To introduce more chemistry into a middle and high school bioengineering camp experience, we developed an educational and entertaining presentation that examines the chemistry in movies about aliens and minerals from outer space. Our goal was to help the campers to think creatively about the bioengineering projects they are doing and about its chemistry. After watching each movie clip, we explain whether the chemistry in the clip is real or fake, and then describe the real chemistry that inspired it. The chemical touchstone for the presentation is the periodic table. First, the campers learn that aliens in five movies are composed of the same elements as those found on Earth, although some do not have the same biochemistry. The second half of the talk is about the utility of extraterrestrial minerals of known composition. The campers learn that moviemakers speculate that people of the future might visit other celestial bodies to collect scarce minerals with known properties. The topics of alien biochemistry and extraterrestrial minerals are not often taught in the classroom. The pairing works well, however, for chemical outreach because it shows students how to bring divergent thoughts together to solve problems and, therefore, encourages creative chemical thinking.
Para introducir más química en una experiencia de campamento de bioingeniería para estudiantes de escuela intermedia y secundaria, hemos desarrollado una presentación educativa y entretenida que examina la química en las películas sobre extraterrestres y minerales del espacio exterior. Nuestro objetivo era ayudar a los campistas a pensar creativamente acerca de los proyectos de bioingeniería que están haciendo y sobre su química. Después de ver cada cinta de video explicamos si la química presentada en el clip es real o falsa y luego se describe la verdadera química que la inspiró. El punto de referencia de la química para la presentación es la tabla periódica. En primer lugar, los campistas aprenden que los extraterrestres en cinco películas se componen de los mismos elementos a los encontrados en la Tierra, aunque algunos no tienen la misma bioquímica. La segunda mitad de la charla es sobre la utilidad de los minerales extraterrestres de composición conocida. Los campistas aprenden que los cineastas especulan que la gente del futuro podría visitar otros cuerpos celestes para recoger minerales escasos con propiedades conocidas. Los temas de la bioquímica alienígena y minerales extraterrestres no se enseñan a menudo en el salón de clases. Sin embargo, la utilización conjunta funciona bien, para la divulgación de la química, ya que muestra a los estudiantes cómo se pueden usar pensamientos divergentes juntos para resolver problemas, estimulando de esta manera el pensamiento creativo en química.
Outreach to the public is critical to the success of chemistry departments. It can increase the public's understanding of important issues and number of students who become chemistry majors, while raising the profile of chemistry faculty within the community. The mechanisms for achieving these informal chemical experiences include public demonstrations, classroom visits, summer science camps, topical research workshops, and topical research lectures. All of these are close encounters with chemistry and each balances entertainment with education in a different way.
Most outreach opportunities involve “magic of chemistry” demonstrations, because the public enjoys watching them almost as much as the faculty and their assistants enjoy doing them (Flynn, 2005; Harpp, Fenster, & Schwarcz, 2011; O’Brien, 1991). The object is to educate while entertaining, with the scales tipped toward the latter. The audience gets to watch colors change rapidly, smell the smoke of inefficient combustion, and feel the sonic boom of explosions. They watch an expert or assistant initiate each demonstration deliberately and, hopefully, safely while explaining what is happening on the theoretical level. The take-home message for everyone is that there is more than meets the eye to these spectacular and reproducible phenomena and, with more studying, you can explain these things to other people.
Science camps and the workshops are focused on education far more than they are on entertainment (Exstrom & Mosher, 2000; Flynn, Johnson, & Penn, 2007; Robbins & Schoenfisch, 2005; Sheridan, Szczepankiewicz, Mekelburg, & Schwabel, 2011). Their typical goal is to increase participant's interest in taking more chemistry courses in high school and college, both of which should raise the number of chemistry majors. Campers get a deeper scientific experience because they carry out more preparations, perform more separations, and use more sensitive instruments of analysis. The complexity of these activities makes it necessary to target a particular audience to ensure sufficient enrollment, and to recruit and train assistants to optimize the experience.
To introduce more chemistry into the camper learning experience, we developed a presentation using movie clips and explanations that is both fun and educational. Our goal was to help the campers integrate the things they were learning at the camp with the chemistry they had seen in everyday life, namely in the movies. We have previously reported that an effective way to arouse interest in learning chemistry was to show a movie clip and then explain the chemistry presented therein (Griep & Mikasen, 2013; Griep, Frey, & Mikasen, 2012). Here, we report the results of a talk based on alien biochemistry and extraterrestrial minerals in the movies that was presented in the evening to summer science camps focused on molecular biology.
MethodologyBefore this informal chemistry project could be launched, three aspects had to be planned. The first was to choose the target audience. The second aspect was to design a presentation especially for the target audience. The third part was to devise a tool to assess the audience's response to the presentation.
Choosing the target audienceBanks et al. (2007) coined the phrase “Life-long, Life-wide, and Life-deep” to encapsulate the notion that most learning takes place throughout our lives, in formal and informal environments, and in ways that are acceptable to our local community (i.e. connected to religious, moral, ethical, and social values). These “life-learning” ideas arise from the realization that a small percentage of people's lives are spent in structured, formal learning environments (18.5 percent for Grades 1–12; 9.7 percent for undergraduates; 5.1 percent for graduates; and occasionally as adults). Since learners learn by asking questions, science learning will happen more often if their informal environments are science-rich. Furthermore, Banks and colleagues noted the majority culture is well served with currently available materials but that there is a need for an equitable amount of materials for diverse audiences. Since movies have an appeal that crosses socioeconomic and geopolitical boundaries, we decided to create a presentation about chemistry in the movies for students who are underrepresented in chemistry.
