Introduction
In the U.S., approximately 25% of hemodialysis patients use catheters for hemodialysis, up from 13% over ten years ago1,2. As many as one in five new dialysis patients start their treatment with tunneled cuffed catheters.3 Dialysis catheters are used either as temporary solutions or as a bridging device while patients wait for fistula or graft maturation or kidney transplantation, or as the sole chronic access method. Complications associated with dialysis access, and especially with dialysis catheters, are on the rise as patients diagnosed with end stage renal disease (ESRD) are increasing both in age and in co-morbidities.
Hemodialysis patients currently have high rates of morbidity and mortality. Chronic tunneled hemodialysis catheters are a contributing factor to poor clinical outcomes. The main complications associated with hemodialysis catheters include poor function due to thrombosis and fibrin sheath, central vein stenosis and occlusion, and infection.4,5 Consequently, newer catheter designs that aim to address these issues have been developed with innovations over existing products.
Thrombosis and fibrin sheath can lead to inadequate hemodialysis by disrupting flow primarily by limiting inflow through the arterial lumen. Catheter exchange or fibrin sheath stripping can improve flow, but also introduce further risk for future complications.6 A catheter's anti-thrombogenic properties might be important for longevity, considering that thrombus and fibrin sheath formation begin as early as 24 hours after insertion.7 The continuing development of catheter biomaterials, coatings, and tip designs reflects the need for better durability, as the current median dwelling time is little more than two months. The majority of unplanned catheter removals are due to infection and poor function.8 Poor function is generally related to flow problems that account for over half of these removals, with 76% of cases exhibiting fibrin sheath formation.8,9
In addition to causing flow problems, catheter thrombosis and fibrin sheath formation have also been implicated in the high rates of catheter-related infection seen in dialysis patients. Infections can be introduced into catheters by various methods such as extraluminally via skin or intraluminally via the catheter hub. Bacteria can move through the skin insertion site along the dermal tunnel and reach the tip of the catheter.10 Fibrin sheath can exacerbate bacterial colonization by providing a habitable environment. In dialysis patients who are already immunocompromised, bacteria can multiply and prosper within the fibronectin coating, rendering them inaccessible to immune cells.11-15 Bacteria can also adhere to the catheter material itself, forming a protective glycocalix biofilm.14,15 The combination of breaking the skin barrier to insert a catheter, exposure to contaminants, and formation of pathogen-trapping fibrin sheaths can cause appreciable bacteremia risk, translating to up to three-fold relative mortality risk.2,16 New catheter design and coatings are often emphasized for their effects on reducing fibrin sheath formation and infection rates, which can ultimately lead to decreased morbidity and cost of care.
Maintaining patients on hemodialysis catheters for the long term is problematic from patient comfort as well as healthcare cost perspectives. NKF-K/DOQI guidelines recommend that less than 10% of chronic renal failure patients be maintained on dialysis catheters, due to the high rates of complications.17 In terms of healthcare cost, the U.S. spends $1-1.5 billion annually on maintaining patients who use hemodialysis catheters.18 However, only 10% of that is the actual cost of the dialysis catheters themselves. A large portion of this cost goes toward the hospitalization and procedural costs necessary to manage post-placement complications and catheter exchanges. Therefore, selecting a dialysis catheter that minimizes complications and excessive procedures can yield patient benefits as well as significant cost savings to the healthcare system.
Catheter design
According to NKF-K/DOQI guidelines, long-term tunneled cuffed catheters should be inserted when anticipated use is three weeks or longer.17 These long-term catheters are designed to be soft so that endovascular trauma can be minimized.19 A rigid shaft and tapered tip, which make the acute hemodialysis catheter easy to insert, also renders it unsuitable for long term use. If left for a long time within the superior vena cava or right atrium, the rigid, sharp material could cause significant tissue injury and subsequent thrombosis and vascular stenosis.20
The majority of modern chronic tunneled hemodialysis catheters are made from polyurethane, which provides an initial stiffness upon insertion, but then softens when exposed to body temperature. Carbothane is a polyurethane/polycarbonate copolymer that affords strength for longevity and softness for flexibility and patient comfort. With slightly greater strength than polyurethane, it can afford to have thinner walls.
