From 1888 till 1892 Alfred Werner, the founder of coordination chemistry, developed together with his friend Arturo Miolati electrical conductivity of ionic complexes as an auxilliary analytical tool for structural elucidations of complexes. The used electrical conductivity device was based on a design of Wilhelm Ostwald consisting of a generator for alternating current (induction apparatus with Wagner hammer), a measuring cell with platinated electrodes and a rheostatic part including a buzzer for balancing resistivity conditions. Electrical conductivities were examined in the ion-isomeric series of Pt(II)(NH3)nCl2 (n = 0 - 4), Pt(IV)(NH3)nCl4 (n = 0 - 6) and Co(III)(NH3)nCl3 (n = 0 - 6) complexes producing approximately V-shaped curves in dependence of the stoichiometry factor n. Replacing in the coordination spheres neutral Lewis base type ligands by ‘anionic residues’ generated charged complexes, which by conductivity measurements laid grounds for Alfred Werner’s coordination theory [primary (from ‘anionic residues’) and secondary valencies (from Lewis bases)] and the Nobel Prize in 1913. From 1893 on seven PhD theses were prepared in Alfred Werner’s group, which dealt with conductivity measurements establishing identification processes of complexes by ‘ion counting’. In 1902 Alfred Werner ceased to apply electrical conductivity in his group switching to meanwhile more timely coordination chemistry fields.
Desde 1888 hasta 1892 Alfred Werner, el fundador de la química de coordinación, desarrrolló junto con su amigo Arturo Miolati la medición de conductividad eléctrica de iones complejos como una herramienta analítica auxiliar para la elucidación de la estructura de complejos. El aparato utilizado para la conductividad eléctrica estaba basado en un diseño de Wilhelm Ostwald, que consistía de un generador de corriente alterna (aparato de inducción con martillo de Wagner), una celda de medición con electrodos platinados y un componente reostático que incluía un timbre para balancear las condiciones de resistividad. Las conductividades eléctricas fueron examinadas en una serie de iones isoméricos complejos de Pt(II)(NH3)nCl2 (n = 0 - 4), Pt(IV)(NH3)nCl4 (n = 0 - 6) y de Co(III)(NH3)nCl3 (n = 0 - 6), que produjeron curvas en forma de V con dependencia en el factor estequiométrico, n. Al reemplazar en la esfera de coordinación ligantes neutros tipo base de Lewis por ‘residuos aniónicos’ se generaron complejos cargados, que por mediciones de la conductividad condujeron a Alfred Werner a la teoría de la coordinación [valencia primaria (proveniente de los ‘residuos aniónicos’) y secundaria (de las bases de Lewis)]. A partir de 1893, siete tesis doctorales fueron elaboradas por miembros del grupo de Werner en las que con mediciones de la conductividad establecieron el proceso de identificacion de complejos por ‘conteo de iones’. A partir de 1902, Alfred Werner cerró la aplicación de la conductividad eléctrica en su grupo cambiando a otros temas más avanzados de la química de coordinación.
Alfred Werner is regarded as the founder of coordination chemistry. He took a whole field of chemistry from a dark and mystical perception to the level of a bright and rational being. To achieve this he was left only a short period of time by living an intense life, which was mainly dedicated to chemistry (Kauffman, 1979; 1994). Alfred Werner was a full heart scientist. All his endeavors were strongly curiosity-driven, which made him a steady searcher for novelties in science. The main sources of his chemical creativity were deductions by intuition and conceptual thinking, which made his research very effective. Connected to that many contemporaries reported that Alfred Werner was a man of great imagination and inspiration.
