Microalbuminuria, defined as urine albumin to urine creatinine ratio of 30 to <300mg/g, is an established risk factor for cardiovascular morbidity and mortality in the general population. Low-grade albuminuria (<30mg/g) is considered a marker for subclinical vascular damage that predisposes to future cardiovascular diseases and death. Lowering urinary albumin excretion reduces the risk of cardiovascular disease. Our study was designed to evaluate the influence of novel calcium channel blocker, cilnidipine in hypertensive chronic kidney disease (CKD) patients with lowgrade albuminuria.
Patients and methodsOur 6-month prospective observation study used a randomized control and open-label design as we examined the effects of cinidipine on blood pressure, urinary albumin excretion and estimated glomerular filtration rate (eGFR) in hypertensive CKD patients. A total of 60 hypertensive CKD patients were enrolled in this study. Patients were randomly assigned to 2 groups: cilnidipine group (n=30), receiving 10–20mg cilnidipine daily for 6 months, and the control group (n=30).
ResultsIn the cilnidipine group, urine albumin excretion was significantly reduced from 25.1±19.9mg/g Cr at baseline to 12.6±9.0mg/g Cr at 6 months after administration. eGFR decreased slightly at 6 months after administration.
ConclusionsThis study reveals that cilnidipine is safe and effective in reducing low-grade albuminuria in hypertensive CKD patients. Thus, early treatment of cilnidipine in hypertensive CKD patients with low-grade albuminuria may prevent cardiovascular disease.
La microalbuminuria, definida como un cociente albúmina:creatinina, analizado en una muestra aislada de orina, de 30 a <300mg/g, es un factor de riesgo establecido de morbilidad y mortalidad cardiovascular en la población general. La albuminuria de bajo grado (<30mg/g) se considera un marcador de lesión vascular subclínica que predispone a futuras enfermedades cardiovasculares y la muerte. La disminución de la excreción urinaria de albúmina reduce el riesgo de enfermedad cardiovascular. El presente estudio se diseñó para evaluar la influencia de un nuevo antagonista del calcio, el cilnidipino, en pacientes con nefropatía crónica hipertensiva asociada a albuminuria de bajo grado.
Pacientes y métodoEn el presente estudio observacional, prospectivo, de 6 meses de duración, se usó un diseño controlado, aleatorizado y abierto para examinar los efectos de cilnidipino sobre la presión arterial, excreción urinaria de albúmina y tasa estimada de filtración glomerular (eTFG) en pacientes con nefropatía crónica hipertensiva. Se reclutó en el estudio un total de 60 pacientes con nefropatía crónica hipertensiva. Los pacientes fueron asignados aleatoriamente a uno de dos grupos: grupo cilnidipino (n=30), tratado con 10–20mg/día de cilnidipino durante 6 meses, y grupo de control (n=30).
ResultadosEn el grupo tratado con cilnidipino, la excreción urinaria de albúmina disminuyó significativamente desde 25,1±19,9mg/g Cr en el período basal hasta 12,6±9,0mg/g Cr a los 6 meses tras la administración. La eTFG disminuyó ligeramente a los 6 meses tras la administración.
ConclusionesEl presente estudio revela que el cilnidipino es bien tolerado y eficaz en la reducción de la albuminuria de bajo grado en pacientes con nefropatía crónica hipertensiva. Por esta razón, el tratamiento precoz con cilnidipino en pacientes con nefropatía crónica hipertensiva asociada a albuminuria de bajo grado podría prevenir la enfermedad cardiovascular.
