Identification of problems associated with kidney transplantation in small weight children is an essential step toward improving graft function and patient survival.
MethodsThis study comprised 63 renal transplant children weighing 25kg or less at time of renal transplantation. All children have received living-donor renal allotransplant between December 1984 and March 2009. These children were retrospectively evaluated regarding their survival, grafts survival as well as associated risk factors.
ResultsOur patient and graft survival rates at 1, 5 and 10 years were (98.4%, 96.8%, and 96.8%) and (94.9%, 82.6%, and 58.4%), respectively. Significant better graft survival was obtained in cases with non-glomerular etiology of the end-stage renal disease, pre-emptive transplantation and when the aorta was used for arterial vascular anastomosis. Significant worse graft survival was documented among those who experienced pre-transplant hypertension, pre-transplant blood transfusion, post-transplant acute rejection, chronic rejection, hypertension and graft obstruction.
ConclusionThe outcome of live donor renal transplantation in children weighing 15–25kg in our locality as a developing country is comparable to other reports. Better graft survival rates might be obtained by adopting pre-emptive transplant policy, avoiding pre-transplant blood transfusions, using aorta for arterial vascular anastomosis in addition to adopting recent tacrolimus-based immunosuppressive regimens that is associated with reduced incidence of post-transplant acute rejection and graft dysfunction.
La identificación de los problemas asociados con el trasplante de riñón en niños de bajo peso corporal es un paso esencial para mejorar la función del injerto y la supervivencia de los pacientes.
MétodosEl presente estudio incluyó a 63 pacientes sometidos a un trasplante de riñón cuyo peso era ≤25kg en el momento del trasplante renal. Todos habían recibido un alotrasplante renal de donante vivo entre diciembre de 1984 y marzo de 2009. Estos niños se valoraron retrospectivamente con respecto a la supervivencia, supervivencia del injerto, al igual que factores de riesgo asociados.
ResultadosLas tasas de supervivencia de nuestros pacientes y del injerto a los 1, 5 y 10 años fueron del 98,4%, 96,8% y 96,8%, y del 94,9%, 82,6% y 58,4%, respectivamente. Se obtuvo una supervivencia del injerto significativamente mayor en los casos sin etiología glomerular de la nefropatía terminal, trasplante preferente y cuando se usó la aorta para la anastomosis vascular arterial. Entre los niños que experimentaban hipertensión pretrasplante, se sometieron a transfusión de sangre pretrasplante, experimentaron rechazo agudo postrasplante, rechazo crónico, hipertensión y obstrucción del injerto, se documentó una supervivencia del injerto significativamente más breve.
ConclusiónEl desenlace del trasplante renal de donante vivo en niños cuyo peso corporal es de 15–25kg en nuestro centro en un país en vías de desarrollo es comparable al descrito en otras publicaciones. Además de adoptar las pautas inmunosupresoras recientes basadas en tacrólimo, que se asocian con una disminución de la incidencia de rechazo agudo postrasplante y disfunción del injerto, pueden obtenerse mayores tasas de supervivencia del injerto adoptando una política de trasplante preferente, evitando las transfusiones de sangre pretrasplante, utilizando la aorta para la anastomosis vascular arterial.
Although there have been many recent advances in conservative renal replacement therapy, renal transplantation remains the best treatment option for children with end-stage renal disease.1
Transplantation results of several large pediatric studies reported from the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) registry,2 and United Network for Organ Sharing (UNOS) scientific registry were quite promising, with outcomes comparable to those of adult recipients.3 Moreover, children with well-functioning graft have a better quality of life, improved cognitive development and near normal growth in comparison with dialysis.4
However, despite advances in immunosuppressive regimes,5 surgical technique as well as pre- and post-operative managements,6 kidney transplantation remains a challenging procedure in small children with a higher risk of peri-operative complications and poorer outcome.7
The aim of this work was to evaluate the outcome of live-donor renal transplantation in children with end stage kidney disease who were weighing less than 25kg at time of transplantation and maintained on different immunosuppressive therapeutic modalities post-transplantation.
Patients and methodsPatientsThis study comprised all children weighing 25kg or less at time of renal transplantation, who received living donor renal allotransplant in Urology and Nephrology Center Mansoura University, Egypt, between December 1984 and March 2009.
MethodsA retrospective review of these patients was carried out and included.
Recipients’ dataClinical records of kidney transplant recipients were reviewed for demographic information, recipient age, sex, cause of end stage renal failure, virology status (hepatitis C antibody, hepatitis B surface antigen), prior blood transfusion, pre-transplant hypertension, pre-transplant dialysis therapy, and human leukocyte antigen matching.
