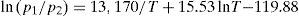Alfred Ditte (1843–1908), a student of Henri Sainte-Claire Deville, carried on a large number of important researches in the areas of mineralogy, chemistry, and thermodynamics, in particular, vanadium and its compounds, properties of aluminum, alumina, and aluminates, the Leclanché cell, dissociation mechanism, and iodic acid and iodates.
Alfred Ditte (1843–1908), alumno de Henri Sainte-Claire Deville, realizó un gran número de investigaciones importantes en las áreas de mineralogía, química, y termodinámica, en particular, vanadio y sus compuestos, propiedades del aluminio, la alúmina, y los aluminatos, la pila de Leclanché, mecanismo de la disociación, y el ácido yódico y los yodatos.
Very little information is available about the early life of Alfred Ditte, his family, and his basic education (Anonymous, 1908). Alfred was born in 1843, at Rennes, northwestern France. After finishing his basic education he entered the École Normale Supérieure where he studied and worked under Henri Sainte-Claire Deville (1818–1881), Henri Jules Debray (1827–1888), and Louis Joseph Troost (1825–1911), and pursued his doctoral studies. In 1864, after receiving his bachelier ès sciences, he was appointed aspirant répétiteur auxiliaire at the Lycée Impérial Charlemagne. In 1870 he received his doctorate (docteur ès Sciences) from the Faculté des Sciences, after successfully defending his thesis about iodic acid and its principals salts, and thermal studies about iodine and iodic acid (Ditte, 1870d). His first post-doctoral paper was the study of the formation and decomposition of hydrogen selenide and hydrogen telluride, using the concepts of dissociation equilibrium developed by Deville (Ditte, 1872). In 1873 Ditte was appointed professor at the Faculté des Sciences de Caen, a position he kept until is return to Paris, in 1888 to occupy the chair of Mineral Chemistry, in replacement of Debray. In 1897 Ditte was elected to the Académie des Sciences replacing Paul Schutzenberger (1829–1897), who had just passed away, and in 1900 he replaced Troost as director of the teaching laboratories at the Sorbonne.
Alfred Ditte passed away on November 7, 1908. He was buried at the Montparnasse cemetery, Paris, and at his request, no speeches were pronounced. Émile Jungfleish (1839–1916) replaced him at the Académie des Sciences.
Ditte worked on many different subjects in chemistry, metallurgy and thermodynamics, among them, the action of metallic nitrates on nitric acid (Ditte, 1879ab), the action of HCl on metallic chlorides (Ditte, 188ab), vanadium and its derivatives (Ditte, 1885ab, 1886abcde, 1887ab), aluminum and its derivatives (Ditte, 1890abc, 1893a, 1899ab, 1900); electrochemistry (Ditte, 1893bc, 1894a), chemical behavior of different saline solutions (Ditte, 1890c, 1896, 1897ab, 1906), uranium (Ditte, 1894c), thermochemistry (Ditte, 1870c, 1894b), etc.
Scientific contributionDitte published over 100 papers describing his scientific findings and several books based on this research and teaching activities (Ditte, 1879c, 1881c, 1891, 1894bc, 1900, 1902, 1906). As customary of all candidates to a position in the Académie des Sciences, he also published a brochure describing his scientific work and results (Ditte, 1897c). He also contributed the chapters about tin and uranium in the Encyclopédie chimique of Edmond Frémy (1814–1894) (Ditte, 1882).
Iodic acid and compoundsAccording to Ditte (Ditte, 1870a), in spite of the work done by several important chemists, the information available about iodic acid and the iodates was quite incomplete and contradictory. Many of the iodates prepared were assumed to be amorphous powders, of ill-defined composition. Humphry Davy (1778–1819) and Joseph-Louis Gay-Lussac (1778–1850) had simultaneously discovered iodic acid in 1813 (Davy, 1814; Gay-Lussac, 1814); in 1832 Georges Simon Sérullas (1774–1832) prepared it in large quantity by decomposing sodium iodate with hydrofluosilicic, H2SiF6 acid (Sérullas, 1832), and in 1843 Eugène Auguste Nicolas Millon (1812–1867) did it by decomposing potassium chlorate with iodine and the potassium iodate with sulfuric acid; he also reported the properties of the acid and of some of its salts (Millon, 1843). For these reasons, Ditte decided to carry on an in depth study of the acids and its salts (Ditte, 1870a).
The first part of the memoir was a description of anhydrous iodic acid (iodine pentoxide, I2O5) and its physical and chemical properties. The pentoxide was a white powder obtained by heating the hydrated variety. It was very soluble in water and in boiling concentrated acids, little soluble in concentrated alcohol, insoluble in ether, chloroform, carbon disulfide, and hydrocarbon essences such as turpentine. Heated to about 300°C it decomposed completely into iodine and oxygen. Its specific gravity was 5.037 at 0° and 5.020 at 51°C. It did not react with hydrogen at room temperature and pressure, but heated to its decomposition temperature in a stream of hydrogen, the iodine sublimed and mixed with the stream of hydrogen and oxygen. Heated in a closed tube up to 250°C, a temperature lower than the dissociation one, the reduction was complete; the iodine separated as crystals and the pressure decreased to about 1.9 atm due of the formation of liquid water. In the presence of platinum sponge, the hydrogen reduction was complete at atmospheric pressure. The pentoxide could be distilled in the presence of bromine, without decomposition (Ditte, 1870ab).
