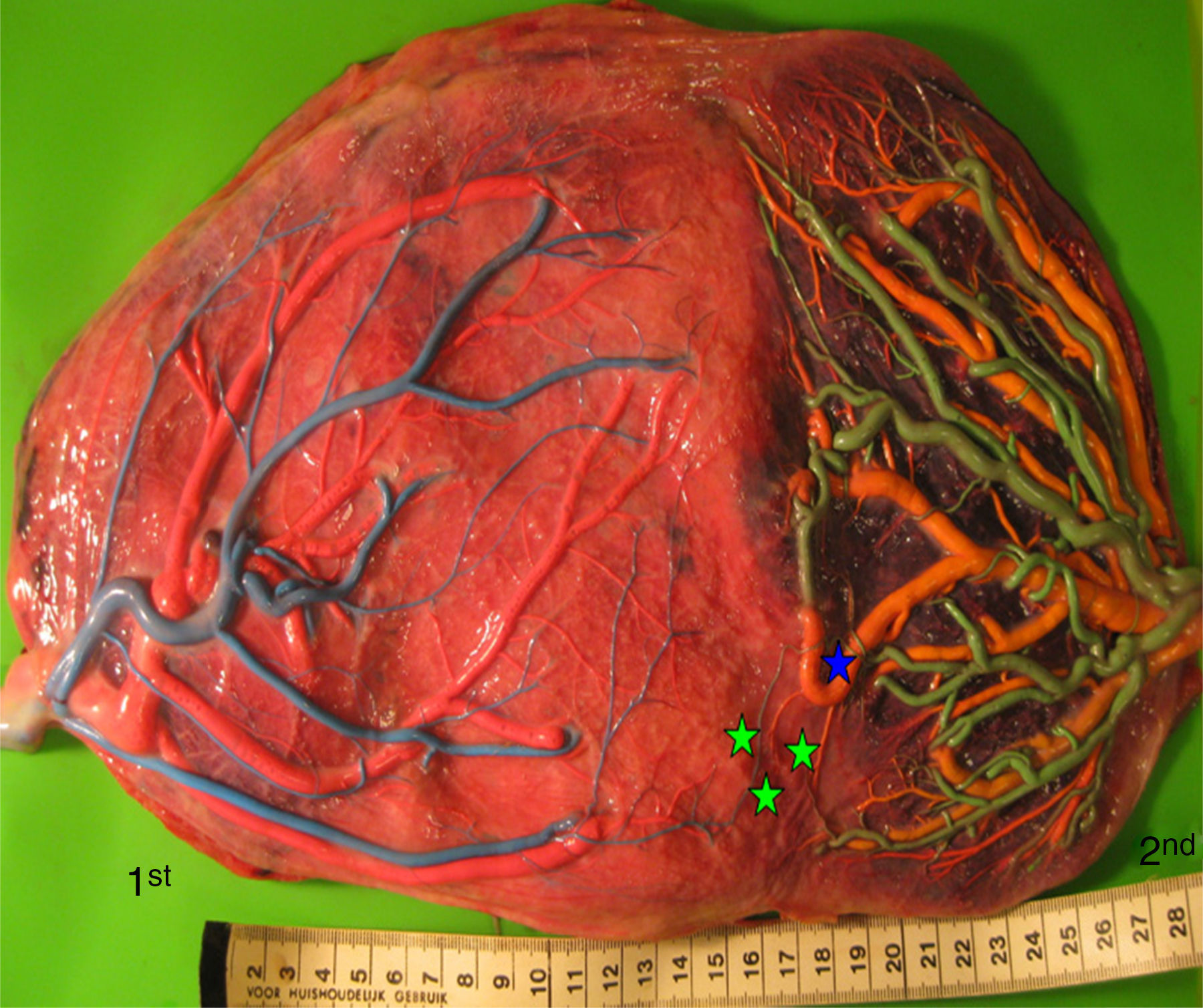
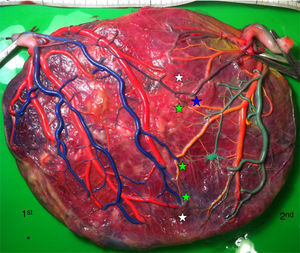
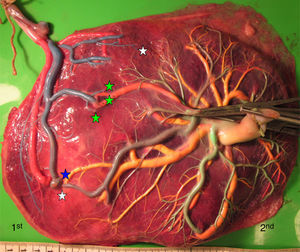
Placentas with sIUGR are characterized by the presence of a large AA anastomosis, large placental sharing discordance and higher rates of velamentous cord insertion.3,32,34,31 The mean number of anastomoses in sIUGR placentas is similar to normal MC placentas. However, nearly all the sIUGR placentas have one AA anastomosis with a significantly larger diameter compared to normal MC placentas. We injected a total of 73 sIUGR placentas (Fig. 6). The mean number of anastomoses per placentas was 9.4±5.6. The prevalence of AV (and VA) anastomoses, AA anastomoses and VV anastomoses was 100% (73/73), 100% (73/73) and 23% (17/73). sIUGR is strongly associated with unequal placental sharing: a large placental share for the large twin and a small placental share for the growth restricted twin. The incidence of velamentous cord insertion in sIUGR placenta is up to 30%, which is significantly higher compared to normal MC placentas. The velamentous cord insertion belongs usually to the growth restricted fetus. The results of sharing discordance and type of cord insertion in sIUGR placentas in our cohort are shown in Table 1.
sIUGR placenta (gestational age at delivery: 29 weeks) showing 3 AV anastomoses (green stars), 2 VA anastomoses (white stars) and 1 large AA anastomosis (blue star). The growth restricted fetus (1st fetus) has a velamentous cord insertion and a small placental share (left side of the picture).
Monoamniotic (MA) twins account for about 1% of MC pregnancies. MA twins share not only their placenta but also the amniotic sac. MA twins are diagnosed on ultrasound examination by the presence of a single amniotic sac and lack of an inter-twin septum.
MA placentas are characterized by the presence of large AA anastomoses, cords insertions that are close together and cord entanglement.35–37 Inter-twin blood flow in MA twins is well compensated due to the large AA, leading to a lower incidence of TTTS (<3%)37. On the other hand, the close insertion of umbilical cords also causes the ubiquitous