Several lumen designs have emerged over the years. The earliest tunneled cuffed catheters were large, oval shapes with two separate lumens. Subsequent improvements have included a round lumen with a central wall, two separate single lumen catheters for differential placement of the inflow and outflow catheter, and fusion of the two single lumens at a distal point along their length for easier insertion.21
Commonly used long-term hemodialysis catheters have a staggered tip design, meaning that the outflow tip extends several centimeters (typically a minimum of 2.5 cm) beyond the inflow tip, to prevent recirculation. Another common design is the split tip or dual catheter design. Two key prospective randomized studies have demonstrated that while double lumen and two single lumen catheters do not lead to appreciable differences in survival, flow rate, or infection rate, double lumen catheters are preferred due to ease of use.21,22 Despite the evolution of tip design, there are few studies on the influence of tip geometry on patient outcomes. Multiple side holes on many catheters reflect the belief that these side holes are necessary to preserve function in case of obstruction. However, side holes can also cause thrombosis due the irregularity of their cut surfaces. In a recent comparison of two similar chronic dialysis catheters with and without side holes, reduced bacteremia rate was demonstrated in the non-side hole catheters. The authors attribute this result to reduced thrombus formation at the catheter tip.23 There is also evidence that side holes can prevent locking solution from reaching the area between the side hole and catheter tip, precipitating clot formation at the tip.24,25 Clots may become firmly anchored to the walls around side holes, presenting a diffcult to manage situation.25