Ditte also described the behavior of the pentoxide in the presence of several reagents, such as Cl2, CO, SO2 (formation of SO3), different nitrogen oxides, H2S, HCl (formation of iodine pentachloride), NH3 (formation of iodine and nitrogen), and water.
The second part of the paper was a description of the hydrated acid (iodic acid), its properties and chemical reactions. According to Ditte, the pentoxide combined with water to give only one compound, containing 94.89% pentoxide, and 5.11% water, corresponding to the formula (IO5, OH). This compound was a colorless, transparent solid, crystallizing as straight rhomboidal prisms. It was soluble in water yielding a colorless viscous and dense liquid, of specific gravity 2.842 at 12.5°C. At 104°C and atmospheric pressure, this solution gradually lost its water until it became a white mass of iodic acid. The acid was not soluble in absolute alcohol, ether, chloroform, carbon disulfide, acetic acid, and hydrocarbon essences. The alcohol, left in contact with the acid, acquired rapidly a deep brown color; crystalline iodine precipitated while the alcohol oxidized. Ditte described the behavior of the acid when treated with many elements such as nitrogen (no reaction), phosphorus (formation of iodine and phosphoric acid), arsenic (formation of iodine and arsenic acid), hot carbon (formation of iodine and CO2), boron (formation of iodine and boric acid) etc. The reaction of metallic elements such as sodium, potassium, magnesium, and aluminum, with a concentrated solution of iodic acid resulted in the formation of hydrogen and iodine; with metals such as bismuth, zinc, cadmium, copper, silver, and iron, resulted in the formation of hydrogen and the pertinent iodate. Contrary to the results reported by Davy, Séroullas, and Millon, mixing sulfuric acid with an aqueous solution of iodic acid did not produce a new compound; heating the solution resulted in the total destruction of iodic acid, with release of oxygen and iodine. Ditte also reported the reaction of aqueous iodic acid with other acid substances, such SO2, H2S, arsenic acid, HCl, HBr, HF, and acetic acid, with metallic oxides, sulfides, cyanides, and hydrocarbons (Ditte, 1870a).
The next section of the memoir was devoted to the preparation of metallic iodates (particularly potassium iodate, KIO3, and bi-iodate, KHI2O6), salts which were not found in nature, except for the small amounts of sodium iodate present in the sodium nitrate fields in Northern Chile. Potassium iodate was prepared according to Gay-Lussac's method: treating aqueous KOH with iodine. The resulting salt appeared as small cubic crystals, of density 2.601, which on heating decomposed into potassium iodine and oxygen. Potassium bi-iodate was obtained by adding the appropriate amount of iodic acid to an aqueous solution of potassium iodate (Ditte, 1870a).
The final section of the memoir reported the measurement of the heat of combustion of iodine to give iodine pentoxide (+110cal/g), the heat of solution in water of iodine pentoxide (−5.7cal/g) and iodic acid (−12.7cal/g), the specific heat of the same (0.1625cal/g.°C), and the heat of reaction of iodine pentoxide with water (0.6cal/g) (Ditte, 1870ac).
In a later work Ditte reported the preparation and properties of a large number of inorganic (Ag, Ba, Ca, Cd, Co, Cu, Fe, Li, Mg, Mn, NH3, Ni, Pb, Sr, Tl, U, and Zn) and organic iodates (methylamine, ethylamine, aniline, toluidine, xylidine, rosaniline, pyridine, quinine, cinchonine, morphine, strychnine, and urea). All of these were well-defined crystalline salts (Ditte, 1890b).
The above research constituted the central subject of Ditte's doctoral thesis (Ditte, 1870d).
Solubility of a salt in its acidAnother research subject was the phenomena that took place when a salt was dissolved in its acid. The first work was related to nitric acid (Ditte, 1879a, 1880, 1881ab). According to Ditte, metallic nitrates contacted with nitric acid behaved in three different ways. The first situation was illustrated by ammonium nitrate. Eighty grams of this salt dissolved rapidly in 12g of fuming nitric acid, with release of heat. The resulting solution could be cooled to +5°C, without solidification, but a little after solidification started, the internal temperature increased to 18°C and remained at that value until all the mass had become solid. The long prisms crystals formed melted at 18°C. Ammonium nitrate and nitric acid had combined to form the acid salt [NO5, NH4O, 2(NO5, HO)], which above 18°C, was a liquid very similar to nitric acid, but non-fuming. It solidified at 18°C but it could be over-cooled to 5°C, without solidification. Addition of further ammonium nitrate and crystallization resulted in the formation of a different salt, having composition [(NO5, NH4O) ((NO5, HO)] (Ditte, 1879a).