The University of Nebraska-Lincoln hosts two different science camps that target students of interest. The Young Nebraska Scientists camp is hosted by the Nebraska EPSCoR (Experimental Program to Stimulate Competitive Research) program and targets middle and high school students from rural areas, low-income urban areas, and favors students who are returning for a second year. The Nebraska College Preparatory camp is linked to an academic program at select high schools with high enrollments of Hispanic and African-American students. Separate camps for rising 10th graders and rising 11th graders take place over three days and two nights in June or July, during which time the campers attend lectures, learn methods, and analyze data.
When we explained to the camp director that our presentation was designed to help campers to think more chemically, she arranged for us to give talks during the special enrichment seminar that takes place in the evening. We have now given presentations over three years to almost twenty summer camps and hundreds of campers. In the first year, all camps experienced our talk, “Everything I Know About Chemistry, I Learned at the Movies.” The majority of clips in that talk were chosen because they were already being used in Griep's introductory chemistry course for non-majors. The student responses to that talk have been published (Griep & Mikasen, 2013; Griep et al., 2012). Since some campers return the following summer, we developed a second talk titled “Alien Biochemistry and Extraterrestrial Minerals in the Movies” (Fig. 1). Both these talks were used during the second and third years of our participation in the summer camps. With the creation of the Alien Biochemistry talk, we sought to connect it more directly to the things they were learning in their day camp experiences.
Graphic for the presentation titled “Alien Biochemistry and Extraterrestrial Minerals in the Movies”. The elements mentioned in the presentation are in movie theater seats arranged in the form of the periodic table. The celestial bodies on the screen were inspired by those in Duck Dodgers in the 24½ Century (1953), one of the movies used in the presentation.
Youth participating in both camps learn how to create transgenic fluorescent microbes. An effective way to transform observations into knowledge that learners can use in new contexts is to anchor it to things they already know (Hofstein & Kempa, 1985; Rivet & Krajcik, 2008; Sherwood, Kinzer, Bransford, & Franks, 1987). Anchoring is necessary because the cognitive load perspective postulates that working memory is limited, making it difficult for learners to process too many ideas at once (Carlson, Chandler, & Sweller, 2003). For example, a recent study demonstrated that a “relevance” intervention was successful in improving interest and grades in chemistry for students with low expectations about their own ability to do well in chemistry (Hulleman & Harackiewicz, 2009). Since our campers were amplifying genes from plasmids, inserting them into vectors, and then expressing the cloned gene inside a bacterium, we reasoned that a presentation about the elements of life would connect very well. Our familiarity with movies about chemistry suggested we would be able to find this material in science fiction movies.
Many of the most-watched movies ever made have a science fiction theme. According to BoxOffice Mojo (www.boxofficemojo.com), Avatar (2009) is the highest grossing film of all time. In fact, about one-fifth of the Top 100 Grossing Films are in the science fiction category. The genre lends itself well to stories about the individual vs. nature that are told within the context of fantastic environments and novel experiences.
The first step in creating a presentation about the chemistry is to assemble a tentative list and then to group the entries into categories. Since the Internet Movie Database (IMDB; www.imdb.com) is the largest movie database, we began searching there first. It was not especially fruitful because IMDB does not have a specific tagging system. Their keywords are created by individual reviewers based on their own interests, and it would appear that the reviewers are not especially interested in chemistry. Instead, our list was created over a period of years. The most productive sources were two science fiction movie encyclopedias and four books about the Saturday morning serials that were popular from the 1930s to the early 1950s (Barbour, 1970; Hardy, 1986; Kinnard, 1998; Rainey, 1999; Warren, 1982, 1986; Weiss & Goodgold, 1972). Supplementing those were more specialized books that described some movies in enough detail to reveal possible chemical scenes (Galbraith, 1994; Glassy, 2001; Noonan, 2005; Sontag, 1974). Some discoveries were made while reading books about two successful science fiction writers, H.G. Wells and Michael Crichton, whose works have inspired numerous screenplays (Smith, 2002; Trembley, 1996). A colleague alerted us regarding The Andromeda Strain (1971), which was released many years ago, and we found it to be such an excellent example of chemistry in the movies that we described it in our book ReAction! Chemistry in the Movies (Griep & Mikasen, 2009). Finally, the most recent movies on these lists were added because we watched them when they were released or because of a tip from another chemist/movie enthusiast.