The main stages of his life are listed in the following compilation, which will be referred to in the later context of this article:
Alfred werner’s ufe| 1866 | Born in Mulhouse |
| 1887 | Enrollment as a student of chemistry at ETH Zürich |
| 1889 | Diploma of Chemistry from ETH Zurich |
| 1890 | Ph.D. with Prof. Hantzsch at ETH Zürich |
| 1889 | Alfred Werner meets A. Miolati |
| 1892 | Habilitation ETH Zürich, unsalaried Privatdozent |
| 1893 | Associate Professor, University of Zürich |
| 1893 | Formulation of Coordination Theory |
| 1895 | Full Professor, University of Zürich |
| 1895 | Marriage and Swiss Nationality |
| 1905 | First edition of Alfred Werner’s book Neuere Anschauungen auf dem Gebiete der Anorganischen Chemie |
| 1909 | New chemistry building in Zurich |
| 1913 | Nobel Prize |
| 1915 | Descent to Illness |
| 1919 | Death |
Alfred Werner was a chemist always trying to look over the fence to other science fields. At his times physics was just at the turn to develop into a quantitative discipline, which attracted him very much. It was maybe his not so consciously expressed desire, but steadily pursued way in chemistry to take chemistry, particularly coordination chemistry, away from the world of qualitative and at the time often even illdefined and mystical perceptions to a more quantitative and better defined science. The marriage of chemistry with physics is physical chemistry, which took place at around the beginning of Alfred Werner’s scientific career in the 1880s along with the quantification trends in physis. Looking over the fence to physics meant for Alfred Werner to treat chemistry more quantitatively through physical measurements, but also to conceive it more conceptually and fundamentally.
A good, but not fully accomplished example for this latter notion are the developments of his coordination theory, which expresses indeed the trend to make the earlier Bloomstrand-Jorgensen coordination theory (Kauffman, 2003; Berke, 2009) more adjusted to the physical reality by transposing coordination chemistry from the two-dimensional space (‘flat molecules’) into the three-dimensional space inventing thus the stereochemistry of coordination compounds.
In this context and with the given conviction in mind Alfred Werner got aware of the fact that his new stereochemical views of coordination compounds needed also a new binding theory for complexes, which indeed he had then eventually created. He tried to reach a more quantitative view of inorganic and coordination chemistry (Werner, 1905), but in its essence Alfred Werner’s binding theory could finally achieve that, even though it was not totally built on solid physical grounds. His coordination theory could serve as a heuristic representative method to extensively match reality with conclusions by analogy, but could not serve for more. An atomistic physical picture of the chemical bond did not exist at Alfred Werner’s time, nor was it existent in fragments, which ‘forced’ Alfred Werner to strive for substitution of this deficiency from the physics side. Even though Alfred Werner’s conceptual thinking was much ahead of his time and his ‘coordination theory’ created at the turn of the year 1892 (published in 1893) had finally attained consistency by itself, it could not cope with the factual physical level of say the times of the 1920th when quantum physics had triggered the developments of a new picture of molecules. Alfred Werner’s coordination theory did not reach the level of quantification and sophistication that it had needed in order to become comprehensive. This was for instance criticized at his time by his PhD student A. Pfeiffer, who became later on his colleague (Werner, 1923). Principally there was nothing wrong with his theory, but as said already the level of sophistication was not high enough to survive for good. Alfred Werner’s theory served for about 20 years as a well-received inductive formula used as a valuable platform for the creation of ideas and explanations.
Another so to speak ‘true’ example, more suited to demonstrate Alfred Werner’s looking over the fence to physics, was UV-vis spectroscopy (at Alfred Werner’s time called spectral analysis (Spektralanalyse)), which he tried to implement as a structural characterization method of complexes in his group (Fox and Berke, 2014). Alfred Werner caught interest in this method in the range of 1912 - 1914, which at the time was known as a method for the spectral characterization of organic compounds and of inorganic solids only. But after 1914 Alfred Werner had stopped carrying out research with UV-vis spectroscopy. We do not know the real reason for that, but we can speculate that at the time UV-vis spectroscopy seemed to be too laborious in view of what could be achieved regarding the elucidation of structures of coordination compounds.
These two examples of physical influence on Alfred Werner’s research were accompanied by the quest to quantify inorganic and in particular coordination chemistry. But these examples were not the only new physical methods, which Alfred Werner fostered to be applied to coordination chemistry in his group: At a very early stage of his scientific career Alfred Werner got contact with conductometry or electrical conductivity measurements, which made him try to tackle the marriage of coordination chemistry with electrolytic dissociation in solution. The application of electrical conductivity to coordination chemistry is the main subject of this article, popularly denoted as the ‘counting of ions’ of ionic complexes in water. The method seemed to be advantageous for the structural characterization of complexes.