Potassium nitrate showed a similar behavior; it dissolved in nitric acid with a significant increase in temperature. The5 solution yielded crystals having the formula [NO5, KO, 2(NO5, HO)], which could not be transformed into [(NO5, KO), (NO5, HO)]. Similar experiments with thallium nitrate and rubidium nitrate yielded crystals of [NO5, TlO, 3(NO5, HO)] and [2(NO5, RbO, 5(NO5, HO)], respectively (Ditte, 1879a).
Magnesium nitrate (originally as hexahydrate) behaved in a different manner; treated with nitric acid and subject to crystallization and melting, formed di and trihydrates. The nitrates of zinc, manganese, aluminum, iron, copper, and uranium exhibited a similar behavior (Ditte, 1879a).
The third category corresponded to the nitrates of Na, Li, Ca, Ba. Sr, Pb, Ni, Co, Bi, Cd, Hg, and Ag. Although soluble in water they became sparingly soluble or totally insoluble in nitric acid at all temperatures. For example, 100 parts of water at 11°C dissolved about 49 parts of lead nitrate. Addition of increasing amounts of nitric acid showed the mounting precipitation of a white precipitate of anhydrous lead nitrate; the concentrated acid retaining less than 0.7g of the salt per liter, even at its boiling temperature. Another example of this category was cobalt nitrate hexahydrate. Heating crystals to their melting point generated a liquid that changed its color from red, to blue, and then to green, as the hydrate lost its water and eventually decomposed into a black deposit of cobalt sesquioxide. The dehydrated salt was soluble in water but sparingly so in nitric acid. Diluting the latter solution showed a gradual and significant increase in solubility (Ditte, 1879a).
An additional paper gave a more detailed information about the solubility of the above nitrates in nitric acid and a summary of the results: (a) The first group of nitrates (K, NH3, Tl, and Rb) were highly soluble in nitric acid and combined with it to form clearly defined salts; (b) the second group of nitrates, after deprived of their water of hydration, were easily soluble in nitric acid; the solubility increased with temperature. Upon cooling they precipitated hydrated nitrates containing less water than the original salt; (c) nitrates slightly soluble or completely insoluble in nitric acid constituted the third category, and (d) the nitrates of sodium and potassium did not belong to the same category (Ditte, 1879b).
A following series of papers analyzed the solubility of salt chlorides in hydrogen chloride (Ditte, 1881ab). These salts could be classified in two categories: (a) the solubility of the chloride increased as the solution became more concentrated in HCl, To this category belonged the chlorides of Hg+1 Au+3, Pt+2, Sb+3, and Bi+3, and the chlorides of Ag and Cu2+. The first group was susceptible of forming a crystalline combination with the acid, decomposable by water; (b) the solubility of the chloride decreased as the solution became more concentrated in HCl (e.g. Ca, Sr, Mg, Cu1+, Ni, Co, Mn). The crystallization of these chlorides was usually accompanied by the formation of hydrates.
The next case studied was more complicated: The influence of the base or the acid on the solubility of its alkaline halide at constant temperature (Ditte, 1896, 1897ab). The experimental results indicated that the successive addition of the acid to the solution of a given neutral salt first decreased the solubility of the halide and then increased it. The position of the minimum and the slope of the two branches depended on the nature of the salt. For example, for the system KF + HF the decrease in solubility was very strong while the increase was more moderate. The solubility of KF in 1000 grams of water at 21°C decreased from 963 grams (no HF) to 289.6 grams when the acid content was 92.5g.
Addition of the base always decreased of the solubility of the corresponding salt. This decrease was continuous and regular when no hydrates were formed at the temperature in question (e.g. KCl, NaCl, KBr, and KI). If a hydrate was formed, the solubility curve comprised two branches, one for the anhydrous salt and another for the hydrate, joined by an almost horizontal stretch (e.g. NaBr, Nal, and KF). The latter portion corresponded to the equilibrium of solutions yielding both the anhydrous and the hydrated salt (Ditte, 1897ab).
The Leclanché cellIn the 1890's Ditte became interested in the Leclanché battery and published a series of papers on the subject (Ditte, 1893abc, 1894). This cell, invented and patented by Georges Leclanché (1839–1886) in 1866, consisted of a carbon cathode surrounded by a mixture of crushed manganese dioxide and a little carbon, enclosed in a porous vase, and a zinc anode immersed in a solution of ammonium chloride. The deoxygenation of the manganese dioxide provided a depolarization effect. This cell, providing 1.4 volts, became very successful in telegraphy, signaling and electric bell work. Ten years later Leclanchée showed that adding 5% of shellac to the mixture of manganese dioxide and carbon, heating everything to 100°C and pressurizing it up to about 300 atm, formed a shock resistant homogeneous solid, having an almost metallic conductivity. Addition of 3–5% of potassium bisulfate contributed to decrease the resistance of the agglomerate by dissolving the oxychlorides that deposited in the pores. The improved pile produced an electromotive force 50% higher than that of the Daniell cell (Leclanché, 1876).