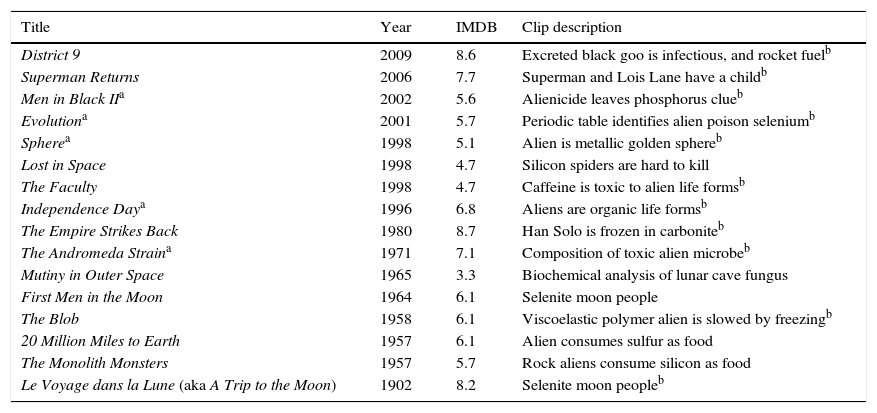
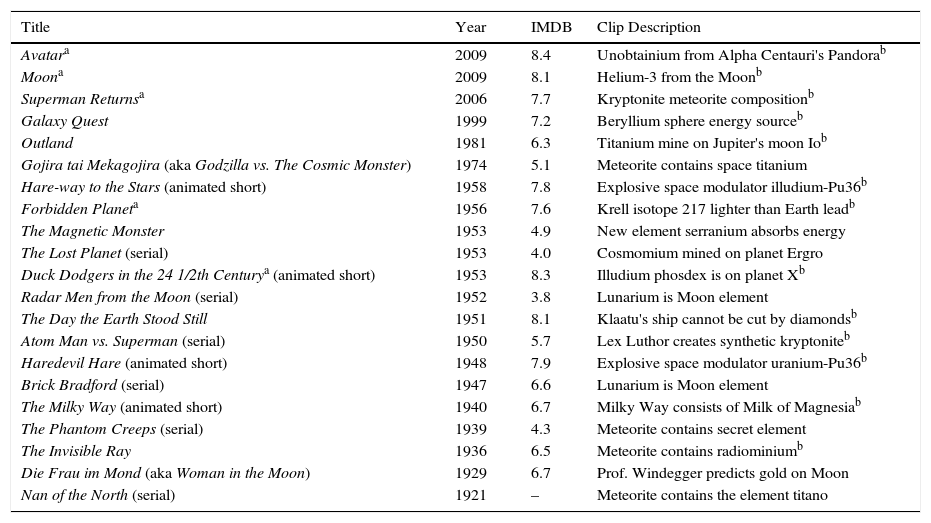
After identifying movies that might have chemical scenes, it was possible to separate the films into three categories: alien biochemistry, extraterrestrial minerals, and aliens are short on fuel (Tables 1–3). There are 14 movies in which alien biochemistry is described in some way and two in which the biochemistry is strongly implied (Table 1). In District 9 (2009), the aliens can eat Earth food and their excreted goo is able to infect a human. In Superman Returns (2006), the alien being Kal-El (aka Clark Kent, aka Superman) is able to have a child with human Lois Lane. These movies, and probably many others, imply that human genetics are very similar to genetics of the aliens, which in turn implies they have virtually the same biochemistry. We decided to focus on movies that mention elements so that we could incorporate the periodic table, the central organizing principle of chemistry, into our informal science education presentation.
Alien biochemistry movies.
| Title | Year | IMDB | Clip description |
|---|---|---|---|
| District 9 | 2009 | 8.6 | Excreted black goo is infectious, and rocket fuelb |
| Superman Returns | 2006 | 7.7 | Superman and Lois Lane have a childb |
| Men in Black IIa | 2002 | 5.6 | Alienicide leaves phosphorus clueb |
| Evolutiona | 2001 | 5.7 | Periodic table identifies alien poison seleniumb |
| Spherea | 1998 | 5.1 | Alien is metallic golden sphereb |
| Lost in Space | 1998 | 4.7 | Silicon spiders are hard to kill |
| The Faculty | 1998 | 4.7 | Caffeine is toxic to alien life formsb |
| Independence Daya | 1996 | 6.8 | Aliens are organic life formsb |
| The Empire Strikes Back | 1980 | 8.7 | Han Solo is frozen in carboniteb |
| The Andromeda Straina | 1971 | 7.1 | Composition of toxic alien microbeb |
| Mutiny in Outer Space | 1965 | 3.3 | Biochemical analysis of lunar cave fungus |
| First Men in the Moon | 1964 | 6.1 | Selenite moon people |
| The Blob | 1958 | 6.1 | Viscoelastic polymer alien is slowed by freezingb |
| 20 Million Miles to Earth | 1957 | 6.1 | Alien consumes sulfur as food |
| The Monolith Monsters | 1957 | 5.7 | Rock aliens consume silicon as food |
| Le Voyage dans la Lune (aka A Trip to the Moon) | 1902 | 8.2 | Selenite moon peopleb |
IMDB is the average viewer rating from 1 to 10 given on the Internet Movie Database website (www.imdb.com).
Movies used in the biochemistry portion of the presentation “Alien Biochemistry and Extraterrestrial Minerals in the Movies”. Movies that assume or imply biochemical or genetic similarity between humans and aliens, such as Prometheus (2012), Alien (1979), The Mysterians (1957), and others, were not included in this list because they did not mention anything chemical about alien composition.
Extraterrestrial mineral movies.