Electrical conductivity of coordination compounds in water and the significance of electrolytic dissociation to the development of alfred werner’s coordination theoryThe fact that many coordination compounds are salts, which separate into ions in water, makes them a perfect study case for electrical conductivity. Alfred Werner the founder of coordination chemistry has recognized this circumstance at a very early stage of his scientific career and carried out research on this subject even before he got associate professor at the University of Zurich in 1893. His curiosity, impetus and affinity for physical and physical chemistry methodologies brought Alfred Werner to think about the usefulness of electrical conductivity in coordination chemistry as soon as he got knowledge of the possibility to determine electrolytic dissociation and this occurred to happen when he had become a member of the group of Arthur Hantzsch, first as a PhD student and then as a ‘Habilitand’ (similar to an untenured assistant professor) at the Polytechnikum in Zurich (today called Eidgenössische Technische Hochschule Zürich (abbreviated ETH Zurich). He met there Arturo Miolati coming from Turin, who was in the group of Arthur Hantzsch from 1899 on and returned to Italy in 1893 to join the group of young chemists at the Institute of Chemistry, University of Rome, headed by S. Cannizzaro. After that Arturo Miolati got professor in Turin and Padua (Kauffman, 1970). Arturo Miolati was an universal ingenious. During in his scientific life he worked in many fields of chemistry, but he never completely left the fields of coordination chemistry and electrical conductivity. He is often designated as the first physical chemist of Italy.
The collaboration of Alfred Werner and Arturo Miolati turned out to be very fruitful culminating in several collaborative papers. The main ones are ‘Beiträge zur Konstitution anorganischer Verbindungen, Abhandlungen I. and II.’, which were published in the Zeitschrift für Anorganische Chemie in 1893 and 1894 (Miolati, 1893; 1894). Other accounts detailing Alfred Werner’s fundametal ideas were also published (Miolati, 1896; 1897) including a paper with C. Herty (1901). All his scientific life Alfred Werner felt very grateful to Arturo Miolati about the collaboration they had. He not only had devoted the first edition of his book (Werner, 1905) to Arturo Miolati, but also was thankful to Arturo Miolati in his Nobel lecture — held in German language — in Stockholm, 1913. In this speach he also gave an explanation for how electrical conductivity measurements influenced his coordination theory. For a better understanding the German text of the respective part of the Nobel Lecture is given here as an excerpt translated into English, the main sentence in the eyes of the author of this paper is also given in German (Werner, 1913; 1914): The diverse function of the acid residues (Comment from the author: ‘acid residues’ means anionic ligands here) in the compounds under discussion could, in accordance with the electrolytic theory of dissociation put forward by your famous member, Professor Arrhenius, also be interpreted by postulating that the acid residues in direct bond with the metal atom do not dissociate in solution, whereas those not directly linked with the metal atom occur as independent ions. It should be possible, therefore, to provide experimental physico-chemical evidence for the conclusions drawn, by determining the electrolytic conductivity. The result of the investigation I carried out jointly with my friend A. Miolati, fully confirmed our conclusions, since we discovered that the compounds of which it was assumed that they contained all acid residues in direct bond with the metal atom, are so little dissociated electrolytically, that they behave practically as non-conductors. This important result was later confirmed again by an investigation carried out jointly with Ch. Herty. In this way we had secured an experimental foundation, on which the new theory of the constitution of inorganic compounds could be based …
The last sentence of the given excerpt of the Nobel Lecture is very important, which is therefore also given in German: Damit war die sichere experimentelle Grundlage gewonnen auf der das neue Lehrgebäude von der Konstitution der anorganischen Verbindungen errichtet werden konnte.
Alfred Werner was saying here that electrical conductivity was crucial to the development of his new coordination theory, which in essence distingushed between the primary and secondary valency of ligands in complexes (Werner, 1905). According to this anoinic ligands, directly bound to the metal atoms, were bound in a primary fashion, while neutral Lewis base type ligands in modern terminology were bound to metal atoms in complexes in a secondary way. It becomes clear now, how Alfred Werner viewed electrical conductivity to be a suitable method for the ‘secure foundation’ of his coordination theory. Following the picture explained in the Nobel Lecture (Werner, 1913), as he did also in all the editions of his book (Werner, 1905), that a primary bound anionic ligand could also be replaced by a secondary valency type ligand; the earlier would then stay outside the coordination sphere, consequently the complex unit had attained by this an electrical charge and the whole complex had become an electrolyte. By this formalism a whole series of subsequent substitutions could be established as shown in Fig. 1 (taken from the Nobel Lecture, p. 6 of the German version) attaining different charges of the complex units and by electrolytic dissociation different total numbers of ions of one compound.