In his first paper, Ditte examined the phenomena that took place in a cell having a zinc and a platinum electrode, immersed in an aqueous solution of 10% NaCl. Several hours after the circuit was closed the upper part of the zinc became covered with a layer of oxide, growing little by little towards a certain depth in the liquid, which separated the latter into two layers having very different compositions. The upper one the saline solution had become alkaline and was free of zinc, while the lower one remained neutral and contained zinc in variable proportion according to the length of the experiment (Ditte, 1893b). From a thermochemical viewpoint, the electrolysis of the salt into chlorine and metallic sodium absorbed 192 calories, the action of the sodium on the water released 86 calories, and the formation of ZnCl2 and its dissolution released 192 calories. The net result was exothermic. Since the density of a 10% aqueous solution of NaCl (1.073) was higher than that of an aqueous solution of less than 9% of NaOH, and lower than the density of an aqueous solution on ZnCl2 containing 8% of the solute, the net result of the electrolytic process was that the NaOH formed at the platinum electrode generated a thin liquid layer that ascended towards the surface while the ZnCl2 formed another that descended towards the bottom. This explained the observation that the upper layer was alkaline. The NaOH reacted with the remaining ZnCl2, and its decomposition generated the gelatinous oxide that was seen to appear on the upper part of the metal (Ditte, 1893b).
If now the platinum electrode was replaced by compacted manganese dioxide, the latter notably decreased the polarization and thickness (and hence the resistance) of the liquid layer that the current had to traverse, and consequently, the phenomena were faster and marked. If the zinc was replaced by cadmium, a metal having a smaller heat of oxidation, the net result was again exothermic, even when taking into account that the process produced the double salt, (2NaCl, CdCl2, 3H2O). Hence it was to be expected that the cadmium-platinum cell would behave like the zinc-platinum cell (Ditte, 1893b).
In the second part of this paper, Ditte discussed the phenomena that took place in the zinc-platinum when the electrolyte was ammonium chloride instead of sodium chloride (Ditte, 1893b). In this situation, the zinc decomposed as before, but its oxide formed and reacted with the alkaline chloride, generating hydrogen and compounds of zinc and ammonia in different proportion, according to the concentration of the solution. These compounds, decomposable by pure water, were soluble in the aqueous solution of ammonium chloride and precipitated as crystals when the solution became saturated. The overall process was exothermic; the density of a 10% aqueous solution of ammonium chloride (1.031) decreased by the dissolution of the ammonia and increased by the dissolution of ZnCl2. As a consequence, the ammonia released at the platinum electrode tended to go up along the strip towards the surface of the liquid while the ZnCl2 moved in the opposite direction. The experimental evidence confirmed these conclusions: the upper part of the liquid became rapidly ammoniacal as the electrolysis went on. The ammonia, on meeting the ZnCl2 could lead to an oxide deposit, because this oxide was soluble both in aqueous ammonia and its chloride. As in the previous case (NaCl), decreasing the length (and hence the resistance) of the liquid layer that the current had to traverse accelerated the reaction. Again, this was easily accomplished by replacing the platinum electrode with a mixture of manganese dioxide and carbon; within one hour the upper layer became strongly ammoniacal, and after 6 to 8 hours it became saturated with the compound (2NH4Cl, 4ZnO, 9H2O), which began to deposit over both electrodes and the walls of the cell, as transparent crystals. After a long enough time, the solid that deposited was a mixture of the above salt and zinc oxide hydrate. Almost the same phenomena were observed when the initial solution contained 15% of ammonium chloride; the only difference was that the compound that deposited was now (2NH4Cl, ZnO) (Ditte, 1893b).
In the next paper, Ditte analyzed the behavior of a cadmium-platinum pile in the presence of an ammonium chloride electrolyte. In his situation the net reaction was also exothermic and the cadmium oxide combined with the chloride to form transparent crystals of 2NH4Cl, CdO, decomposable by water. The upper layer became ammoniacal while the cadmium chloride accumulated at the bottom. Substitution of the platinum electrode by the mixture of manganese dioxide-carbon accelerated the reaction and a white sheath was seen to form on the upper section of the cadmium electrode. This sheath was not composed of cadmium oxide but of crystals of 2NH4Cl, CdO. Mixture of the two layers, in the presence of an excess of ammonium chloride resulted in the- precipitation of small crystals of 2NH4Cl, CdO (Ditte, 1893c).
According to Ditte, all the experimental evidence described above pointed to the same behavior: an exothermic electrolysis of the solution and production of a density gradient going from an upper layer charged with alkali, to a lower one containing a high concentration of zinc or cadmium chloride. The different layers did not present the same resistance to the passage of the current so that the intensity of the electrolysis varied according to the depth. The alkali formed reacted with the metallic salt forming an oxide or crystalline compounds more or less complex, which deposited in certain regions of the liquid (Ditte, 1883c).