A common drawback of many of the current catheter designs is high levels of recirculation upon lumen reversal, leading to subsequent flow failure. Reversal of the lumens in long-term dialysis catheters is usually performed to correct inadequate inflow, where the inflow through the arterial lumen is inadequate.26 However, reversal of flow also leads to the undesirable effect of recirculation, whereby dialyzed blood exiting from one lumen directly enters the other lumen, bypassing systemic circulation. Recirculation of blood during dialysis reduces treatment effciency and can lead to adverse health outcomes.27,28
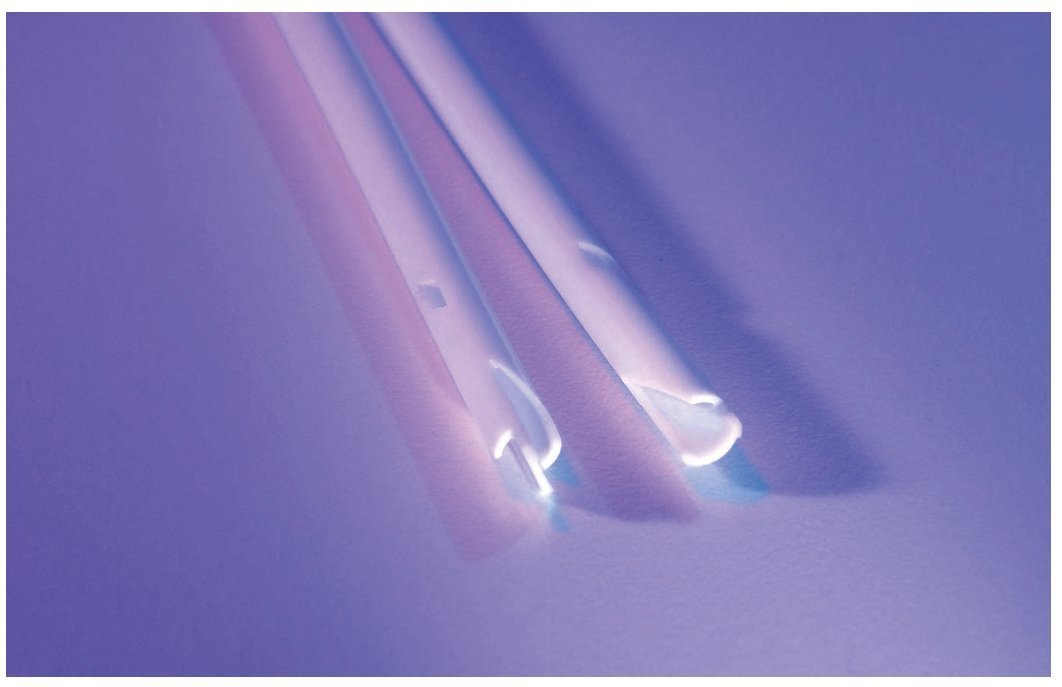
A more recent catheter designed to address the problem of recirculation is the Tal PalindromeTM catheter (fig. 1). In this design, the arterial and venous tracts have the same length. While inflow occurs through the side slot and the most proximal portion of the end hole, outflow occurs as a jet directed away from the catheter tip. This design was found to prevent recirculation in a swine model.29 A recent study comparing two groups of catheters inserted in 200 patients demonstrated improved patency and reduced re-interventions with the Palindrome design compared to the split tip design.30 Catheter patency was significantly higher in the Palindrome. Primary-assisted patency was significantly reduced with the split tip (71% and 61% at 90 and 180 days, respectively) compared with the Palindrome (94% at 90 and 180 days, P<.0001).
Figure 1. Tip of the Tal palindrome catheter demonstrates a symmetric tip with a Z shape design. Notice the side slots. (Image provided by Covidien).
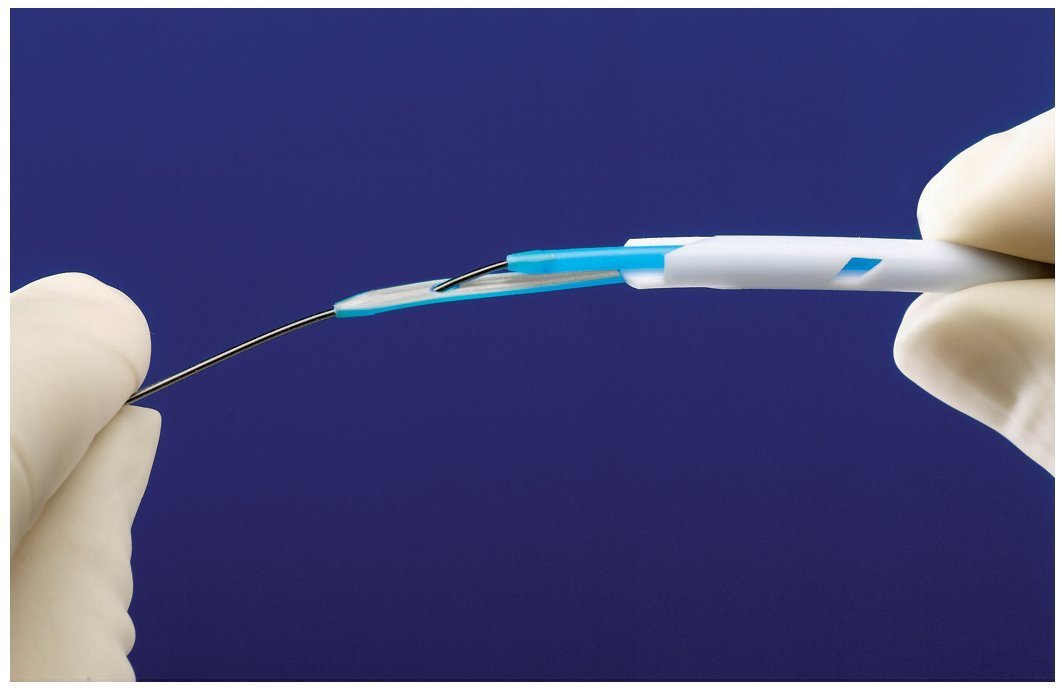
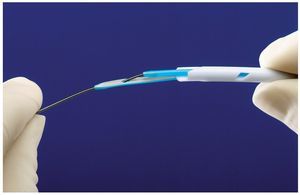
Insertion of the Tal Palindrome catheter is performed either by utilizing the provided valved peel away sheath or over-the-wire utilizing the VenaTracTM device (fig. 2). The VenaTrac device is composed of two D shaped stylets that effectively occlude both lumens of the dialysis catheter, thus preventing air embolism or bleeding during over the wire catheter insertion or exchange. One stylet of the VenaTrac is longer than the other. The wire is passed through the tip of the longer stylet and into the shorter one. This allows catheter insertion or exchange over a single wire by providing transition to the catheter tip.