As sketched in Alfred Werner’s Nobel Lecture a sequence of substitutions of ammine ligands by X = ‘acid residue’ (anionic group). In all complexes the metal center (= Me in Alfred Werner’s terminology) has retained its threefold primary valency, however, the total charge of the complex units and the total number of ions of the complexes have changed. From the top to the bottom the number of ions of the complexes range from 4, 3, 2 to 0.
Each of the species of Fig. 1 could pricipally be distinguished by electrical conductivity, which in a first order approximation seems in its magnitude to be dependent only on the total number of ions, into which each individual compound can electrolytically dissociate.
Besides the stereochemical aspects of coordination compounds, the distinction between primary and secondary valencies of complexes was the essence of Alfred Werner’s coordination theory and a complex series as shown in Fig. 1 validated in conjunction with conductivity measurements the notion of his theory. After a short description of Alfred Werner’s device for conductivity measurements in Chapter 3, Chapters 4 and 5 will report about several examples of complex series as depicted in Fig. 1.
Principal setting of alfred werner’s apparatus for conductivity measurements of solutions of coordination compoundsAs mentioned before the early conductivity measurements of Alfred Werner were carried out together Arturo Miolati in the years 1898 -1891 (period of time of Arturo Miolati’s thesis as an egineer of chemistry and PhD thesis) in Arthur Hantzsch’s group at the ETH Zurich. During a 2 year’s period Alfred Werner, at the time also a member Arthur Hantzsch’s group, was the senior to Arturo Miolati. The conductivity apparatus used and shown in Fig. 2 existed already in Arthur Hantzsch’s group, which was for instance used to determine the acidity of oximes (Hantzsch, and Miolati, 1893) constituting also part of the scientific work of Arturo Miolati.
Left: Schematic sketch of the Ostwald conductivity device for the conductivity measurement of electrolyte solutions (Ostwald, 1892): Resistor box with various fixed value resistors (1), the measuring cell in a thermostatic bath (2), rheostat containing an iridium alloyed platinum resistor wire (3), telephone = buzzer (4), electrolytic cell with platinated electrodes (5), source of alternating current consisting of an induction apparatus combined with a Wagner hammer (6). Right: Schematic drawing of the electrolytic cell with platinated electrodes according to Arrhenius (Ostwald, 1892).
The conductivity apparatus was based on a construction suggested by Wilhelm Ostwald from the University of Leipzig, Germany, occupying one of the first physical chemistry chairs in Europe there since 1887. The apparatus was an advancement of the so-called Kohlrausch bridge. It is schematically sketched in Scheme 1 as taken from Wilhelm Ostwald’s original publication (Ostwald, 1892).
The conductivity measurement device consisted of a source of alternating current (induction apparatus combined with a Wagner hammer) (6), the electrolytic cell (in a thermostatic bath) (2) with the platinated electrodes (5) to be immersed into the cell. The measurements were physically based on parallel resistors from the cell and the rheostatic device made up by the fixed value resistors (1) and the variable resistor of the rheostat (3). When the so-called telephone (4), which was physically equivalent to a buzzer, indicated the lowest level of humming noise (tone dependent on the frequency of the alternating current) the known resistivity or its reciprocal value, the conductivity, of the rheostatic device and the unknown resistivity of the measuring cell including the electrodes were apparently the same. Since the water used as solvent was at the time often not very pure, its conductivity had to be measured separately and substracted from the determined values for the electrolyte. According to Wilhelm Ostwald the given apparatus could be built without great financial investment and the measurement could be carried out quickly (Ostwald, 1892). This situation would have made it also easy for Alfred Werner to built a conductivity apparatus at a later stage of his career after he had moved to the University of Zurich as an associate professor in 1893 establishing his own research group. Whether he finally built a conductivity device at the University of Zurich in his group or used an apparatus from the Physics Institute of the University of Zurich (Fig. 3) or whether he continued to use the apparatus from the Hantzsch group at ETH is unknown.
Left: Photograph of a historic induction apparatus of about 1874 taking the function of (6) of Fig. 2. Right: photograph of a resistor box taking the function of (1) of Fig. 2. The age of this box is unknown. It could however correspond to a commercial model available at Alfred Werner’s time. Both instruments were located in the archives of the Physics Institute of the University of Zurich. In principle these instruments could have been used by Alfred Werner and his group, whether they were used by them is not reported.