The last paper was a long document (30 pages), which repeated in more detail the information reported in the previous papers, for example, the variation with time of the density and composition of the two layers: For a zinc cell with NaCl, after 25 hours, 48 hours, and 14 days of operation, the amount of free NaOH in the upper layer, and of ZnCl2 in the lower layer, increased to 17.7, 51.9, and 173.3mg/100cm3, and 16.7, 20.0, and 66.0mg/100 cm3, respectively (Ditte, 1894).
Dissociation of hydrogen selenide and hydrogen tellurideIn 1887 Hautefeuille had shown that heating a mixture of sulfur and selenium in a sealed tube heated to 440°C produced hydrogen sulfide and hydrogen selenide, while, arsenic trioxide yielded arsenic pentoxide, and sulfur dioxide yielded sulfur trioxide and sulfur (Hautefeuille, 1867).
Ditte decided to study the formation and decomposition of hydrogen selenide (H2Se) between 150° and 700°C to determine its characteristics and the accompanying phenomena. To do so he introduced inside a glass tube an excess of selenium and hydrogen at a certain pressure, sealed the tube and contacted the mixture during a certain time at a given temperature. The glass had a high fusion temperature and was not attacked by the selenium or the hydrogen (Ditte, 1872).
His experiments indicated that the amount of hydrogen selenide produced was a function of the temperature and achieved a maximum about 250°C, the fusion temperature of the metal, This maximum did not change if the pressure of the hydrogen was doubled or by introducing a porous material inside the tube. At any given temperature, it was observed that the amount of acid formed increased until it reached a maximum value after some hours. He investigated the effect of heating all the tube to the same temperature, or submerging in the heating medium only the part containing the selenium. Although in both cases the maximum conversion was the same, in the first case, liquid selenium was seen at the bottom of the tube, or spread as liquid drops on the walls of the same. In the second case, the excess selenium solidified as brilliant crystals in an upper section of the tube.
In another series of experiments, Ditte heated two tubes until the maximum conversion was achieved and then rapidly quenched one of them while letting the other cool slowly to room temperature. His results indicated that in the second case the amount of hydrogen selenide was smaller than that present in the quenched tube. This he interpreted saying that slowly cooling destroyed part of the hydrogen selenide formed, that is, it was an example of dissociation caused by a temperature decrease.
In order to study methodically this phenomenon, he repeated it at different temperature levels. His results indicated that the amount of selenide destroyed increased substantially as the initial temperature was lowered towards 270°C. The maximum amount present was the same, independent if it was obtained by combining selenium with hydrogen (forward reaction), or by destroying by cooling, part the initial amount of selenide (backward reaction). Below the above temperature the rate of both reactions was too slow enough to give credible results (Ditte, 1872).
Another interesting result was obtained when heating the elements in very strong fire. The amount of selenide formed increased up to about 520°C and then started diminishing. If one of two tubes (at 520°C), containing this maximum of hydrogen selenide, was rapidly quenched, while the other was heated to an even higher temperature, it was found that the second tube contained less selenide, that is, an example of dissociation by increasing the temperature.
Ditte then analyzed in more detail the phenomena occurring when heating only part of a tube containing selenium and hydrogen. As mentioned above, hydrogen selenide was formed in the lower part while this compound dissociated in the upper section, with deposition of selenium. The extreme mobility of the hydrogen and the temperature gradient in the tube led to its continuous movement. The hydrogen originating from the decomposition of the selenide combined with selenium in the bottom section, and separated from it in the upper one. Hence, in a certain annular section of the tube selenium was constantly being deposited, it became liquid when the temperature was higher than its fusion temperature, and became solid in the opposite case. If the tube were totally heated to the same temperature, selenium would deposit as liquid drops; if the temperature was below 250°C, it deposited as crystals. This particular annular section did not appear if a gas that did not react with selenium replaced the hydrogen. If the temperature was high enough the selenium vaporized rapidly; if part of the tube was not at the same temperature, the selenium condensed as a red powder that became black if heated (Ditte, 1872).
Ditte found that tellurium behaved similarly; it combined directly with hydrogen forming hydrogen telluride (H2Te). Within a tube partially heated, the acid formed in the colder part decomposed into brilliant white crystals of tellurium, but at a lower temperature than hydrogen selenide. Thus, hydrogen should be considered the mineralizing agent that transformed amorphous selenium or tellurium into their crystalline forms (Ditte, 1872).
Ditte's results were challenged by Henri Pélabon after discovering that hydrogen selenide was highly soluble in melted selenium (Pélabon, 1893). Pélabon repeated Ditte's experiences and observed that during the slow cooling of the contents of the tube, the free surface of the liquid selenium seemed to be in a boiling state with gas being released in decreasing amounts as the temperature went down to the point were the metal became pasty and its surface almost solid. The few gas bubbles being discharged would then erupt through the solid and splash selenium around. Pélabon compared the fracture of the resulting selenium with that of pure selenium and observed that while the surface of the latter was smooth and brilliant, that of the former was rough and full of cavities. Crushing this metal under water resulted in the release of gas having the characteristic smell of hydrogen selenide. These results led Pelabon to make a detailed study of the formation of the acid, in order to find how its solubility in liquid selenium affected equilibrium temperature (Pélabon, 1895ab).