| Title | Year | IMDB | Clip Description |
|---|---|---|---|
| Avatara | 2009 | 8.4 | Unobtainium from Alpha Centauri's Pandorab |
| Moona | 2009 | 8.1 | Helium-3 from the Moonb |
| Superman Returnsa | 2006 | 7.7 | Kryptonite meteorite compositionb |
| Galaxy Quest | 1999 | 7.2 | Beryllium sphere energy sourceb |
| Outland | 1981 | 6.3 | Titanium mine on Jupiter's moon Iob |
| Gojira tai Mekagojira (aka Godzilla vs. The Cosmic Monster) | 1974 | 5.1 | Meteorite contains space titanium |
| Hare-way to the Stars (animated short) | 1958 | 7.8 | Explosive space modulator illudium-Pu36b |
| Forbidden Planeta | 1956 | 7.6 | Krell isotope 217 lighter than Earth leadb |
| The Magnetic Monster | 1953 | 4.9 | New element serranium absorbs energy |
| The Lost Planet (serial) | 1953 | 4.0 | Cosmomium mined on planet Ergro |
| Duck Dodgers in the 24 1/2th Centurya (animated short) | 1953 | 8.3 | Illudium phosdex is on planet Xb |
| Radar Men from the Moon (serial) | 1952 | 3.8 | Lunarium is Moon element |
| The Day the Earth Stood Still | 1951 | 8.1 | Klaatu's ship cannot be cut by diamondsb |
| Atom Man vs. Superman (serial) | 1950 | 5.7 | Lex Luthor creates synthetic kryptoniteb |
| Haredevil Hare (animated short) | 1948 | 7.9 | Explosive space modulator uranium-Pu36b |
| Brick Bradford (serial) | 1947 | 6.6 | Lunarium is Moon element |
| The Milky Way (animated short) | 1940 | 6.7 | Milky Way consists of Milk of Magnesiab |
| The Phantom Creeps (serial) | 1939 | 4.3 | Meteorite contains secret element |
| The Invisible Ray | 1936 | 6.5 | Meteorite contains radiominiumb |
| Die Frau im Mond (aka Woman in the Moon) | 1929 | 6.7 | Prof. Windegger predicts gold on Moon |
| Nan of the North (serial) | 1921 | – | Meteorite contains the element titano |
IMDB is the average viewer rating from 1 to 10 given on the Internet Movie Database website (www.imdb.com).
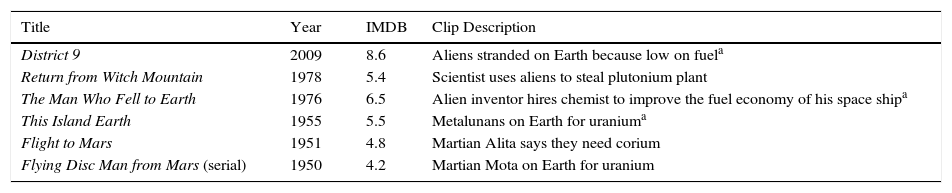
Aliens are short on fuel.
| Title | Year | IMDB | Clip Description |
|---|---|---|---|
| District 9 | 2009 | 8.6 | Aliens stranded on Earth because low on fuela |
| Return from Witch Mountain | 1978 | 5.4 | Scientist uses aliens to steal plutonium plant |
| The Man Who Fell to Earth | 1976 | 6.5 | Alien inventor hires chemist to improve the fuel economy of his space shipa |
| This Island Earth | 1955 | 5.5 | Metalunans on Earth for uraniuma |
| Flight to Mars | 1951 | 4.8 | Martian Alita says they need corium |
| Flying Disc Man from Mars (serial) | 1950 | 4.2 | Martian Mota on Earth for uranium |
IMDB is the average viewer rating from 1 to 10 given on the Internet Movie Database website (www.imdb.com).
The extraterrestrial minerals mentioned in some movies are composed of the same elements as found on Earth, such as helium, beryllium, and titanium (Table 2). A related theme is that aliens ran out of their own fuel and have come to Earth for our uranium (Table 3). This implies that the universe is composed of the same building blocks. On the other hand, most of the minerals in Table 2 and one of them in Table 3 are fictional elements, often with unusual properties. Examples are that unobtainium is a room-temperature superconductor, serranium converts energy into matter and grows, and lunarium is a special element found only on our Moon. In fact, lunarium found its way into two Saturday morning serials.
Feature films (and theatrically released cartoons) were created to entertain rather than educate so that many of them get very creative when including chemistry. We feel it is important to address all examples of chemistry in the movies to let our audience know what is real and what is fake. This is especially important for audiences with limited prior exposure to chemistry in the classroom because they do not know how to distinguish whether it is real or fake. In our book ReAction! Chemistry in the Movies (2010), we note that the narrative for most chemistry shown in movies is described correctly and is based on real chemistry. But, we also describe some movies with fictional molecules that can be related to real chemistry. In our presentations, it does not matter whether the movie clip shows real or fake chemistry, as long as it has a high Wow! to provoke the audience to want to know whether it is real or fake. In our presentations, we satisfy this thirst for knowledge through a mini-lesson following each clip. Our hidden agenda is to cause our audience members to ponder on the chemistry they observe in the next movie or television show they watch.
There are several reasons for the popularity of fictional elements in books, comics, and science fiction stories. One example is from the comic book Uncle Scrooge No. 17 (1957) in which Walt Disney's miserly old duck handles bombastium, the “rarest element known to man” that “nobody knows what it is good for (Carter, 1988).” In the 1980s, the writer of that comic explained that he was making a joke about all of the long-named actinides that were being discovered in the 1950s but for which there was no known use. In fact, Krell lead isotope 217 from Forbidden Planet (1956) is also used for comic effect as are the beryllium spheres in Galaxy Quest (1999). Although beryllium is a real element, the idea that one can harvest large spheres of them from a planet's surface is absurd. Isaac Asimov described two more reasons for using fictional elements—to launch the story or to get the hero out of a jam (Asimov, 1974). A recent example of story launch is Avatar (2009), where we learn about unobtainium in the first few minutes of the film but which is hardly mentioned again in the next 2h.