At Alfred Werner’s time all these institutes or institutions were located in Zurich in close vicinity, which provided no great obstacles to use ‘each other’s’ instrumentation. The conductivity measurements were conducted measuring molar conductivities. This condition made it possible to count the number of ions per mole of complex, but it seemed often not possible to compare the absolute values of these measurements with those obtained by other settings (Peterson, 1901). In his publications and in all the editions of his book Alfred Werner (1905) was refering to μ values without denotations, which are supposed to be refering to the molar conductivity entity as established by Friedrich Kohlrausch (1927). However, molar conductivities have in modern times the denotation of [S-L/cm-mol] meaning that there is always a constant set by the device to be taken into consideration. If this constant is explicitely not reported, the measurements are expected to produce only comparable numbers when the measurements were carried out with the same apparatus, but there would be incomparable numbers when changing the measurement device. This presumably was Alfred Werner’s main difficulty, when he compared his work with the work of others at his time (Peterson, 1901).
Examples of alfred werner’s and arturo miolati’s conductivity measurements of complexesAlfred Werner and Arturo Miolati descibed in their first respective publication ‘Beitrage zur Konstitution anorganischer Verbindungen’ ‘I. Abhandlung’ (Werner, and Miolati, 1893) the conductometric determination of molar conductivities of the coordination complex series of ammine chloro cobalt and ammine chloro platinum(II) and (IV) compounds. They derived these from measurements the existence of a constant coordination number for each of these complex series. This actually was the birth act of the term coordination number, which became very fundamental to coordination chemistry. It should be mentioned at this point that in the earlier coordination theory of Blomstrand and Jörgensen (Kauffman, 2003) the term coordination number was not known. Further, as explained in the earlier context of this article this number is distiguished from the valency number, which refers to the number of the maximum of covalently bound partners in a complex as formulated by Alfred Werner shortly later. However, this paper of the ‘I. Abhandlung’ constituted a first exploratory study in the field. It should also be mentioned that Alfred Werner and Arturo Miolati had in these first experiments (as in many experiments later also) to fight with technical problems of the conductivity measurements, i.e. that the measurements changed in many, if not in most cases, somewhat or often to a great extent with time. About the reasons of this phenomenon we can only speculate, but it looks very rational to assume that as a physical reason the given induction apparatus of Fig. 2 and 3 did not provide a stable alternating currency. Furthermore, for chemical reasons the complexes were often thermodynamically and kinetically not stable enough so that by the time of measurement the complexes changed in their compositions and led to formation of various species with different molar conductivities.
The second paper in this collaboration series with Arturo Miolati was published under the same title as the first paper (,Beiträge zur Konstitution anorganischer Verbindungen’ II. Abhandlung’) (Werner and Miolati, 1894). Apparently for this paper the experimental finish was prepared with more routine so that more reliable conductivity values were obtained and more definite conclusions could be drawn. This copes also with the fact that many of the experiments of the second paper were repititions of the first paper.
It is not at all accidential that Alfred Werner and Arturo Miolati had chosen kinetically quite stable complex series of Pt(II)chloroammines of a), Pt(IV)chloroammines of b) and Co(III)nitrotoammines of c) for their measurements (Fig. 4). The higher the involved number of ions of a complex, the higher the molar conductivities were within one of the series and the molar conductivity changes by one ion were within a certain range independent of the type of ions. Thus the counting of ions via conductivity measurements could really be used to establish the constitutions of complexes telling us with the assumption of a constant coordination number how many ions were ligands and how many counterions. It should be noted at this point that the gross independence of the molecular conductivity from the type of the ion caused the ‘almost’ V-shapes of the curves of Fig. 4 and allowed structural assignments within the square planar four-coordinated series a) or the octahedral six-coordinated complex series b) and c) merely based on elemental analysis and electrical conductivity. It should be mentioned in addition that in certain cases of Fig. 4 a full series of substitution of the anionic residues could not be obtained due to the fact that the compounds required for measurement were not known.
Series of conductivity measurements of platinum(II)chloroammines of a) platinum(IV)chloroammines, of b) and Co(III)nitritoammines, and of c) taken from Werner, and Miolati (1894). The missing members of certain conductivity plots were complexes unknown at Alfred Werner’s time.