Pélabon's results indicated that the final composition of the gaseous mixture depended on the amount of selenium present in the tube (Ditte had always used an excess of selenium). For this reason, he conducted additional experiences using the minimum amount possible of selenium, but such that some was always present in the liquid state (Pélabon, 1895a). For every temperature, Pélabon determined the value of the ratio p1/p2, where p1 represented the partial pressure of pure hydrogen and p2 the partial pressure of hydrogen selenide in the final mixture, and T the absolute temperature, and correlated them very well with the equation
This equation indicated that the ratio p1/(p1+p2) reached a maximum value at 575°C.
According to Pélabon, since the experiments were conducted using the minimum amount of selenium the above equation represented the true equilibrium of the system in the temperature range 320°–720°C. Ditte had already reported that independently of the direction in which the equilibrium was approached (forward or reverse reaction), the slow rate of reaction below 320°C led to unreliable results. Hence, Pélabon followed the formation or decomposition of hydrogen selenide at temperatures below 320°C for considerable longer times (up to 20 days), and calculated the corresponding values of p1/(p1+p2) for the temperature range 250°C to 320°C. Not surprisingly, he found that the values of the parameter decreased as the temperature was increased, achieved a minimum value at about 270°C, and then began to increase; above 300°C they overlapped the values given by the equation (1). Hence, all the determinations made below 320°C represented states of false equilibrium and not of thermodynamic equilibrium, because they were substantially affected by the solubility of hydrogen selenide in selenium (Pélabon, 1895b).
Pélabon found the same behavior during the formation of hydrogen sulfide by the reaction between hydrogen and sulfur (Pélabon, 1897).
Vanadium and compoundsVanadium and its salts were widely studied by Ditte. His first work was devoted to the study the properties of vanadic acid (Ditte, 1885a). When heating ammonium vanadate in a platinum crucible he observed that the acid was reduced by the gases released, leaving a strongly non-homogeneous colored powder, containing the oxides VO4 and VO3. Treatment of this residue with hot nitric acid, followed by evaporation to dryness and calcinations, transformed it into a yellow red matter composed of pure anhydrous vanadic acid. In contact with air the acid changed its color to dark red while absorbing two equivalents of water (VO5, 2H2O). Placed inside a glass bell having moist walls, the dihydrate absorbed 6 additional equivalents of water (VO5, 6H2O). The later returned to the state of dihydrate when contacted again with atmospheric air. Mixing the anhydrous vanadic acid or its hydrates, with a small amount of cold or hot water, transformed it immediately into a viscous gelatinous paste, completely soluble in water. Treatment with different reagents showed that vanadic acid could exist in three different forms: (a) an ochre red, capable of absorbing the humidity of air and yielding dark red hydrates soluble in water. The aqueous solution, containing about 8g of acid per liter, was precipitated by acids and salts; (b) a yellow variety, unable to absorb water from the air and little soluble in water (about 0.5g/liter), and (c) a crystalline form, highly insoluble in water (about 0.05g/liter). In Ditte's words, “these curious reactions led me to begin a comprehensive study about the combinations of vanadium” (Ditte, 1885a).
Thus he found that under the action of hydrogen, sulfur, ammonium oxalate, arsenic, phosphorus, and SO2, the anhydrous vanadic oxide or its ammonium salt were reduced to a variety of other oxides, which he identified as VO4, VO5, V2O6, V2O7, and V2O8 (Ditte, 1885b). He prepared five different ammonium vanadates and described their properties (Ditte, 1886b), and studied the action of vanadic acid on ammonium phosphate, arsenate, molybdate, tungstate, sulfate, chromate, iodate, oxalate, borate, acetate, perchlorate, chloride, and vanadate (Ditte, 1886c). He also studied the combinations of vanadic acid with oxygenated acids (Ditte, 1886a) and with hydrogenated acids (Ditte, 1886d). Acids of the first category were found to form definite combinations with vanadic acid; some of these could be isolated in the free state as crystals (e.g. sulfuric, phosphoric, arsenic, iodic, and molybdic), others (e.g. tungstic, silicic, oxalic, and tartaric) only as salts. Acids of the second category (e.g. SO2 and the hydrogen halides) reduced vanadic acid forming the crystals of (2VO3I, 3HI, 20H2O), (VO3I, HI, 8H2O), (VO3Br, 3HBr), and (VO3Cl, 4H2O), according to the halogen. All of these compounds were derivatives of hypovanadic acid VO4 and not of the oxide VO3. Ditte found that the alkaline halides also reacted with vanadic acid yielding hypovanadates such as (2VO4, KO, HO) (Ditte, 1886e).