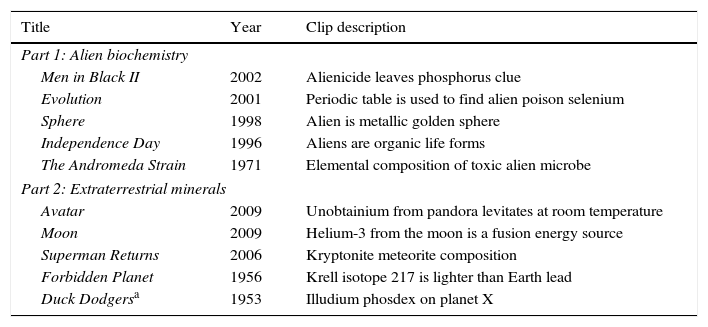
Although some reviews and summaries are very well written, they rarely describe the chemistry scenes in any detail. For this reason, it is usually not possible to determine how useful the chemistry will be until we watch it. Therefore, the next step is to watch some movies so we can critique them from a chemical perspective. We watched as many as were available to purchase. It is important that there is a single short scene (about 3min in length) in which the chemistry is sufficiently described to be useful for instructional purposes. Then, the specific movie clips are extracted and edited for the presentation and a correlating chemical education explanation developed. These explanations are given in Griep (2015). The first clips to emerge were from The Andromeda Strain, Men in Black II, and Duck Dodgers in the 24½ Century. The last two were discovered early enough to become part of our presentation titled “Everything I Know About Chemistry, I Learned at the Movies” that we described previously (Griep & Mikasen, 2013; Griep et al., 2012). With these three clips as the nucleus for a presentation, we assembled a talk about the chemistry of aliens and minerals from outer space while maintaining a focus on the periodic table during the explanations (Table 4).
Extraterrestrial biochemistry and chemistry in the movies.
| Title | Year | Clip description |
|---|---|---|
| Part 1: Alien biochemistry | ||
| Men in Black II | 2002 | Alienicide leaves phosphorus clue |
| Evolution | 2001 | Periodic table is used to find alien poison selenium |
| Sphere | 1998 | Alien is metallic golden sphere |
| Independence Day | 1996 | Aliens are organic life forms |
| The Andromeda Strain | 1971 | Elemental composition of toxic alien microbe |
| Part 2: Extraterrestrial minerals | ||
| Avatar | 2009 | Unobtainium from pandora levitates at room temperature |
| Moon | 2009 | Helium-3 from the moon is a fusion energy source |
| Superman Returns | 2006 | Kryptonite meteorite composition |
| Forbidden Planet | 1956 | Krell isotope 217 is lighter than Earth lead |
| Duck Dodgersa | 1953 | Illudium phosdex on planet X |
The fictional material “illudium phosdex” was chosen as the last movie clip in the presentation because it is make-believe and unexpected. As we reach the end of the presentation, we expect the audience will be wondering how we are going to mine some educational content out of a theatrically released cartoon. During the 1:32min long clip, the audience learns from Dr. I.Q. Hi (voice of Mel Blanc), Secretary of the Stratosphere, that the “World's supply of illudium phosdex, the shaving cream atom, is alarmingly low.” After the clip, the students are asked one question—“What type of material is illudium phosdex: Element, Compound, or Mixture?” When someone answers mixture or compound, they are asked how they reached that conclusion. The typical answers are that shaving cream has to be a mixture or that it must be a compound based on its two-part name. They are told these are reasonable conclusions but that this is a cartoon and you need to be careful when drawing a conclusion. They are then asked to remember what else they learned from the clip. Inevitably, one of the campers remembers the phrase “shaving cream atom” and realizes that illudium phosdex is an element.
Assessing the experienceThe clips and accompanying explanations were assembled into a 55-min Powerpoint® presentation, the script of which is available online (Griep, 2015). After watching each movie clip, campers were asked to rate its amount of Wow! from 1 to 5, where 5 is high (Griep et al., 2012). Next, campers listened to and participated in each explanation and rated “How much chemistry did you learn?” also from 1 to 5, where 5 is high. At the end of the presentation, they were asked to place a code name on their ratings sheets, which were then gathered, shuffled, and randomly chosen to determine the order in which door prizes were awarded.
This method was described in a recently published book chapter (Griep & Mikasen, 2013). Briefly, the Wow! rating provides an assessment of audience feelings about the entertainment value for each clip. The “How much chemistry did you learn?” rating measures their feelings about how much chemistry they learned. Our original hypothesis was that movie clips with high Wow! would encourage the audience to be more attentive while learning the real chemistry associated with the clip and yield a correspondingly high Learning rating. Instead, we found that students and teachers showed a high correlation for Learning or Pedagogical Utility but not for their Wow! ratings. Furthermore, there is a high correlation for Learning and Pedagogical Utility whether the responses were from middle school students in the classroom, middle and high school students in summer science camps, college students attending an evening lecture, or senior citizens attending a life-long learning course. In short, there appears to be a universal interpretation of whether chemistry was learned or taught that is independent of the personal response to the Wow! of the clip. Movies and the scenes within them connect with us strongly because they arouse our emotions. We want the audience's opinions about how much Wow! a particular scene holds for them so that we know which movie clips connect most strongly. When chemistry is part of the narrative, it carries a complex emotional content related to the actors in the scene, the dialog they use to describe the chemistry, and the realism of the chemical apparatus.