Donors’ dataIncluding donor age, sex, consanguinity, and blood group.
Operative dataIncluding ischemia time, time to dieresis, number of renal arteries and site of anastomosis and type of urinary recontinuity.
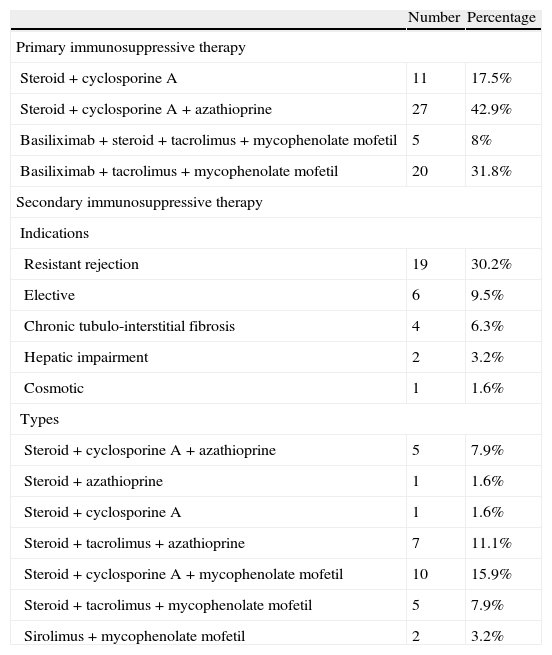
ImmunosuppressionPrimary immunosuppression regimens includedOlder regimens included either prednisolone and cyclosporine A maintenance regimen or the subsequent triple therapy regimen of prednisolone, cyclosporine A and azathioprine. More recent regimens, started since more than one decade and included either basiliximab induction and maintenance on prednisolone, tacrolimus, mycophenolate mofetil (MMF) or steroid avoidance protocol in which children were maintained on tacrolimus and MMF after basiliximab induction.
Secondary and tertiary immunosuppressionSecondary and tertiary immunosuppression protocols were defined respectively as the first and second modification of maintenance (primary) immunosuppressive protocol.
Postoperative morbidities and surgical complicationsIncluding, acute tubular necrosis, graft artery or vein thrombosis, renal artery stenosis, lymphocele, wound dehiscence and secondary urinary recontinuity. Hypertension was defined as either systolic and/or diastolic BP ≥95th percentile measured upon three or more occasions,8,9 diabetes mellitus, infections, hepatic impairment, gastrointestinal tract troubles, acute rejection episodes (type and number) and chronic rejection.
Laboratory parametersAssessment of the graft function was performed by measuring the serum creatinine, urine analysis and creatinine clearance using Schwartz's formula.10 Immunosuppressive drug level for cyclosporine A (CsA), tacrolimus and sirolimus, complete blood count, liver function tests, fasting and post prandial venous plasma sugar viral, profile for hepatitis C virus, hepatitis B virus, cytomegalovirus, and human immunodeficiency virus were performed.
Evaluation of graft biopsiesRenal allograft histopathological examination was carried out in cases of: nephrotic range proteinuria, episodes of renal dysfunction (25% increase in creatinine from base line) unexplained by pre-renal, post-renal causes or high calcineurin inhibitor trough level. Histologic examination was performed according to Banff Schema 1993 criteria.11
Statistical analysis of the dataQualitative data were displayed in cross tabulation and quantitative data were described in terms of arithmetic mean and standard deviation. Bivariate techniques were used for initial evaluation of contrast. Thus, the Chi-square and Fisher's exact test were used for comparison of frequencies of qualitative variables and the unpaired t-test was used for comparisons of means two quantitative variables. The endpoints were patient death or return to dialysis. Graft and Patient survival rates were evaluated by means of Kaplan–Meier survival curves. When evaluating graft survival, patients who lost their grafts were excluded at the time of graft loss. When evaluating patient survival, patients who died with functioning grafts were excluded at the date of death. Significant variables in the univariate analysis were further analyzed by the multivariate Logistic regression method. P value<0.05 was considered statistically significant. All analysis were carried out using the computer package SPSS for windows, release 16 SPSS inc., Chicago, IL, USA.