Ditte also prepared and described the properties of a large number of alkaline and metallic vanadates (e.g. K, Na, Li, Mg, Ba, Ca, Co, Ag, Cu, Pb, Zn, Ni, and Cd), the vanadates of methylamine, ethylamine, the double vanadates of ammonium, and ammonium-magnesium (Ditte, 1887a) and reached the general conclusion that all vanadates prepared by the dry or wet procedure corresponded to one of the following three simple formulas: (a) 3VO5, MO; 2VO5, MO; or 2VO5, 2MO, for the acid vanadates; (b) ) VO5, MO, for the neutral vanadates, and (c) ) VO5, 2MO; VO5, 3MO; or VO5, 4MO, for the basic salts.
Ditte published a long memoir (more than 80 pages) summarizing all his findings about vanadium and its compounds, as well as giving a more detailed description of their preparation and properties (Ditte, 1887b).
Aluminum and its compoundsIn his well-known memoir about aluminum, Deville showed that the metal was hardly attacked by cold diluted sulfuric acid and was not affected by nitric acid; however, the formation of hydrated alumina released 195.8cal, hence the metal should be able to decompose water at diluted acids at room temperature (Deville, 1855). In the case of pure water, it was possible to accept that during the first moments of the reaction the metal became covered with a layer of alumina that protected it from further attack by the water. Nevertheless, this explanation was unacceptable when operating with acids capable of dissolving alumina instantly and forming salts soluble in water. The results of the Ditte's experiences seemed to prove that the metal in contact with acid solutions that seemed unable to attack it, became covered by a layer of gas that hindered its contact with the liquid. Removal of this coating resulted in dissolution of the metal. Ditte gave several examples that proved is contention: Sulfuric acid containing 2.5g of SO3/100g of water, seemed to be without action over a strip of aluminum, but if the experience was continued for several days, bubbles were seen to disengage from the free edges, the brightness of the metal slowly disappeared until it became matt, and finely grained or covered by shimmering were seen that emphasized all the irregularities. Simultaneously, hydrogen bubbles were seen all over the surface. Hence, aluminum behaved like zinc; the hydrogen generated forming an impenetrable layer, the more adherent, the more polished the surface (Ditte, 1890ab).
A similar behavior was observed in the presence of certain metallic chlorides. For example, addition of traces of platinum chloride to the sulfuric acid resulted in the immediate formation of large bubbles of hydrogen; the reduced platinum deposited on the surface of the aluminum and spiked its surface with small asperities that did not allow the hydrogen to form a continuous layer over the surface. An additional interesting phenomenon took place. The rate of generation of hydrogen and the accompanying formation of aluminum sulfate was seen to decrease rapidly and after same days, a new white granular solid was seen to deposit increasingly on the walls of the vase, as well as on the surface of the aluminum. This new solid was composed of a basic alumina sulfate. This sulfate did not seem to attack aluminum, but, once again, addition of traces of a chloride repeated the effects seen with a mixture of chloride and dilute sulfuric acid: the aluminum strip became covered with large bubbles of hydrogen, while the metal dissolved. This process explained what happened when aluminum was contacted with certain metallic sulfates, such as cupric or zinc sulfate. The latter alone, did not seem to act on aluminum, even in the presence of sulfuric acid. Addition of traces of a chloride, in the absence or presence of an acid, resulted in immediate attack. Similar results were obtained with nitric acid and organic acids (acetic tartaric, citric, oxalic, etc.). All these reactions were markedly exothermic (Ditte, 1890abc).
In another publication of the subject, Ditte repeated his claims that the reason was aluminum seemed to be not attacked by water and acids was the fact that it became covered immediately by a highly adhesive layer of alumina, hydrogen, or NO2, that suppressed contact with the surrounding liquid layer (Ditte, 1899e). This effect could be eliminated by carrying on the operation with boiling water or acid, or adding to the solution an aluminum salt soluble in water. Similarly, although the metal seemed to be not attacked by a solution of an alkaline (e.g. NaCl, KBr)) or alkaline-earth (e.g. CaCl2, MgBr2) halide, addition of a few drops of an acid such as acetic acid, was enough to observe the release of hydrogen bubbles (Ditte, 1899e). Alkaline carbonates showed a different behavior; they strongly attacked aluminum, releasing hydrogen and CO2, and a large amount of heat (164cal). The CO2 reacted the alkaline carbonate transforming it into bicarbonate.