Calcium channel blockers (CCBs), which target voltage-dependent Ca channels, are widely used in the field of hypertension therapy. Conventional CCBs elicit marked increases in the glomerular filtration rate and renal blood flow1–3 by dilating afferent arterioles preferentially,4–8 whereby glomerular hypertension and subsequent renal injury are expected to ensue unless systemic blood pressure is sufficiently controlled.9–12 Voltage-dependent Ca channels are widely distributed throughout the body and play a critical role in the maintenance of vascular tone. Ca channels are classified into several subtypes, including L-type, T-type, N-type, P/Q-type and R-type Ca channels based on their electrophysiological properties.13 The blockade of L-type Ca channels dilates the systemic vasculature and substantially reduces blood pressure. In the renal microvasculature, however, the vasodilator response to L-type CCBs is observed only in preglomerular microvessels (e.g., afferent arterioles), whereas efferent arterioles are refractory to the dilator action of these agents. The renal microvascular response thus supports the speculation that glomerular hypertension may develop following the administration of these agents. In the kidney, several Ca channel subtypes are reported to be present, including L-type, T-type, N-type and P/Q-type Ca channels.14,15 Cilnidipine (marketed as Cilacar in India), an L-type and N-type CCB, has been reported to exert antiproteinuric action when administered in patients with essential hypertension.16 Collectively, the role of Ca channels may differ depending on the subtype expression within the kidney, and the specific blockade of Ca channel subtypes would be anticipated to exert a salutary action on the renal microvasculature. In this article, we analyze the efficacy of cilnidipine in hypertensive CKD patients with low grade proteinuria.
Materials and methodsThis is a 6-month prospective observation study used a randomized control and open-label design, to study the CCB cilnidipine versus control.
This study enrolled hypertensive CKD patients whose spot urine albumin specimens collected at an outpatient visit contained 30mg/g of creatinine or less. Patients with stable renal function in the last 6 months were included in the study.
The patients with severe renal dysfunction (eGFR<30ml/min per 1.73m2) or severe hypertension (baseline blood pressure>180/100mmHg) or on angiotensin coverting enzyme inhibitors or angiotensin receptor blockers were excluded from the study. Patients with diabetes mellitus and those with suspected or documented stenosis of the renal artery were excluded. A total of 60 hypertensive CKD patients were enrolled and assigned randomly into 2 groups: cilnidipine group (n=30), receiving 10–20mg cilnidipine daily for 6 months, and the control group (n=30).
All subjects gave informed consent for participation in the study, which was approved by the Human Ethics Committee of Vaatsalya Hospital. Cilnidipine was administered in a single daily oral dose of 20mg in the cilnidipine group. Other antihypertensive medications were not changed during the study. All of the procedures were in accordance with the Helsinki Declaration of 1975.
We examined the effects of the cilnidipine on blood pressure, urinary albumin excretion, S-Cr and eGFR of the participants. Changes in blood pressure, urinary albumin excretion, levels of S-Cr and eGFR from baseline to 6 months were compared between the 2 groups.
MeasurementsFasting blood samples were obtained in the early morning for biochemical studies, including serum creatinine (S-Cr), serum potassium and hematocrit. Estimated glomerular filtration rate (eGFR) was estimated by the modified Modification of Diet in Renal Disease (MDRD) Study equation. As a marker of renal damage and diffuse endothelial dysfunction, urinary albumin excretion (the ratio of spot urine albumin to creatinine) was measured in the participants. Blood pressure was reported as the average of 5 automated measurements taken at 3-min intervals. Clinical parameters such as age, sex and vital statistics were recorded. Body mass index (BMI) was calculated as body weight in kilograms divided by the square of height in meters (kg/m2).
Statistical analysisAll analyses and calculations were performed using the Stat View V Statistical System. The results were presented as means±standard deviation (SD) for continuous variables and as the proportion for categorical variables. Comparisons between groups at baseline were performed using Student's t-test or Mann–Whitney U-test. Categorical variables were compared using chi-square analysis. Changes within each group were evaluated by paired Student's t-test or Wilcoxon's test. Statistical significance was defined as a p value<0.05.