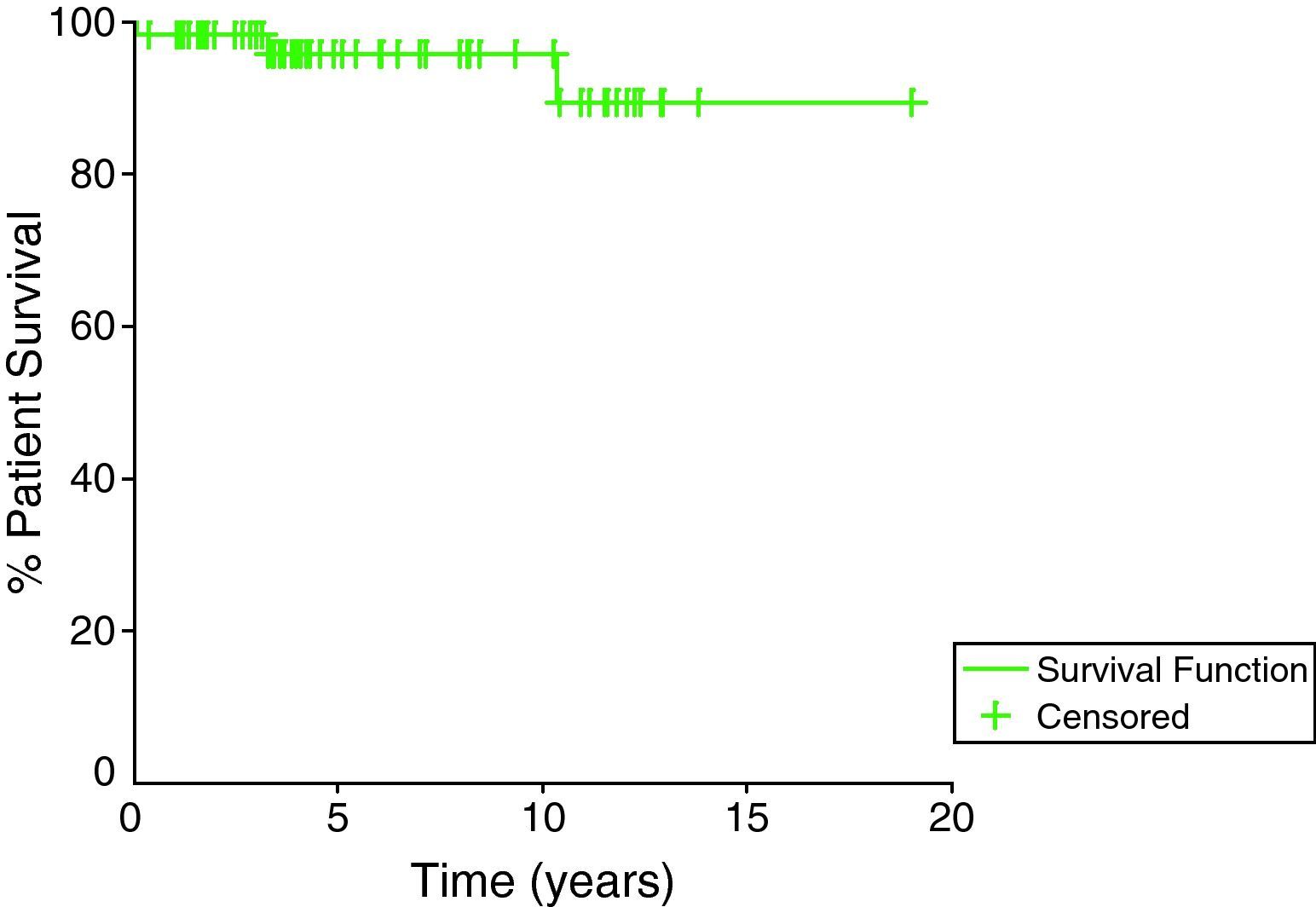
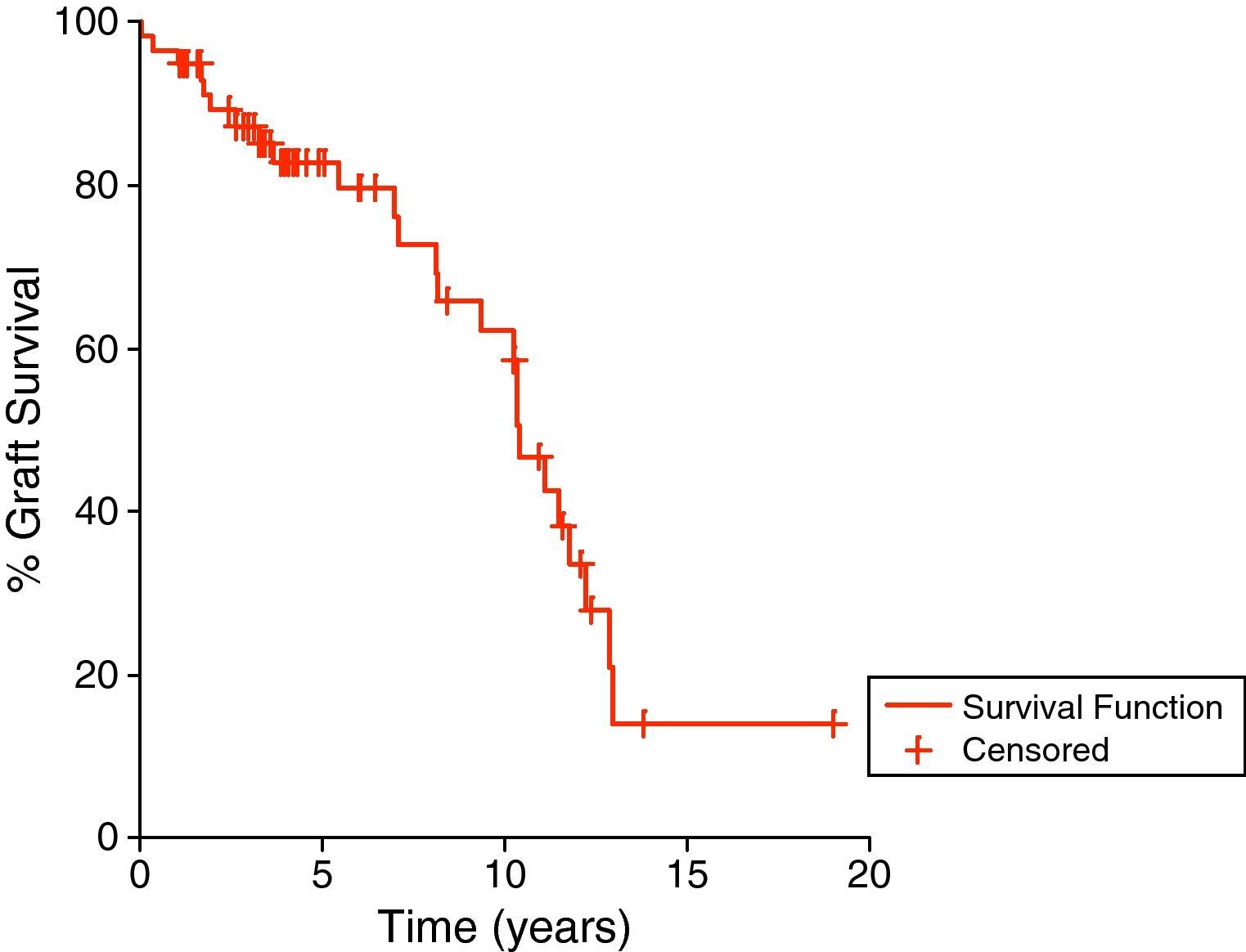
ResultsWe studied all children weighing 25kg or less at time of renal transplantation, the lower body weight limit was 15kg. Sixty-three cases were included in our study, for whom the mean follow up duration after transplantation was 74.2±52.8 months (range 1.1–231.5 months). The patient survival rates at 1, 5, and 10 years were 98.4%, 96.8%, and 96.8%, respectively (Fig. 1) while the graft survival rates were 94.9%, 82.6%, and 58.4% for the same time periods (Fig. 2).
