Leptospirosis is an emerging worldwide zoonotic disease. Renal involvement is common; it may vary from sub-clinical course with mild proteinuria and urinary sediment changes to severe renal failure. Leptospirosis-induced acute kidney injury is usually non oliguric and hypokalaemic. Renal impairment is mainly characterized by an association of interstitial and tubular damages. The most severe form of Leptospirosis, characterized by jaundice, acute kidney injury and haemorrhagic diathesis, is referred as Weil's disease. We describe a Weil's disease case with oliguric and hypokalaemic acute renal failure, hypotension, jaundice and lung haemorrhage. We performed haemodialysis treatment, steroid and antibiotic therapy with complete recovery of renal function.
La leptospirosis icterohemorrágica es una zoonosis emergente en todo el mundo. La afectación renal es frecuente, y puede variar desde un curso subclínico con proteinuria y ligeros cambios del sedimento urinario hasta una insuficiencia renal grave. La lesión renal aguda inducida por la leptospirosis suele ser no oligúrica e hipopotasémica. El deterioro renal se caracteriza sobre todo por la asociación de una lesión intersticial y tubular. La forma más grave de leptospirosis, caracterizada por ictericia, insuficiencia renal aguda y diátesis hemorrágica, se conoce como enfermedad de Weil. Describimos un caso de enfermedad de Weil con insuficiencia renal aguda oligúrica e hipopotasémica, hipotensión, ictericia y hemorragia pulmonar. Sometismos al paciente a hemodiálisis, tratamiento con esteroides y antibióticos con el restablecimiento completo de la función renal.
Leptospirosis is an emerging worldwide zoonotic disease caused by pathogenic Gram negative Spirochaetes of the genus Leptospira.1,2 Its clinical expression includes subclinical infection, an undifferentiated febrile illness and the most severe form, characterized by jaundice, acute kidney injury (AKI) and haemorrhagic diathesis, referred as Weil's disease. Leptospirosis has a biphasic course: initially the leptospiremic phase (lasting 3–10 days) with acute fever, severe headache, anorexia, diarrhoea; later the immune phase with more severe symptoms, such as meningitis and uveitis. Renal involvement is common in leptospirosis.3 In developed countries leptospirosis is an uncommon cause of AKI, but in tropical countries, where the disease is endemic, it is a frequent cause of AKI. Mortality in leptospirosis-associated AKI is around 22%.4