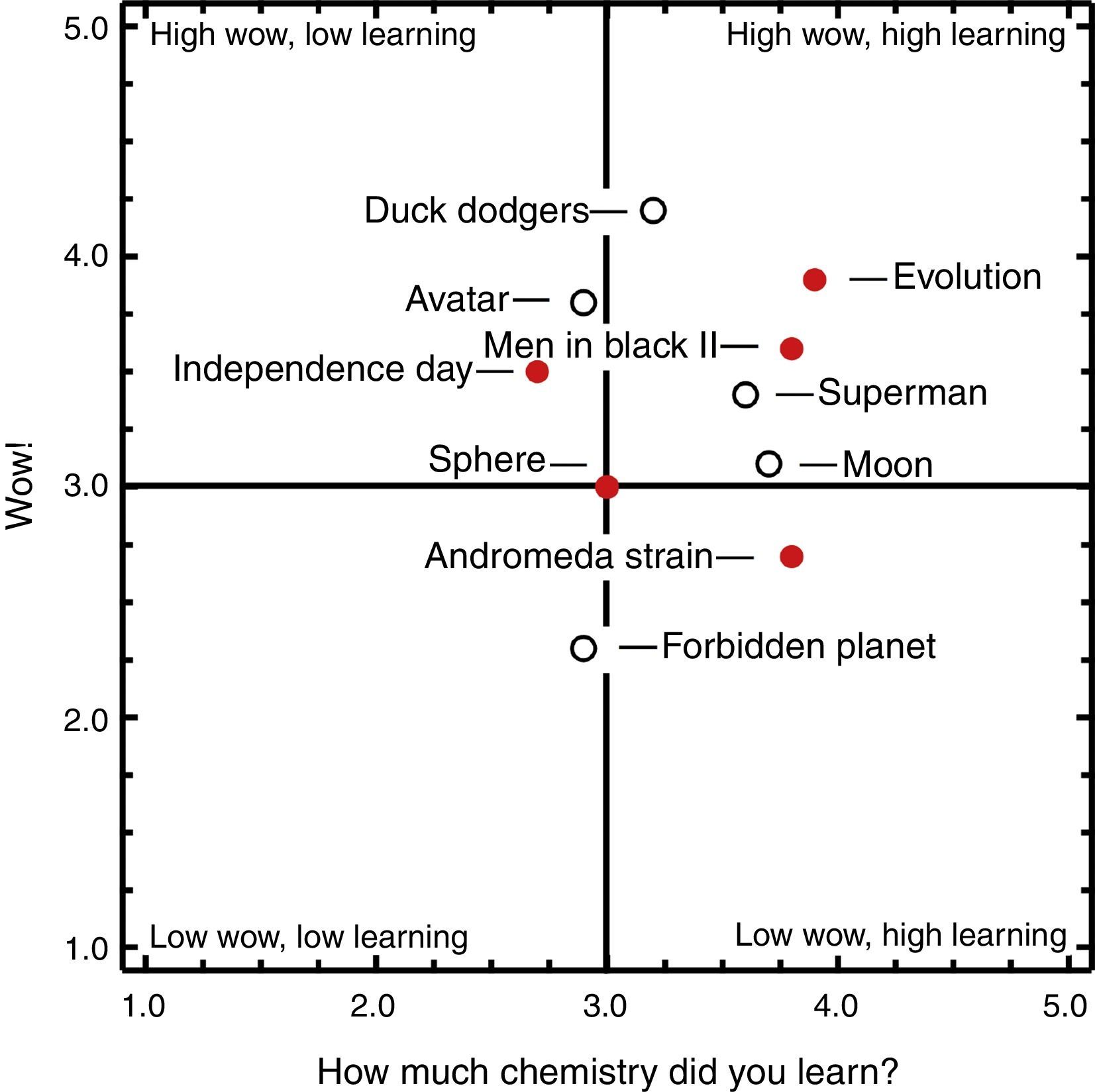
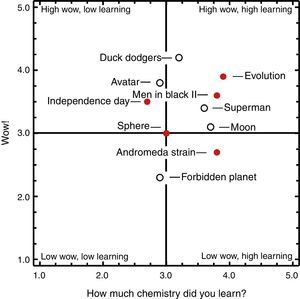
ResultsThe scores from 115 campers in six camp presentations were aggregated to create the camper average ratings (Fig. 2). The ratings were placed onto a Wow! and Learning quadrant plot, the creation of which was described in a prior article (Griep et al., 2012). Five of the ten clips fall within the “High Wow, High Learning” quadrant. This indicates the campers enjoyed the experience while learning some chemistry. Duck Dodgers in the 24 and ½ Century had the highest Wow!, with an average rating of 4.2. There were at least two reasons for this. First, the audience is familiar with the iconic Warner Brothers cartoons and cartoon imagery connects with us very powerfully. Secondly, prior to showing the clip, we described how this is one of the highest rated cartoons by cartoonists and cartoon lovers. It is important to note here that we previously showed that Wow! and Learning ratings are not related to the quality or age of the entire movie (Griep et al., 2012). This point is demonstrated by these ratings because Forbidden Planet has the lowest Wow! even though it is a funny scene from an iconic movie made in the same decade as Duck Dodgers.
Quadrant analysis of the movie Wow! vs. “How much chemistry did you learn?” for 10 movie clips shown to 115 youth attending summer science summer camps. Approximately half the campers were rising 10th graders and the other half were rising 11th graders. Both factors were rated from 1 to 5, where 5 is the highest. The filled circles signify movie clips related to extraterrestrial biochemistry. The open circles signify movies related to extraterrestrial minerals.
We define impact as the sum of Wow! and Learning because the Wow! draws learners into the chemical story and the Learning determines how much they feel they learned (Griep & Mikasen, 2013). Our previous study showed that impact factors are robust with seven out of ten movies being ranked in the same order by a wide range of audiences: middle school students in the classroom, middle and high school students in summer science camps, college students attending an evening lecture, and senior citizens attending a life-long learning course. From the current presentation (Fig. 2), Evolution had the greatest impact of all the clips. The amount of Wow! was a slight surprise to us because the action in this particular clip is a bit slow. Next most impactful were Men in Black II, Duck Dodgers, and Superman Returns. This finding was gratifying because the first two of these clips are used in our “Everything I Know About Chemistry” presentations (Griep & Mikasen, 2013; Griep et al., 2012).