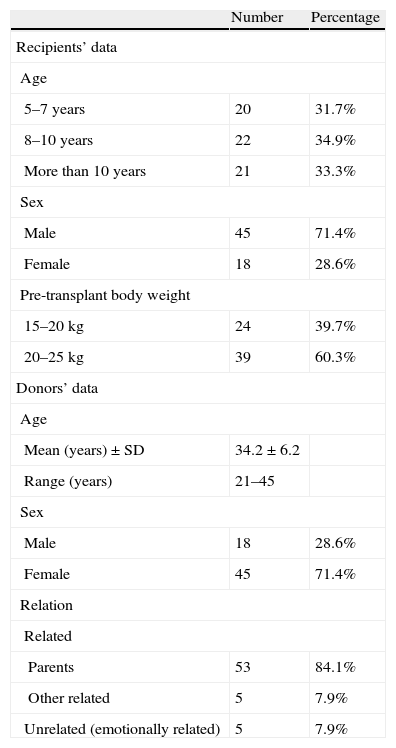
The mean age of our cases was 8.9±2.4 years, their mean body weight was 20.5±3kg, the majority of them were boys 71.4% and the majority were maintained on hemodialysis 71.4% (Table 1). On the other hand the mean age of the donors was 34.2±6.2 years; the majority of them were females 71.4%, parents 84.1% (Table 1) and had the same blood group of the recipient (71.4%).