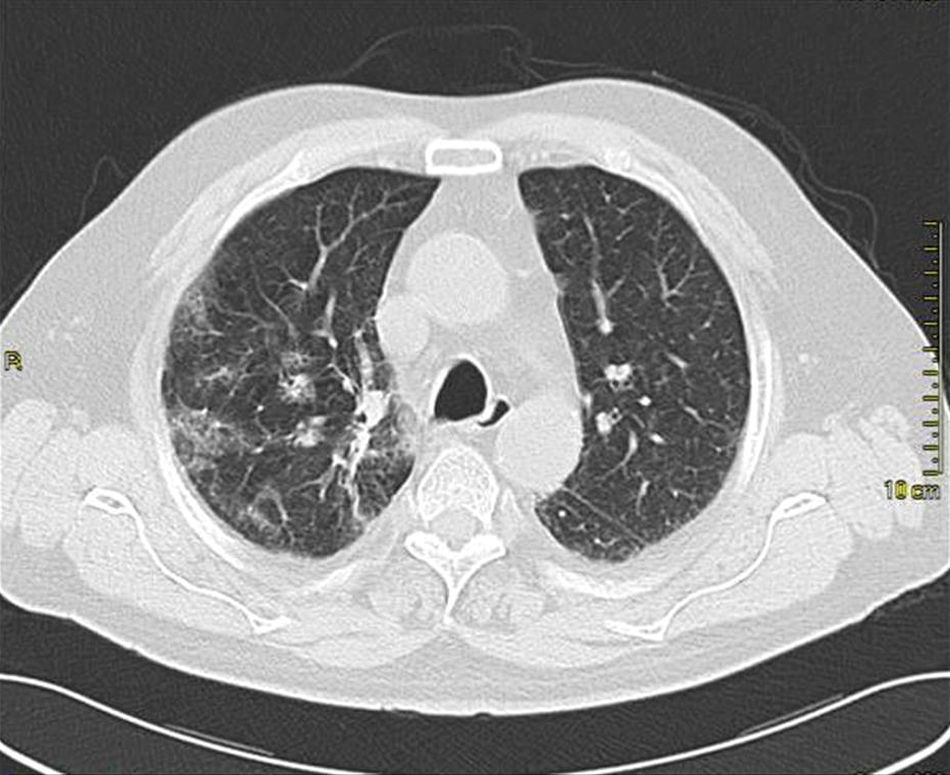
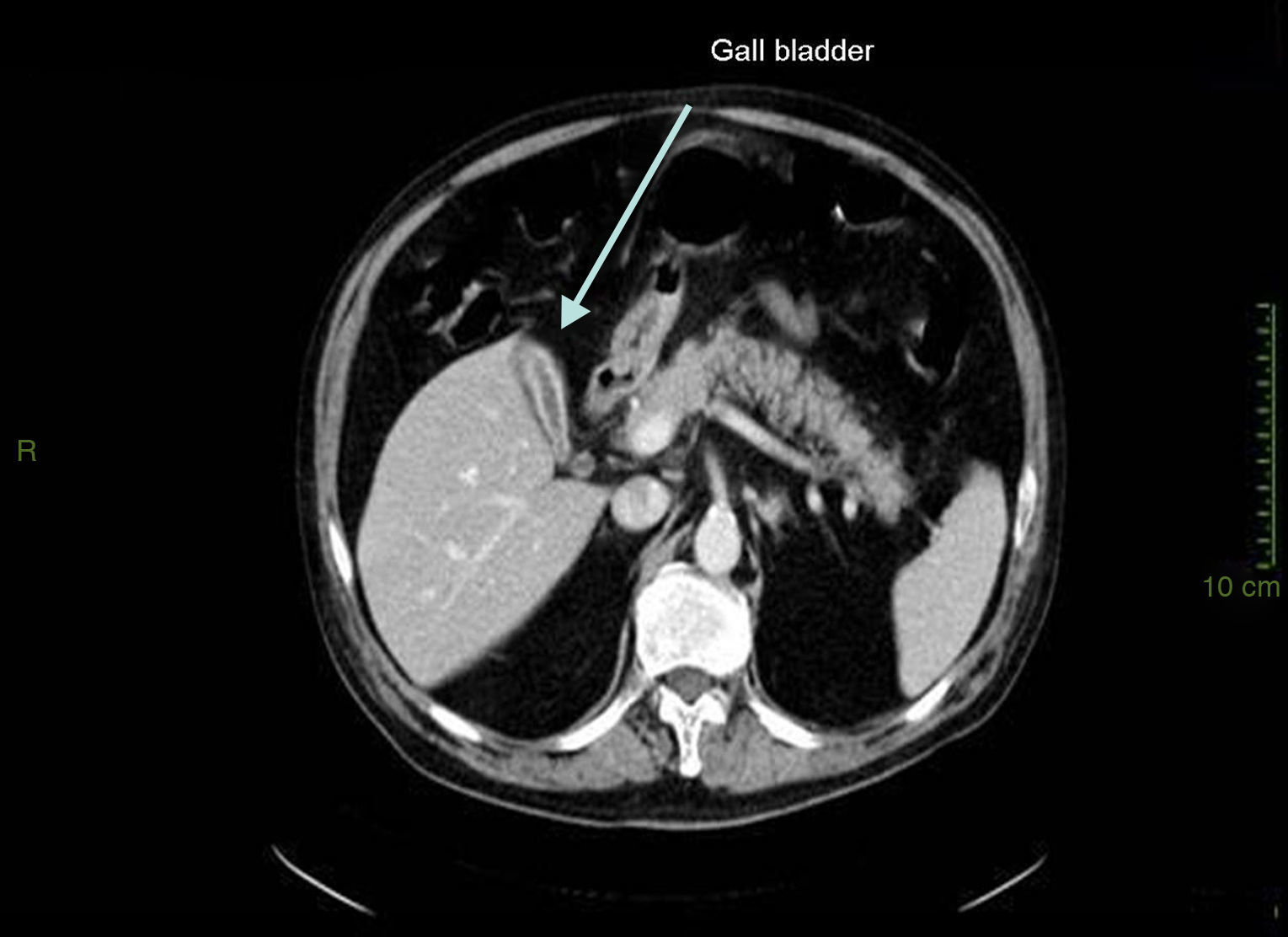
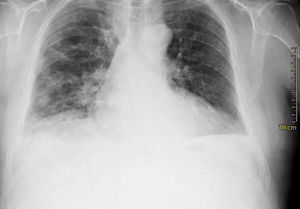
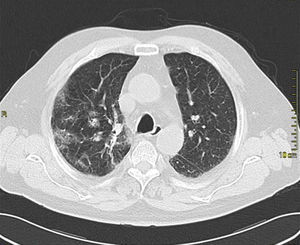
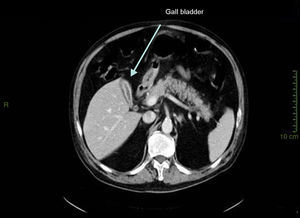
Case presentationIn October 2010 a 74-year-old Caucasian male was admitted because of fever, anuria and mialgia lasting for 2 days. He had no history of renal disease and reported previous normal values of renal function. A month earlier he had travelled to a region of Southern Italy. Physical examination showed normal blood pressure, dyspnoea and jaundice without other dermatological findings; the patient was anuric with 2+pedal oedema. Initial laboratory work-up indicated a total leucocyte (WBC) count of 11,300/μL (neutrophils 88.3%, lymphocytes 11%, eosinophils 0.7%), a haemoglobin level of 9.7g/dL, platelets count of 13,000/μL, serum creatinine 5.25mg/dL, blood urea nitrogen (BUN) 67g/dL, total protein 6.7g/dL, albumin 2.9g/dL, serum sodium 130mEq/L, serum potassium 3.3mEq/L, serum calcium 8.4mg/dL; total cholesterol 254mg/dL; LDL cholesterol 48mg/dL; HDL cholesterol 22mg/dL, procalcitonin 45.71ng/mL, normal value (n.v.)<0.09, Reactive C Protein 22.70mg/dL, n.v.<0.30, ferritin 1300μg/L. Complement C3 and C4, Immunoglobulin G, A and M, kappa and lambda light chains, clotting profile, AST and ALT levels were in the normal range. Total and direct bilirubin values were respectively 4.3 and 2.1mg/dL. Immunological (anti-neutrophil cytoplasm antibodies, anti-nuclear antibodies, anti-double stranded DNA antibodies, anti-extractable nuclear antigen antibodies, anti-phospholipids antibodies, rheumatoid factor) tests were unremarkable. A chest X-ray showed multiple bilateral ground-glass opacities (Fig. 1). A thoracic CT-scan revealed multiple bilateral consolidations and bilateral pleural effusion (Fig. 2). There was to electrocardiogram evidence of normal sinus rhythm and transthoracic echocardiography demonstrated no wall motion abnormality. An abdominal ultrasonography and a CT-scan revealed no abnormalities of liver, gallbladder, kidneys, spleen and pancreas. At the same