In Alfred Werner’s group seven PhD theses were prepared, which contained at least in parts also conductivity measurements of coordination compounds (Inaugural-dissertations related to conductivity measurements from Alfred Werner’s group, 1896-1902).
It should be mentioned that all of these PhD theses cited were prepared at an early stage of Alfred Werner’s career within the first 10 years of his stay at the University of Zurich. The results of only two of the theses were published in scientific journals (Werner and Gubser, 1901; 1906; Werner, Kalkmann and Gubser, 1902) and most of the PhD theses used the conductivity measurements as an analytical method in the context of structural assignments for complexes meaning that they were just occasionally applied in the PhD theses and had not the main goal to establish the physical chemistry field of conductometry in inorganic chemistry. It should be mentioned at this point that for a short period of time in 1900 Alfred Werner had C. H. Herty in his group (name mentioned in Alfred Werner’s Nobel Lecture, Werner, 1913), who came on a sabbatical leave from the Chemistry Department, Franklin College, Franklin, USA. From this collaboration resulted one publication (‘Beiträge zur Konstitution anorganischer Verbindungen (IV. Abhandlung)’) (Werner, 1901), in which C. H. Herty tried to establish mainly conductivity series of cobalt ammine and ethyleneamine complexes with chloride and rhodanide as anionic residues. In the majority of cases the complexes were kinetically unstable and changed molar conductivites during the experiment.
In an exemplary fashion two of the ‘unpublished’ PhD theses were selected to be reviewed here in parts. Their headpages are shown in Fig. 5.
F. Beddow and A. R. Klien both came to the University of Zurich from the United Kingdom to prepare a PhD thesis in Alfred Werner’s group. Both theses are remakable in that Beddow’s thesis was written in English, a quite uncommon language at the University of Zurich at the time, while Klien’s thesis was written in German, which presumably was an uncommon language for the PhD candidate. Their main tasks were to prepare new complexes. However, both used for structural characterizations of certain ionic complexes also electrical conductivity.
Fig. 6 (left) illustrates that the conductivity measurements of F. Beddow were carried out establishing dilution rows (columns v) in two series obtaining the molar conductivities (column μ)which were averaged over two series of measurements. Beddow’s structural proposal as given in Fig. 7 would indeed be a 3-ion electrolyte per cobalt. But the suggested bridging oxo complex does not seem to cope well with reality by modern times knowledge. Therefore the author of this article proposes that the structure of the complex in solution may correspond to an unstable [(NH3)5(OH)Co] Cl2 complex, which can exist in solution for a certain period of time (Mironov et al., 1995; Isaev et al., 1990; Norman, 1988).
Left: Beddow’s measurement — directly taken from the PhD thesis written in English — indicating from the magnitude of the electrical conductivity a 3-ion electrolyte, but in contrast to Beddow’s structural assignment presumably corresponding to an unstable [(NH3)5(OH)Co]Cl2 complex. Right: Klien’s measurements of a cis-nitrito(thiocyanato)tetramminecobalt complex — directly taken from the PhD thesis written in German — indicating from the magnitude of the electrical conductivity a 2-ion electrolyte.
From left to right: a) Beddow’s structural proposal of a μ-oxodicobalt complex as taken from his dissertation (Inaugural-dissertations related to conductivity measurements from Alfred Werner’s group, 1896-1902); b) Proposal of Beddow’s complex to be a [hydroxy(pentammine)cobalt(III)]Cl2 complex; c) Klien’s structural proposal of a pentamine(rhodanato)cobalt(III)]nitrate complex (Inaugural-dissertations related to conductivity measurements from Alfred Werner’s group, 1896-1902), and d) its stereochemical drawing of modern times.
Fig. 6 (right) illustrates that the conductivity measurements of A. R. Klien were again carried out with dilution rows (column v) in two series obtaining molar conductivities (column μ) which were averaged over two series of measurements. Klien’s structural proposal as given in Fig. 7 would indeed indicate a 2-ion electrolyte. This proposal seems to be very realistic and would actually cope with the structure of a cis-nitrito(thiocyanato)tetramminecobalt complex.