Characteristics of kidney transplant recipients and donors.
| Number | Percentage | |
| Recipients’ data | ||
| Age | ||
| 5–7 years | 20 | 31.7% |
| 8–10 years | 22 | 34.9% |
| More than 10 years | 21 | 33.3% |
| Sex | ||
| Male | 45 | 71.4% |
| Female | 18 | 28.6% |
| Pre-transplant body weight | ||
| 15–20kg | 24 | 39.7% |
| 20–25kg | 39 | 60.3% |
| Donors’ data | ||
| Age | ||
| Mean (years)±SD | 34.2±6.2 | |
| Range (years) | 21–45 | |
| Sex | ||
| Male | 18 | 28.6% |
| Female | 45 | 71.4% |
| Relation | ||
| Related | ||
| Parents | 53 | 84.1% |
| Other related | 5 | 7.9% |
| Unrelated (emotionally related) | 5 | 7.9% |
The commonest identified cause of renal failure among our cases was reflux nephropathy (20.6%), while glomerular causes constitute 15.9%. Other causes included developmental disease (4.8%), hereditary nephritis (3.2%), cystic kidney disease (4.8%), oxalosis (3.2%), chronic pyelonephritis (14.3%), and other non-identifiable causes.
Regarding surgical aspects, the mean ischemia time was 57.3±16.1min, the majority of cases had immediate diuresis (63.5%), the majority of arterial anastomosis was to the common iliac artery (44.4%), and the venous anastomosis to the inferior vena cava (95.2%). The primary urinary recontinuities were mainly through Leich Grigoir uretero-vesical anastomosis (88.9%) and secondary urinary recontinuity was performed for 4 cases through uretero-ureteral anastomosis.
For immunosuppression, the commonest primary immunosuppressive therapy used was the triple therapy (steroid, cyclosporine, and azathioprine) (42.9%), the secondary immunosuppressives were needed for 50.8% of cases mainly due to resistant rejection (30.2%). The type of secondary immunosuppression was mainly based on tacrolimus, MMF or both (Table 2).
Immunosuppressive therapy.
| Number | Percentage | |
| Primary immunosuppressive therapy | ||
| Steroid+cyclosporine A | 11 | 17.5% |
| Steroid+cyclosporine A+azathioprine | 27 | 42.9% |
| Basiliximab+steroid+tacrolimus+mycophenolate mofetil | 5 | 8% |
| Basiliximab+tacrolimus+mycophenolate mofetil | 20 | 31.8% |
| Secondary immunosuppressive therapy | ||
| Indications | ||
| Resistant rejection | 19 | 30.2% |
| Elective | 6 | 9.5% |
| Chronic tubulo-interstitial fibrosis | 4 | 6.3% |
| Hepatic impairment | 2 | 3.2% |
| Cosmotic | 1 | 1.6% |
| Types | ||
| Steroid+cyclosporine A+azathioprine | 5 | 7.9% |
| Steroid+azathioprine | 1 | 1.6% |
| Steroid+cyclosporine A | 1 | 1.6% |
| Steroid+tacrolimus+azathioprine | 7 | 11.1% |
| Steroid+cyclosporine A+mycophenolate mofetil | 10 | 15.9% |
| Steroid+tacrolimus+mycophenolate mofetil | 5 | 7.9% |
| Sirolimus+mycophenolate mofetil | 2 | 3.2% |
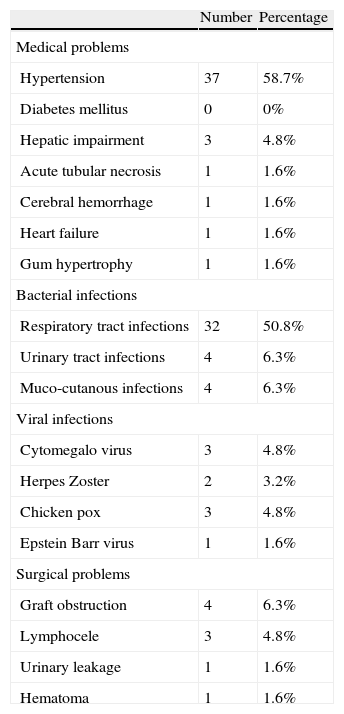
On reviewing the post-transplant complications, the commonest medical problems were bacterial infections (63.5%), and post-transplant hypertension (58.7%), while the commonest surgical problems were graft obstruction (6.3%) and Lymphocele (4.8%) (Table 3).