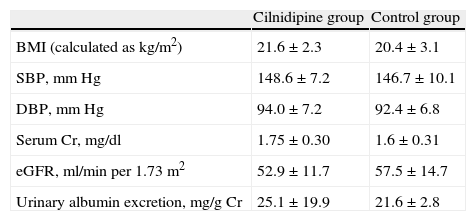
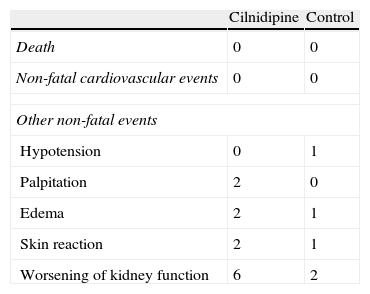
ResultsClinical characteristics of participants at baseline are reported in Tables 1 and 2. As a result of the randomization procedure, the 2 groups had similar biochemical and clinical parameters. There were no significant differences between the 2 groups regarding age, sex and underlying disease. The changes in clinical and biochemical data of recipients in the cilnidipine group before and after cilnidipine administration are shown in Table 4. Blood pressure decreased significantly in recipients of the cilnidipine group from 148.6/94.0±7.2/7.2mmHg (systolic/diastolic) at baseline to 142.0/87.0±6.5/6.0mmHg at 6 months. The urinary albumin excretion in recipients of cilnidipine group reduced markedly from 25.1±19.9mg/g Cr at baseline to 12.6±9.0mg/g Cr at 6 months. S-Cr levels in the cilinidipine group increased from 1.75±0.30mg/dl at baseline to 1.80±0.30mg/dl, and eGFR decreased from 52.9±11.7ml/min per 1.73m2 at baseline to 49.8±8.7ml/min per 1.73m2 at 6 months. However, there were no patients with 30% elevation of S-Cr. No patients undergoing treatment required discontinuation of cilnidipine (Table 3). There were no significant changes in S-Cr level, eGFR and urinary albumin excretion in the study period. Changes in clinical and biochemical parameters of the control group before and after the initiation of this study are shown in Table 5. There was a significant difference in percentage change of urinary albumin excretion between the 2 groups. The result suggests that the reduction of albuminuria in the cilnidipine group was significantly greater than that in the control group.
Patient characteristics.
| Cilnidipine group | Control group | |
| BMI (calculated as kg/m2) | 21.6±2.3 | 20.4±3.1 |
| SBP, mmHg | 148.6±7.2 | 146.7±10.1 |
| DBP, mmHg | 94.0±7.2 | 92.4±6.8 |
| Serum Cr, mg/dl | 1.75±0.30 | 1.6±0.31 |
| eGFR, ml/min per 1.73m2 | 52.9±11.7 | 57.5±14.7 |
| Urinary albumin excretion, mg/g Cr | 25.1±19.9 | 21.6±2.8 |
Results are expressed as means±SD.
BMI=body mass index; Cr=creatinine; DBP=diastolic blood pressure; eGFR=estimated glomerular filtration rate; SBP=systolic blood pressure.
Changes in the mean parameters during this study and statistical analysis of the administration of cilnidipine between before and 6 months after administration.
| Parameters | Before | After 6 months | Statistical differences |
| SBP (mmHg) | 148.6±7.2 | 147.0±6.5 | NS |
| DBP (mmHg) | 94.0±7.2 | 87.0±6.0 | p<0.05 |
| BMI (calculated as kg/m2) | 21.6±2.3 | 21.2±3.1 | NS |
| Serum Cr (mg/dl) | 1.75±0.30 | 1.80±0.30 | p<0.05 |
| eGFR (ml/min per 1.73m2) | 52.9±11.7 | 49.8±8.7 | p<0.05 |
| Urinary albumin excretion, mg/g Cr | 25.1±19.9 | 12.6±9.0 | p<0.05 |
Results are expressed as means±SD.
BMI=body mass index; Cr=creatinine; DBP=diastolic blood pressure; eGFR=estimated glomerular filtration rate; NS=not significant; SBP=systolic blood pressure.