cords entanglement. Cords entanglement is prone to the cord compression, which primarily contributes to the intrauterine fetal demise in MA twins.36 We injected a total of 18 MA placentas (Fig. 7). The mean number of anastomoses per placentas was 8.5±5.6. The prevalence of AV (and VA) anastomoses, AA anastomoses and VV anastomoses was 91% (38/43), 98% (41/43) and 43% (18/43). The results of sharing discordance and type of cord insertion in MA placentas in our cohort are shown in Table 1.
TRAP placentasTwin reversed arterial perfusion sequence (TRAP) complicates about 1 out of 35.000 pregnancies.38 TRAP is defined as the blood flow pumped from one twin (referred to as the pump twin) into the other twin (referred to as the perfused twin).6 The perfused twin is malformed without a functional heart (acardiac twin).38 The pathogenesis of TRAP is related to the presence of a large AA anastomosis and returning back to the pumped twin via a large VV anastomosis (Fig. 8). TRAP is diagnosed by the detection of color Doppler ultrasound showing the reversed blood flow in the umbilical artery.
Bipartite placentasNearly all MC placentas are composed of a single mass. However, in our cohort, 2% (13/608) of MC twins have two separate placental masses (so-called bipartite MC placenta) (Fig. 9). Vascular anastomoses were detected in 69% (9/13) of bipartite placentas and TTTS occurred in 23% (3/13) of bipartite placentas.40 Therefore, detection of two distinct placental masses on prenatal ultrasound or gross examination after delivery does not rule out monochorionicity.39
ConclusionsPlacental vascular anastomoses and sharing discordance result in several specific complications in MC pregnancies such as TTTS, TAPS, sIUGR and TRAP. These complications primarily contribute to the risk of mortality and morbidity in MC twin fetuses and neonates. However, the pathogenesis of these disorders still needs to be elucidated. Routine examination and injected of all MC placentas can provide insight into the development of these complications and optimal management.
Conflict of interestThe authors have no conflict of interest to declare.