Post-transplant complications.
| Number | Percentage | |
| Medical problems | ||
| Hypertension | 37 | 58.7% |
| Diabetes mellitus | 0 | 0% |
| Hepatic impairment | 3 | 4.8% |
| Acute tubular necrosis | 1 | 1.6% |
| Cerebral hemorrhage | 1 | 1.6% |
| Heart failure | 1 | 1.6% |
| Gum hypertrophy | 1 | 1.6% |
| Bacterial infections | ||
| Respiratory tract infections | 32 | 50.8% |
| Urinary tract infections | 4 | 6.3% |
| Muco-cutanous infections | 4 | 6.3% |
| Viral infections | ||
| Cytomegalo virus | 3 | 4.8% |
| Herpes Zoster | 2 | 3.2% |
| Chicken pox | 3 | 4.8% |
| Epstein Barr virus | 1 | 1.6% |
| Surgical problems | ||
| Graft obstruction | 4 | 6.3% |
| Lymphocele | 3 | 4.8% |
| Urinary leakage | 1 | 1.6% |
| Hematoma | 1 | 1.6% |
By univariate analysis none of the demographic data were significant as a risk factor for graft failure. On the other hand, the glomerular etiology of end-stage kidney disease, pre-transplant blood transfusions, pre-transplant dialysis, and pre-transplant hypertension were found to have significant risk for graft failure (Table 4).
Impact of pre-transplant variables on graft survival.
| Functioning grafts no. (34) | Failed grafts no. (25) | P value | |
| Recipient age | 8.7±2.9 | 9±2.1 | 0.647 |
| Donor age | 34.2±3 | 34±2 | 0.975 |
| Recipient sex | |||
| Male | 27 | 15 | |
| Female | 7 | 10 | 0.104 |
| Donor sex | |||
| Male | 8 | 8 | |
| Female | 26 | 17 | 0.470 |
| Donor relationship | |||
| Related | 33 | 21 | |
| Unrelated (emotionally related) | 1 | 4 | 0.075 |
| Pre-transplant weight | |||
| 15–20kg | 14 | 9 | |
| 20–25kg | 20 | 16 | 0.687 |
| Original kidney disease | |||
| Glomerular | 1 | 7 | |
| Non glomerular | 33 | 18 | 0.005 |
| Blood group | |||
| Same | 23 | 18 | |
| Different compatible | 11 | 7 | 0.720 |
| Pre-transplant blood transfusion | |||
| Yes | 5 | 15 | |
| No | 29 | 10 | 0.001 |
| Pre-transplant dialysis | |||
| Yes | 26 | 24 | |
| No | 8 | 1 | 0.039 |
| Type of dialysis | |||
| Hemodialysis | 23 | 19 | |
| Peritoneal dialysis | 3 | 5 | 0.113 |
| Pre-transplant hypertension | |||
| Yes | 9 | 18 | |
| No | 25 | 7 | 0.001 |
| Recipient HCV infection | |||
| Yes | 23 | 2 | |
| No | 6 | 3 | 0.066 |
Analysis of post-transplant risk factors for graft failure revealed that incidence of acute rejection, chronic rejection, post-transplant hypertension and graft obstruction were highly significant by univariate analysis (Table 5).