Avatar and Independence Day fall into the “High Wow, Low Learning” quadrant, although both received nearly average ratings for the amount of learning. Sphere received an average rating for both Wow! and Learning. The Andromeda Strain was in the “Low Wow, High Learning” quadrant, indicating it would be acceptable for use in the classroom but that the campers were not especially impressed by the 1970s pacing, instrumentation, television monitors, earnest dialog, and lack of A-list movie stars. The lowest impact clip was Forbidden Planet with its low Wow! and average learning. Our expectation was that it would perform better than Superman Returns and Independence Day; so, this demonstrates the need to test each movie clip for its reception by the audience. It is the shortest of the clips at under a minute in length and features a robot carrying a slab of metal. The robot pauses to talk to two spacemen about the lightness of Krell isotope 217 to explain that it hardly weighs ten tons. It ends with the two spacemen smiling at each other because they know ten tons is still quite a lot.
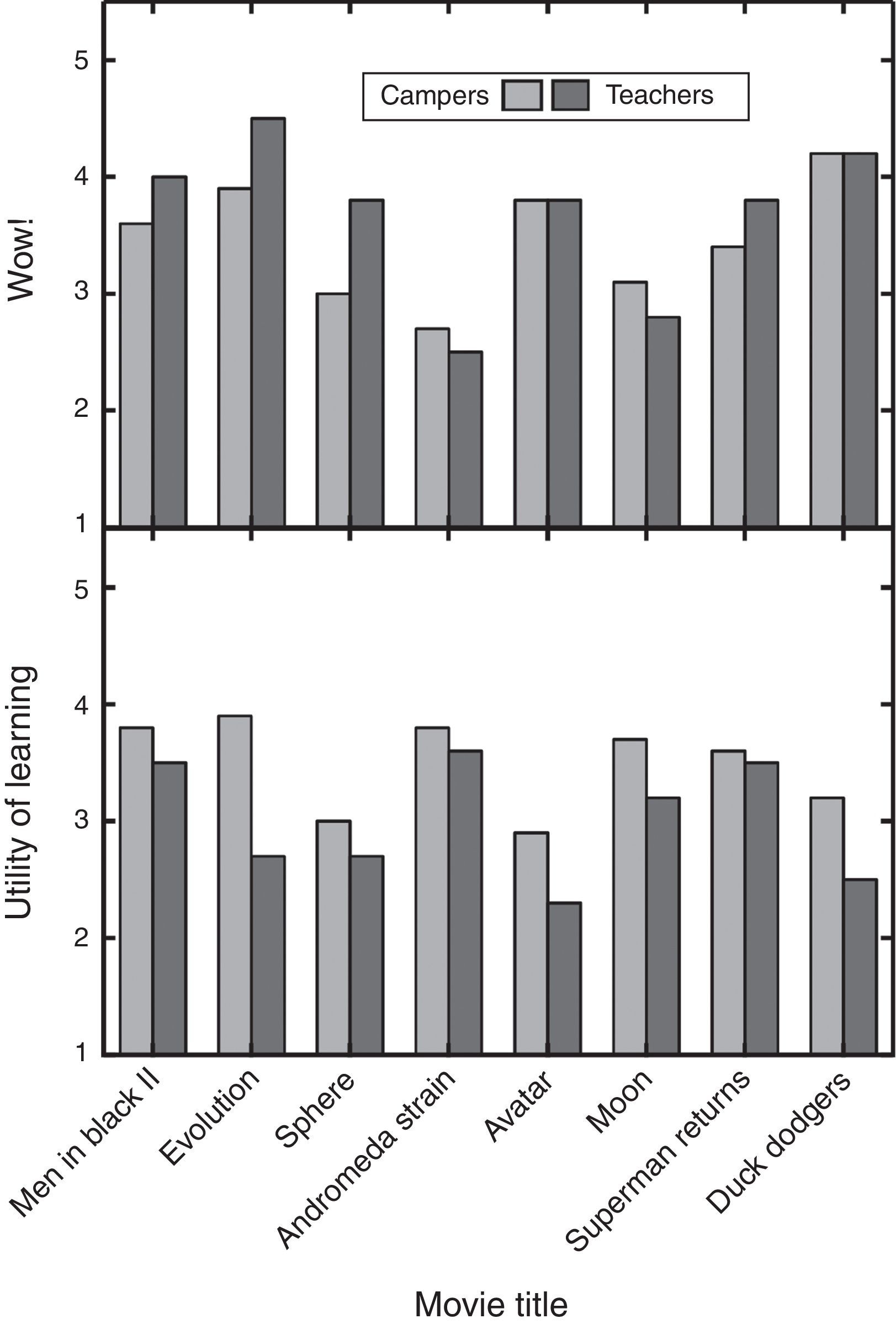
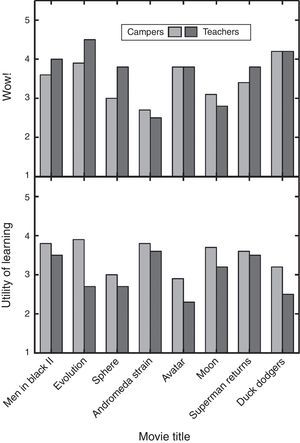
The clips and explanations were also rated by chemistry teachers from middle and high schools during Griep's summer professional development course titled Matter Matters. The teachers were ratings experts in deciding the best material for use in the classroom (Griep & Mikasen, 2013; Griep et al., 2012). They were not asked to rate the material for use in outreach activities related to creative chemical thinking. The teachers rated eight of the same clips. The teachers did not rate either Independence Day or Forbidden Planet, because these two clips were added to the presentation later. When teachers rated the clips, they were asked to give their personal Wow! for the clip and to rate the “Pedagogical Utility” of the explanation given by Griep. Utility was defined as “How much chemistry could you teach using my explanation?”