An additional paper described the alteration of the aluminum and its copper alloy caused by atmospheric oxygen and CO2. In addition to the scientific aspects, Ditte was also interested in finding how these processes affected the possible industrial applications of the metal (Ditte, 1899b). Submerging partly an aluminum strip in an aqueous solution of NaCl and acetic acid, showed a strong chemical attack on the section located immediately above the liquid surface. After a period of time, dependent of the thickness of the strip, this section was strongly corroded and separated from the submerged portion. In the upper section, where the oxygen strengthened the corrosive action of the salt and acetic acid, the strip became perforated while the aluminum chloride and acetate dissolved in the solution. The action of atmospheric CO2 was similar to adding acid to the solution; the non-submerged section of the strip became covered by a thick gelatinous layer of aluminum oxide, while the chemical attack was slower in the submerged section. These results explained what happened when aluminum was in simultaneous contact with the atmosphere and with saline, brackish, or seawater: a thick layer of alumina, mixed with other soluble or insoluble salts, covered the metal. If the aluminum were removed from the liquid without eliminating this layer, the alteration would slowly continue on; the attached material being hygroscopic would facilitate the attack (Ditte, 1899b).
According to Ditte, since aluminum salts did not seem to have a sensitive toxic effect, it was of interest to study the wear resulting from using aluminum ware for handing liquids containing acid and saline materials, particularly in objects intended for military use, such as mess kits, pot, and water tanks, usually made of an aluminum-copper alloy. Ditte subjected these objects to the action of heat, dilute sulfuric acid, alkaline carbonates, saline water, and saline water. His results indicated that, in general, the surface of the object became altered, and this spoilage continued even in a dry state, by the action of aluminous lumps composed of alkaline substances, due to a continuous series of exothermic reactions that took place in every part of the metal covered by theses lumps. In addition, there were also electrochemical alteration produced by contact with other metals; a particularly strong in the case of the ware were the handles, rings, chains, etc., were attached to the main body by rivets having a different composition (Ditte, 1899b).
In another work Ditte investigated the decomposition of potassium and sodium aluminates in the presence of alumina or CO2, as well as the industrial production of alumina (Ditte, 1893a). It was known that alumina hydrate dissolved in a cold solution of KOH but this solubility was quite different depending on the nature of the alumina considered. The nature of the hydrate initially precipitated from one its salts were different from what it became afterwards, if kept under water. For example, consider the alumina precipitated by a slight excess of ammonia, from a cold diluted sulfate solution. After filtration and washing the precipitate to eliminate the accompanying salts, the precipitate appeared as a transparent voluminous jelly; if kept under water, it changed its appearance little by little, its volume decreased and became white matt, and could be easily filtrated. Examined under the microscope it looked like very small white non-crystalline grains. These grains, dried with dry air at room temperature, left a white powder having the formula Al2O3, 3H2O. The same hydrate could be obtained by decomposing certain aluminates with water, but now it crystallized like the gibbsite (hydroargillite), found in nature, granular or crystallized. The latter variety was the more soluble in aqueous KOH. With highly concentrated KOH solutions, the dissolution became a chemical reaction with formation of potassium aluminate trihydrate.
Ditte studied in detail the behavior of the fresh precipitated gelatinous alumina, having the largest solubility in KOH, and observed the following characteristics: (a) Addition of a KOH solution to an excess of alumina. Initially all the alumina dissolved but after a short time, it begun to deposit until it reached the concentration corresponding to the solubility of crystallized alumina, at the temperature of the experiment. This transformation went through an intermediate state of potassium aluminate; (b) in the presence of a slight excess of KOH, the solution remained unchanged indefinitely, but behaved like the previous situation upon heating or addition of a small amount of alumina hydrate crystals (Ditte, 1893a).
In the second part, Ditte examined the effect of CO2. The atmospheric gas was able to decompose the solution of potassium aluminate with formation of crystals of hydrated alumina. Continuous addition of a saturated solution of potassium carbonate resulted in the formation of a substantial amount of a precipitate corresponding to the composition of a double carbonate of potassium and alumina (5CO2, 3K2O, 2Al2O3). Bubbling CO2, through an alkaline solution of potassium aluminate, crystallized alumina hydrate precipitated or not, depending on the excess of KOH present at the beginning. In the opposite case of a solution rich in aluminate and poor in KOH, the water destroyed the aluminate, precipitating crystals of alumina hydrate. The KOH released became potassium carbonate and gave place to the formation of the double carbonate of potassium and alumina.
In general, sodium hydroxide exhibited the same behavior as KOH, except that no sodium crystalline aluminate was formed. Solution of the alumina yielded a very viscous liquid that could only be crystallized by seeding with solid sodium aluminate in the temperature range −5° to 20°C. Purification of the crystals indicated that they were small transparent needles, highly deliquescent and very soluble in water (Ditte, 1893a).
Ditte used his results to explain the phenomena that took place during the industrial manufacture of alumina. The mineral bauxite was treated with NaOH and the sodium aluminate was then mixed with a small amount of alumina. The reaction occurred at room temperature helped by agitation. The resulting alumina hydrate crystals precipitated and washed easily. As explained above, it was enough to seed the alkaline solution of aluminate with a few crystals of alumina hydrate to establish the equilibrium state leading to the precipitation of the alumina. This also explained why this alumina hydrate was in a very pure state. The impurities of silica and phosphate present in the bauxite were converted into salts of the alkali employed, and remained in the mother liquor (Ditte, 1893a).