Impact of post-transplant variables on graft survival.
| Functioning grafts no. (34) | Failed grafts no. (25) | P value | |
| Site of vascular anastomosis of main renal artery | |||
| Aorta | 17 | 1 | 0.002 |
| Common iliac artery | 15 | 11 | |
| Internal iliac artery | 2 | 13 | |
| ATN | |||
| Yes | 0 | 1 | |
| No | 34 | 24 | 0.240 |
| Acute rejection | |||
| Yes | 9 | 19 | |
| No | 25 | 6 | <0.001 |
| Type of acute rejection | |||
| Acute cellular rejection | 5 | 14 | |
| Acute vascular rejection | 4 | 5 | 0.337 |
| Chronic rejection | |||
| Yes | 5 | 19 | |
| No | 29 | 6 | <0.001 |
| Hypertension | |||
| Yes | 13 | 21 | |
| No | 21 | 4 | <0.001 |
| Bacterial infections | |||
| Yes | 20 | 18 | |
| No | 14 | 7 | 0.296 |
| Graft obstruction | |||
| Yes | 0 | 4 | |
| No | 34 | 21 | 0.016 |
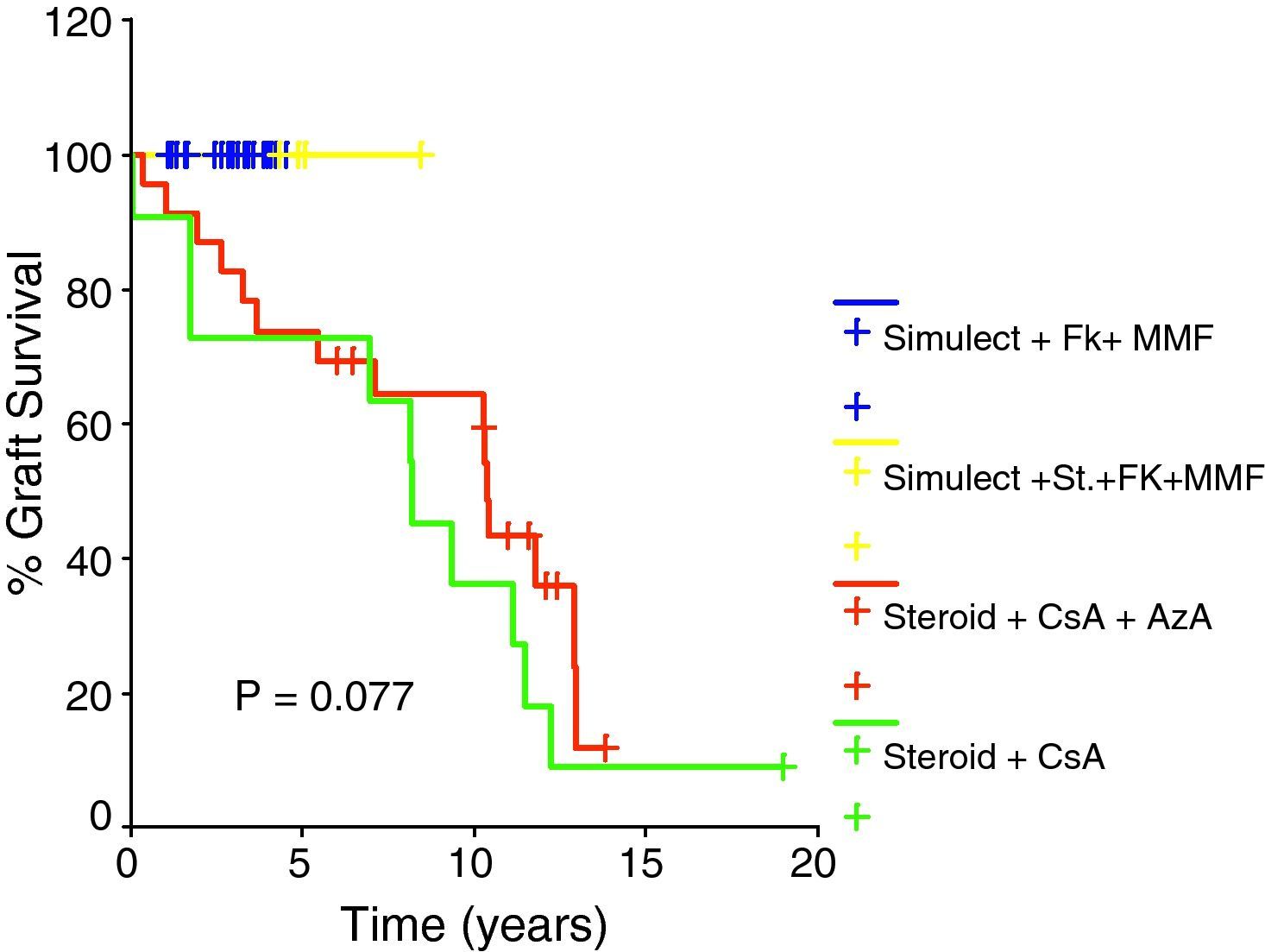
Table 6 shows that the type of primary immunosuppressive regimen has a significant impact on incidence of acute rejection being lower among tacrolimus and MMF based regimens. Subsequently, tacrolimus and MMF based primary immunosuppressive regimens were found to have a better graft outcome than cyclosporine based primary regimens; however, this did not rank to statistical significance as shown in Fig. 3.
Impact of primary immunosuppressive regimen on incidence of acute rejection episodes.
| Acute rejection | P value | ||
| No | Yes | ||
| Primary immunosuppressive regimen | |||
| Steroid+cyclosporine A | 5 | 6 | |
| Steroid+cyclosporine A+azathioprine | 9 | 18 | |
| Basiliximab+steroid+tacrolimus+mycophenolate mofetil | 4 | 1 | |
| Basiliximab+tacrolimus+mycophenolate mofetil | 16 | 4 | 0.008 |
Finally, Table 7 shows that by multivariate analysis only the incidence of acute rejection and chronic rejection were significant risk factors for graft survival.
DiscussionThe aim of this study was to present our center results of kidney transplantation in children weighing less than 25kg at time of transplantation throughout 25 year-period.
All studies show a survival advantage for pediatrics who receive transplants in comparison with those who undergo dialysis.12 In addition, all reports show a small but consistent benefit of living donation on mortality at all ages up to 5 years after transplantation.13–15 However, thereafter, there are few long-term data available, with one center showing a benefit14 and one not13 at 10 years after transplantation.