time of antibiotic, platelet transfusion and diuretic therapy we started continuous venovenous haemofiltration (CVVHF) with weight loss and rapid regression of dyspnoea. Two days after admission the patient showed melena. Subsequent laboratory tests indicated: normal oncologic screening; no signs of haemolytic anaemia; red blood cells and WBC in urinary sediment; a total of 475mg of protein was excreted during a 24-h urine collection; blood cultures examination, tuberculin reaction and HBV, HCV, CMV, EBV, Adenovirus, Chlamydia Pneumoniae and Mycoplasma Pneumoniae screening were negative. During hospitalization we observed a worsening of liver function (total and direct bilirubin respectively 17.30 and 10.50mg/dL, gammaGT 267IU/L, n.v. 7–50, LDH 1027IU/L, n.v. 266–530, AST: 67IU/L, n.v. 10–42, ALT 96IU/L, n.v. 10–45) and inflammation parameters (WBC count 25,250/μL, neutrophils 76.6%, ferritin 4200μg/dL). An abdomen CT-scan highlighted greatly thickened gallbladder and bile duct walls with contrast enhancement, peritoneal effusion and swelling of the visceral adipose tissue (Fig. 3). We performed the Leptospira IgM test (enzyme immunoassay) with positive result. After platelets and erythrocytes transfusions and omeprazole therapy, we started 3g/day ceftazidime and 50mg/day prednisone therapy with a progressive improvement in renal function and general conditions; after 10 days of admission we stopped CVVHF. A thoracic CT scan showed resolution of previously observed lesions. The patient was discharged on day 18. Final laboratory parameters were: creatinine: 0.93mg/dL, BUN: 26mg/dL, total and direct bilirubin respectively 6.50 and 3.40mg/dL, AST: 47IU/L, ALT: 84IU/L, gammaGT: 137IU/L, LDH: 609IU/L, WBC 3420/μL, Hb: 8.3g/dL, and PLT: 262,000/μL.
DiscussionLeptospirosis is an infectious vasculitis. Its pathogenesis is related both to the ability of the leptospira to damage the small blood vessels wall and to systemic immune response.5 Renal involvement is common in leptospirosis.6 It may vary from sub-clinical course with mild proteinuria and urinary sediment changes to severe renal failure. Haemodynamic alterations, bacterial invasion, inflammatory process and direct toxicity of bacterial products are thought to be responsible for nephropathy development. Renal