ConclusionsThe article could descriptively demonstrated that at the time from 1888 on Alfred Werner and Arturo Miolati and Alfred Werner’s group could determine quite quantitatively electrolytic dissociation of ionic complexes by electrical conductivity measurements. The application of this type of measurements by Alfred Werner was interpreted in terms of his special inclination and curiosity for quantification of coordination chemistry using physical methodolgies. Via these measurements it became possible to ‘count ions’ of a specific electrolyte, which then also allowed to determine structures of mononuclear complexes based on their coordination numbers. The given structural analysis concluded on the number of ‘anionic residues’, which could act as ligands in the complexes or as counterions of the complexes. The measurements were conducted by Alfred Werner or his group with an Ostwald type conductometer, which at the time was a cheap device and the measurements could be carried out quickly. The question therefore arises, why Alfred Werner applied this method only between the years of 1889 and 1902 and not generally over all the period of his scientific activities. The answer can only be speculated, because Alfred Werner had — to the author’s knowledge — never given a statement about this. One reason seemed to be that Alfred Werner had realized that one could have drawn ‘safe’ structural conclusions by this method only for mononuclear complexes. But from 1900 on Alfred Werner started also to carry out research on polynuclear complexes (Kauffman, 1973;Blacque, and Berke, 2014), for which the ‘counting of ions’ may have helped more or less only in the identification process of a known coordination compound, but not for structural predictions of such complex species. Furthermore from 1900 on Alfred Werner moved also into the field of chiralility of complexes (Ernst, and Berke, 2011; Ernst, Wild, Blacque, and Berke, 2011). For the structural assignment and the distinction of enantiomers electrical conductivity was for sure not the right method. Thus the more coordination chemistry advanced in Alfred Werner’s hand, the more electrical conductivity lost importance as an analytical methos for his chemistry. His advancing chemistry dealt also more with kinetically unstable (labile) complexes prone for facile ligand exchanges. If a primary and secondary ligand could be exchanged by another ligand of the same type, electrical conductivity would not indicate this properly, since changes in electrical conductivity were often minor. For instance water as a ligand in all the cases present in excess as a solvent makes in the end the method inappropriate for structural assignments if the complexes had labile ligands like ammines. Finally it should be reasoned that Alfred Werner stay-away from electrical conductivity later in his career could also have to do with the physical instability of the electrical conductivity devices, particularly the induction apparatus, which may have sometimes led to great experimental frustration and even misinterpretations could have been a consequence of this circumstance. Todays conductivity devices do not have this disadvantage anymore, they are physically stable. Despite all these not so positive judgements on electrical conductivity measurements of coordination compounds, the method merits great acknowledgements, since the measurements of kinetically stable complexes became for Alfred Werner the method, on which he could base and then verify his ideas of a coordination theory. In the end this achievement got him awarded with the Nobel prize.
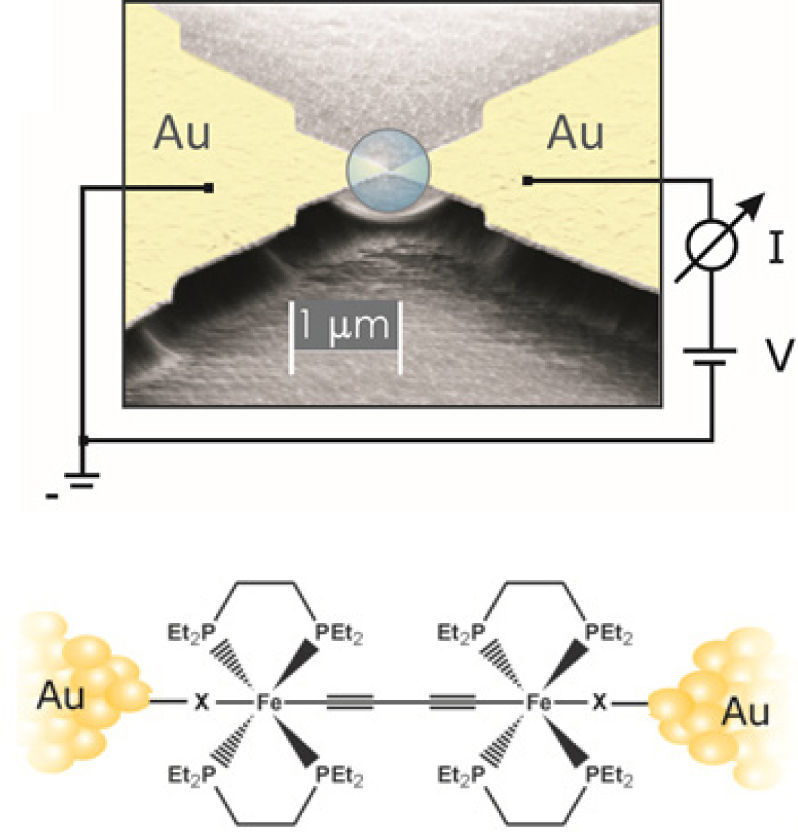
EpilogueMore than 100 years after Alfred Werner’s conductivity measurements of a bulk of complexes in solution, it became possible to measure the conductivity of one molecule of a complex (molecular conductivity) squeezed in between gold electrodes using for instance the controlled break junction method (CBJ) (Fig. 8, top). In contrast to Alfred Werner’s conductivity measurements, the experimental efforts of the CBJ experiments are enormous and can by no means be conducted as routinte-type experiments of chemistry. The author and his group are collaborating with the group of H. Riel and E. Lörtscher, IBM Research, Rüschlikon, Switzerland, to determine molecular conductivities of organometallic compounds, like the ones depicted schematically in Fig. 8, bottom (Schwarz et al., 2014) following thus in some ways Alfred Werner, who was always going for the most in the marriage of physics and chemistry, or say it more specifically in coordination chemistry.