In our series, the overall patient cumulative survival rates were 98.4%, 96.8%, and 96.8% at 1, 5, and 10 years post transplantation respectively. These results were comparable if not better to the overall patient survival rates in other studies which vary between 70% and 100% at 5 years,13–23 75% and 95% at 10 years.13,14,18–23
The estimated graft survival for our pediatrics was 94.9%, 87.1, 82.6%, and 58.4% at 1, 3, 5, and 10 years, respectively. In comparison, the overall transplant survival varies between 44% and 95% at 5 years,13,14,18–23 23% and 95% at 10 years.13,14,18–23 Living related donation has been shown to benefit outcome, compared to deceased donation.14,24,25 In comparison with our adult transplants from the same center, the graft survival was 92%, 76%, and 51.9% at 1, 5, and 10 years, respectively.26
The mean recipient age at time of transplantation was 8.9±2.4 years (ranging from 5 to 13 years). It was not found to have any impact on graft survival. This comes in accordance with data from Scientific Registry of Transplant Recipients (SRTR) which documented that pediatric recipients younger than 11 years old who received living donor transplants had 5-year graft survival rates that were as good as, if not better than recipients in older age groups.27
In our study, glomerular causes of ESKD were associated with poorer graft outcome; this may be attributed to higher rate of recurrence post transplantation. This comes in accordance with a study based upon the NAPRTCS database which compared transplant outcomes of 752 FSGS pediatric patients with 5732 control patients. Graft loss from recurrent FSGS among children was found to be 6.1% at five years.28
The graft failure rates were found to increase by up to 40% for recipients with more than five blood transfusions before transplantation.27 In our study, we found that children with pre-transplant blood transfusion have significant inferior graft outcome.
In our study the graft survival of pre-emptive transplants was better than those transplanted while already commenced dialysis. Several large analyses have demonstrated that preemptive transplantation leads to substantial improvements in patient and graft survival when compared to transplantation after a period of dialysis therapy.28 The benefit of pre-emptive renal transplantation is likely a result of the avoidance of the cardiovascular consequences of long-term dialysis.
We found a significant impact of pre-transplant hypertension on graft survival. Cosio et al.,29 reported that patients with poorly controlled pre-transplant blood pressure had worse graft survival than patients with well-controlled pre-transplant blood pressure. This may be explained by the fact that hypertension in childhood contributes to premature atherosclerosis and the early development of cardiovascular diseases such as left ventricular hypertrophy, hypertensive cardiomyopathy and congestive heart failure.30
As regard the vascular anastomosis, the arterial anastomosis was to the common iliac artery (44.4%), aorta (30.2%), and Internal iliac artery (25.4%), while the venous anastomosis to the inferior vena cava was in 95.2% of cases. We noticed that the site of anastomosis of main renal artery has a significant impact on graft survival; this comes in accordance with Adams et al.,31 who found better outcome in renal transplantation in small children with the use of the aorta and the distal caval vein to perform vascular anastomoses.
In our series, in spite of the tendency toward improvement in graft function in patients treated with tacrolimus based immunosuppressive regimens in comparison to cyclosporine based regimens, the impact on graft outcome did not rank to statistical significance. This could be explained by shorter duration of follow up in recipients treated with tacrolimus.
Nearly half of our cases experienced acute rejection episodes (46%), and the majority of them had one rejection episode. This was slightly lower than that of El-Husseini et al.,32 who reported acute rejection episodes in 52.4% of his series. This may be explained by recent administration of tacrolimus based immunosuppressive regimens among all recent transplanted children who showed significant lower acute rejection episodes. We found that the incidence of acute rejection was associated with lower graft survival, which was statistically significant by both univariate and multivariate analyses. Many studies8,33 have previously demonstrated that acute rejection is associated with poor outcome.
Chronic rejection is one of the common causes of graft loss among transplant recipients, including children. Forty-two percent of our cases suffered from chronic rejection. It was found to have a significant impact on graft survival by both univariate and multivariate analysis. Other studies like Joosten et al.,34 and Chapman et al.,35 had demonstrated that chronic rejection was one of the leading causes of graft failure.
In summary, renal transplantation is the best treatment option for end-stage renal disease in children and should be considered in every child when renal replacement therapy is indicated. Pre-emptive transplantation can avoid long-term dialysis complications and was found to be associated with superior graft outcome. Every effort should be made to avoid pre-transplant blood transfusion and post-transplant complications particularly acute rejection which was proved to be significantly reduced by primary tacrolimus-MMF based immunosuppressive regimens and induction therapy.
Conflicts of interestThe authors declare no conflicts of interest.