Figure 2. Image of the VenaTrac device. The device allows over the wire placement and exchange of the catheter. The VenaTrac consists of two D shaped, blue stylets. The wire is passed through the tip of the long stylet and into the short stylet and out of the back end of the catheter. (Image provided by Covidien).
Catheter coatings
Heparin-coated catheters present a way to decrease infection rate without the risks of systemic antibiotic exposure or bacterial resistance. Heparin exhibits anticoagulant activity via interaction with the plasma protein antithrombin as well as some electrostatic repulsion of charged platelets. Therefore, it can reduce bacterial trapping within fibrin clots and sheaths.15 Hydrophobic and electrostatic interactions also decrease direct bacterial adhesion onto catheter polymer.
Covidien offers the Tal Palindrome Emerald hemodialysis catheter, which incorporates a non-eluting heparin coating technology. Through in vitro and in vivo testing, this coating has been shown to reduce platelet adhesion and thrombus accumulation on the catheter surface. The coating design adopts a multifaceted approach to hemocompatability, by containing functional groups that have demonstrated performance in hydrophilicity, minimizing platelet adhesion and enhancing non-thrombogenicity and anti-thrombogenicity.31
The use of other coating materials such as silver and antibiotics has also been advocated. While results were promising for acute use, studies on chronic hemodialysis catheters have yielded inconclusive results. However, data from a more recent large-scale prospective study reveal that silver coating can have an anti-microbial benefit: in long-term coated catheters, bacterial colonization was observed on 11% of catheter tips, versus 44% for uncoated catheters.32 The key difficulty with coating chronic hemodialysis catheters is that the bonded substance can disappear over time, rendering them ineffective over long periods.33
Covidien also offers the Tal Palindrome Ruby® which incorporates an anti-microbial silver ion sleeve bonded between the hub and the cuff. This sleeve delivers silver ions to the surface of the catheter to reduce microbial colonization on the catheter surface within the tunnel track. In vitro testing with clinical isolates of Staphylococcus aureus, Coagulase-negative Staphylococcus, Candida albicans, and Escherichia coli has shown a 99.2%-99.999% (2.1-5.5log10) reduction in microbial colonization.31
Covidien is also offering a chronic dialysis catheter that provides both anti-thrombogenic and anti-microbial technologies on one catheter, the Tal Palindrome Sapphire®. The catheter combines heparin coating technology with an anti-microbial silver ion subcutaneous sleeve.34
Conclusion
The increasingly important role of central venous catheters in delivering long-term hemodialysis has lead to the development of many generations of dialysis catheters. These devices carry risks of many serious complications, but they are also a necessary tool for managing renal failure patients. The physician has many choices in catheter design. The decision of what catheter to place is made by the inserting physician and can include factors such as ease of insertion, user preference, relationship to vendor and cost of catheter. However, the most important feature to consider is the long term function of the catheter and complication rates. Every small improvement in the complication or re-intervention rate will have a profound impact on individual patient care and cost to society of dialysis catheter management.
Nonetheless, the answer to catheter coating and tip design is not definitive, and future randomized controlled human clinical trials are necessary to substantiate the clinical benefits, if any, of new catheter designs and coatings.
Conflict of interests
Dr. Michael Tal is a Consultant for Covidien Inc.
* Autor para correspondencia.
Correo electrónico:michael.tal@yale.edu (M. Tal).
INFORMACIÓN DEL ARTÍCULO
Historia del artículo:
Recibido el 20 de noviembre de 2009
Aceptado el 30 de noviembre de 2009