impairment is a frequent complication in patients with severe form of leptospirosis, mainly characterized by an association of interstitial and tubular damage. Pathologically all renal structures are involved but interstitial nephritis is the basic lesion of leptospirosis. Glomerular changes are usually not remarkable. Tubular function abnormalities precede a decline in the glomerular filtration rate. AKI can manifest after several days of illness in oliguric or non-oliguric form, with serum electrolyte abnormalities reflecting proximal renal tubular dysfunction. Hypokalaemia is the most characteristic laboratory finding of leptospirosis-AKI. The considerable tubulo-interstitial involvement explains hypokalaemia such as constant and relevant characteristic of leptospirosis-AKI at the time of diagnosis, regardless of hypercatabolism, rhabdomyolysis, acidosis or oliguria. Leptospirosis-AKI can also have a prerenal component. Arterial hypotension is common, because of the reduction in systemic vascular resistance and dehydration. The haemodynamic status and alterations in most patients with severe leptospirosis are similar to those observed in patients with sepsis. Recovery of renal function is usually complete in most patients.3 The most severe form of leptospirosis is Weil's disease, characterized by jaundice, AKI, hypotension, pulmonary oedema and haemorrhage, most commonly involving the lungs but also potentially affecting the gastrointestinal tract, retroperitoneum, pericardium and brain.7 In the present case we observed a mixed jaundice cholestatic and hepatocellular, hypotension, thrombocytopenia, alveolar haemorrhage and oliguric hypokalaemic acute renal failure. We performed laboratory diagnosis by leptospira-IgM detection during the second week of the disease. Prognosis of leptospirosis-AKI is usually favourable unless complicated by multiple organ involvement. Pulmonary complications, hyperbilirubinaemia, oligo-anuria, diarrhoea, hyperkalaemia, old age and associated infection or underlying diseases carry bad prognosis with mortality ranging from 12% to 36%. Based on the recommendation of the World Health Organization of 2003, severe leptospirosis should be treated with intravenous penicillin or cephalosporin.8
Conflict of interestNone declared.