A comparison of teacher and camper responses reveals a number of differences (Fig. 3). In general, teachers gave higher or the same Wow! as the campers but then gave lower Pedagogical Utility than the campers. Since teachers do not typically teach these topics, they rated the explanations significantly lower because they would not choose to use them in the classroom. On the other hand, this double disparity epitomizes the differences between teachers searching for teachable material and campers searching for entertainment after a long day of learning. For instance, teachers gave Evolution the highest Wow! rating but also their second lowest Pedagogical Utility rating. Its appealing features are the periodic table T-shirt, the use of a periodic table to propose a solution to a problem, an example of group work, and that the scene occurs in a classroom laboratory. The Evolution clip and explanation are not especially useful in the classroom because the hypothesis is absurd, students in the classroom do not like dealing with fictional hypotheticals, and the explanation put forward to justify the absurd hypothesis is ad hoc. Even so, the Evolution clip and explanation fosters creative thinking by connecting unrelated things first and then wondering how well they fit together second. Finally, the audience reaction to this movie clip demonstrates how important the audience views creative thinking in solving problems.
Bar chart comparison of the ratings for eight movie clips by 115 high school-aged youth and 14 middle and high school teachers. The campers rated the clips as described in Fig. 2 legend. The teachers rated the clips for Wow! and the explanations for “Pedagogical Utility” as part of a professional development course. Both factors were rated from 1 to 5, where 5 is the highest.
The innovation of this study is the methodology to find and assess movie clips as part of an outreach presentation for science camps. Prior to this, movie clips have been used to animate a chemical topic during lecture in a way that grabs student attention so there is a strong anchor upon which to contextualize the rest of the lesson (Griep et al., 2012; Hofstein & Kempa, 1985; Wink, 2001). Movie clips are also useful in enlivening a serious activity such as lab safety video without detracting from its message (Matson, Fitzgerald, & Lin, 2007).
We have shown that a presentation based on movie clips meets the same objectives as the chemist's outreach stock-in-trade, the chemical demonstration show. Colors, bangs, and smells have gotten us this far even though their effectiveness has not been assessed. Now we need to explore new ways to reach millennials, the people born after 1982, who are less likely to choose chemical careers than their predecessors. To address this question, Wirthlin Worldwide was contracted by the American Chemical Society to learn what millennials think about chemistry and their profession (Dagani, 2004). Among their recommendations was that chemists should develop educational programs that are exciting and fun. Then, they recommended challenging the stereotype that chemists are not goal-oriented, outgoing, or team players. As we have shown here, it is possible to create presentations using movie clips that are fun, educational, and give the speaker an opportunity to use Hollywood movies to address stereotypes by showing a plethora of examples (Masciangioli, 2011).
The presentation itself raises a number of interesting issues for campers to ponder after the talk is over. With regard to the extraterrestrial minerals, the focus is on engineering rather than science. We visit other celestial bodies to collect minerals with known properties that are scarce on Earth. We know where the minerals are located and we know how to extract them. After launching the story in Avatar, Moon, and Duck Dodgers in the 24 and ½ Century, these minerals become unimportant. In Superman Returns, the elemental composition of the mineral is given to add realism. It is an example where chemistry is used to anchor the story in reality. The composition of the mineral does not play a role in the story. In Forbidden Planet, Robby the Robot has a very dry wit and is the primary vehicle for humor. The mention of isotope 217 in the clip anchors the joke in reality. Chemists who watch the movie will find the joke funny without realizing Robby made an error when he says it is lighter than Earth's lead. Nevertheless, chemists can compound their enjoyment by wondering how the screenwriter chose a heavier isotope but then said it was lighter.
With regard to alien biochemistry, the focus is on science and the scientific method. We are not in control of the visitors to our watery planet but we can use our skills to investigate these living entities. Every time, we learn they are composed of the same elements as those found on Earth even when they may not have the same biochemistry. An overlapping theme is the desire to control or kill the aliens. These invaders are spreading in their new environment and there does not seem to be anything to stop them. The alien biochemistry section also taps into one of the biggest questions of our time—does life exist anywhere else in the universe? Movies about aliens have not been as shy about finding and analyzing the chemistry of aliens, thereby providing a tangible link to insert more chemistry into the dialog. If only we had some real materials to analyze.
ConclusionThe topic of alien biochemistry and extraterrestrial minerals is novel to the students because these topics are rarely encountered in the classroom. The use of movie clips work well for chemical outreach because they often show how a fictional character brings divergent thoughts together to solve a problem. Because the students are entertained by the presentation, they are more likely to remember the experience longer, to have more positive feelings toward chemistry, and to identify chemistry in other aspects of their everyday lives.
Conflict of interestsThe authors declare no conflict of interest.
This research was funded by the UNL Kelly Fund to Improve Teaching. We gratefully acknowledge the cooperation of the UNL EPSCoR program for giving us access to their campers, and of the Nebraska Local Section of the American Chemical Society for allowing us to speak to its members. Most of all, we thank the campers and teachers who gave us their responses to the presentations.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.