Above: Schematic drawing of the measurement of molecular conductivities between gold electrodes using a controlled break junction device (CBJ). Below: Sketch of an organometallic dinuclear iron complex with gold-binding endgroups X, mounted between gold electrodes, which is ready for electrical conductivity measurement by CBJ.
















![Headpages of the dissertations of F. Beddow from 1895 [b] and of A. R. Klien from 1899 [c]. Headpages of the dissertations of F. Beddow from 1895 [b] and of A. R. Klien from 1899 [c].](https://static.elsevier.es/multimedia/0187893X/00000025000000S1/v1_201412221110/S0187893X14705671/v1_201412221110/es/main.assets/thumbnail/gr5.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Left: Beddow’s measurement — directly taken from the PhD thesis written in English — indicating from the magnitude of the electrical conductivity a 3-ion electrolyte, but in contrast to Beddow’s structural assignment presumably corresponding to an unstable [(NH3)5(OH)Co]Cl2 complex. Right: Klien’s measurements of a cis-nitrito(thiocyanato)tetramminecobalt complex — directly taken from the PhD thesis written in German — indicating from the magnitude of the electrical conductivity a 2-ion electrolyte. Left: Beddow’s measurement — directly taken from the PhD thesis written in English — indicating from the magnitude of the electrical conductivity a 3-ion electrolyte, but in contrast to Beddow’s structural assignment presumably corresponding to an unstable [(NH3)5(OH)Co]Cl2 complex. Right: Klien’s measurements of a cis-nitrito(thiocyanato)tetramminecobalt complex — directly taken from the PhD thesis written in German — indicating from the magnitude of the electrical conductivity a 2-ion electrolyte.](https://static.elsevier.es/multimedia/0187893X/00000025000000S1/v1_201412221110/S0187893X14705671/v1_201412221110/es/main.assets/thumbnail/gr6.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![From left to right: a) Beddow’s structural proposal of a μ-oxodicobalt complex as taken from his dissertation (Inaugural-dissertations related to conductivity measurements from Alfred Werner’s group, 1896-1902); b) Proposal of Beddow’s complex to be a [hydroxy(pentammine)cobalt(III)]Cl2 complex; c) Klien’s structural proposal of a pentamine(rhodanato)cobalt(III)]nitrate complex (Inaugural-dissertations related to conductivity measurements from Alfred Werner’s group, 1896-1902), and d) its stereochemical drawing of modern times. From left to right: a) Beddow’s structural proposal of a μ-oxodicobalt complex as taken from his dissertation (Inaugural-dissertations related to conductivity measurements from Alfred Werner’s group, 1896-1902); b) Proposal of Beddow’s complex to be a [hydroxy(pentammine)cobalt(III)]Cl2 complex; c) Klien’s structural proposal of a pentamine(rhodanato)cobalt(III)]nitrate complex (Inaugural-dissertations related to conductivity measurements from Alfred Werner’s group, 1896-1902), and d) its stereochemical drawing of modern times.](https://static.elsevier.es/multimedia/0187893X/00000025000000S1/v1_201412221110/S0187893X14705671/v1_201412221110/es/main.assets/thumbnail/gr7.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