Changes in the mean parameters during this study and statistical analysis of control group between before and 6 months after.
| Parameters | Before | After 6 months | Statistical differences |
| SBP (mmHg) | 146.7±10.1 | 145.5±9.0 | NS |
| DBP (mmHg) | 92.4±6.8 | 91.3±6.7 | NS |
| BMI (calculated as kg/m2) | 20.4±3.1 | 20.2±3.4 | NS |
| Serum Cr (mg/dl) | 1.6±0.31 | 1.57±0.31 | NS |
| eGFR (ml/min per 1.73m2) | 57.5±14.7 | 59.4±15.1 | NS |
| Urinary albumin excretion, mg/g Cr | 21.6±2.8 | 25.7±3.6 | NS |
Results are expressed as means±SD.
BMI=body mass index; Cr=creatinine; DBP=diastolic blood pressure; eGFR=estimated glomerular filtration rate; NS=not significant; SBP=systolic blood pressure.
In this study, we revealed that urinary albumin excretion was significantly reduced by administration of the cilnidipine, in our hypertensive CKD patients. Our results further confirm those of previous reports showing that cilnidipine had greater antiproteinuric effect than other CCB's.17,18
Cilnidipine is a representative L-type and N-type CCB and dilates both afferent and efferent arterioles through the inhibition of N-type Ca channels in nerve terminals innervating renal afferent and efferent arterioles. Evidence has been accumulating that dual calcium channel blockers have greater antiproteinuric effects than single channel blockers.19–21 Cilnidipine has been shown to exhibit renal protective effects in terms of urinary protein reduction in various animal models and in patients with renal dysfunction.22,23 These effects are independent of the hypotensive effect.16 It has been reported that cilnidipine exerts antiproteinuric action when administered in patients with essential hypertension.16 Recently, Fujita et al.19 demonstrated that add-on therapy with cilnidipine reduces proteinuria more potently than that with amlodipine in CKD patients treated with renin–angiotensin blocking agents (CARTER study). They suggest that the beneficial action of cilnidipine on proteinuria is mediated by the inhibition of sympathetic nerve activity and the resultant amelioration of glomerular hypertension.
Several clinical studies demonstrate that proteinuria is a predictor of subsequent progression of kidney disease; for example, in multivariate analysis of data from the AASK study,24 baseline proteinuria correlated to decline of GFR independently. Importantly, proteinuria has been recently recognized to be one of cardiovascular risk factors. In the Framingham cohort, proteinuria showed a three-fold increase of the mortality rate and was strongly associated with other risk factors for cardiovascular disease.25 Experimental and clinical data converge to indicate that, in CKD, proteinuria reduction protects against renal and cardiovascular failure.26 In the present study, urinary albumin excretion was significantly reduced by administration of cilnidipine in hypertensive patients with low levels of urine albumin excretion – well below the current microalbuminuria threshold. Reduction of urinary albumin excretion by administration of cilnidipine may translate to reduction in cardiovascular events even in hypertensive CKD patients with low-grade albuminuria. As a result, administration of cilnidipine may induce the improvement of patient's survival in hypertensive CKD patients.
In this study, none of the patients discontinued cilnidipine because of its adverse effects. Blood pressure slightly but significantly in the cilnidipine group. Hypotension (systolic pressure; 80–90mmHg) occurred in 2 patients but was controlled by a decrease in the dose of cilnidipine. This study demonstrated that administration of cilnidipine was considered safe and beneficial in the hypertensive CKD patients with low grade proteinuria.
The present study has several limitations. Firstly, the number of study group was small. Secondly, the relationship between cardiovascular events and the reduction in marginally elevated microalbuminuria by cilnidipine was not clarified. Thirdly, kidney biopsy was performed in only selective group of patients (where the diagnosis was in doubt) in our study population.
ConclusionIn conclusion, the present study is the first of its kind in India, demonstrated that urinary albumin excretion was significantly reduced by administration of the cilnidipine in hypertensive CKD patients with low-grade albuminuria. Reduction of urinary albumin excretion by administration of cilnidipine may translate into reduction of cardiovascular events in hypertensive CKD patients. Further prospective well-controlled and long-term follow-up trials with a large number of patients are needed to confirm the clinical significance of cilnidipine administration for treating low grade albuminuria in hypertensive CKD patients.
Conflict of interestThe authors have no conflict of interest to declare.