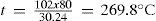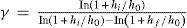Nicolás Clément (1779-1841) fue un brillante científico e industrial, aun cuando no tuvo una educación formal. Solo, o en colaboración con su suegro, Bernard Desormes, desarrolló la teoría de la fabricación del ácido sulfúrico por el método de las cámaras de plomo, descubrió la fórmula correcta del monóxido de carbono y del disulfuro de carbono, y la composición del pigmento lapislázuli, así como realizó la fabricación industrial del alumbre, sulfato ferroso, alcohol, etc.
Nicolas Clément (1779-1841) was a brilliant chemist and entrepreneur, although he lacked a formal education. Alone, or in collaboration with his father-in-law, Bernard Desormes, he developed the theory of the manufacture of sulfuric acid by the lead chamber method, discovered the correct formula of carbon monoxide and carbon disulfide, and the composition of lapis lazuli, as well as carrying on the industrial manufacturing of alum, ferrous sulfate, alcohol, etc.
Nicolas Clément was born in Dijon on January 12, 1779, to Jean Rebelet and Nicolas Clément, who worked as a keeper (porte-clefs) of the Saint-Pierre gate. After finishing his basic education at Dijon, at the age of 15, the modest financial conditions of his parents forced him to leave his studies and take a low government job making an inventory of the books of several libraries that had been confiscated by the Revolution authorities. While doing so he discovered the books of Joseph Priestley (1733-1804) (Priestley, 1774-1777) and Jean-Antoine Nollet (1700-1770, abbé Nollet) (Nollet, 1747) on chemistry and physics, which so attracted his attention that he devoted many hours to their reading (Dunoyer, 1842; Payen, 1842).
A well-to-do uncle, a former senior attorney at Châtelet de Paris who continued to practice his profession in Paris, offered him a clerk position at his office, where he was to copy and file the acts of the proceedings. During his free time Clément continued to deepen his chemical knowledge by reading Jean-Antoine Chaptal's (1756-1832) book on chemistry (Chaptal, 1790) and taking the public courses given at the Jardin des Plantes. After three years of work he was lucky enough to win the lottery, an event that allowed him to abandon his job, live without financial worries, and dedicate all of his time to science. The lectures at the Jardin des Plantes and his strong personality allowed him to develop a close relation with the first group to be promoted at the École Polytechnique, as well as many of the leading scientists of the time, among them, André-Marie Ampère (1775-1831), François Arago (1785-1853), Joseph-Louis Gay-Lussac (1778-1850), Louis-Jacques Thenard (1777-1857), Alexander von Humboldt (1769-1859), and particularly Bernard Desormes (1777-1862), who was working as assistant in the laboratory of Louis-Bernard Guyton de Morveau (1737-1816) at the École Polytechnique. Eventually Nicolas Clément married Desormes's daughter. A lasting scientific collaboration was established between the two men, and Clément often used the name Clément-Desormes, a decision that led many historians to assume that Clément and Desormes was the same person (Lemay, 1949).
Desormes introduced Clément to Bernard Courtois (1777-1838) (Note 1), who let him know his discovery of iodine and asked him to continue the research on this element that he had initiated (Wisniak, 2002). As a result, it was Clément-Desormes who on November 29, 1813, announced the discovery of iodine to the Académie des Sciences (more details below) (Anonymous, 1813; Clément, 1813). The detailed results were later published under Courtois's name (Courtois, 1823).
Clément-Desormes became a successful industrialist who was owner or partner in several large chemical companies and a sought-after industrial consultant. Napoleon appointed him to direct the Imperial sugar industry, which he had ordered to be installed in Rambouillet and which was destroyed shortly after its inauguration, during the events of 1814. Forced to break up with this enterprise, he quickly mounted new ones where the principal activities were chemical. He established successively an alum refinery, in association with Jean Baptiste Marie Chaptal (1782-1833; the son of Jean-Antoine Chaptal), and an industry of alcohol from potato starch, in society with Jean-Baptiste Say (1767-1832) (Dunoyer, 1842). During his later years Clément acquired the habit of traveling during the summer to study the principal business enterprises in France and abroad. As a consequence of his competence, he was much sought as a consulting engineer by the Salines de l’Est and the Compagnie de Glaces de Saint·Gobain, among others. As a result of his contributions the latter company made very large profits. His contract with Saint-Gobain provided him with a percentage of the benefits, which proved to be significant. Unfortunately for him, the Compagnie believed that it could now go on without Clément's assistance, and consequently, when it did not renew his contract when it came to an end. Clément was so mortified by this affront that it affected his health and led to a brain stroke that left him mentally incapacitated and to his early death on November 21, 1841 (Lemay, 1949).
Clément's well-known industrial activities led the government to appoint him to the chair of Applied Chemistry (Arts et Métiers), at the Conservatoire des Arts et Métiers. This chair was one of the three in higher technical education established by the French government in 1819. Together with Thenard and Arago, Clément was among the first to promote the idea of free education at the Conservatoire. There he created a course in industrial chemistry, which he taught for about 20 years and used to transmit his extended experience and to emphasize the application of the thermodynamics of steam to the understanding and development of steam engines. During his many trips to England he was favorably impressed by the services rendered to industry by civil engineers, men, which he believed, combined very efficiently empiricism with theoretical knowledge. As a result, he promoted the establishment of a similar profession in France.
Clément was appointed Chevalier of the Légion d’Honneur and member of the General Council of Arts et Manufactures (Lemay, 1949).
Scientific achievementsClément published some 30-odd papers and reports devoted to pure and applied science, covering areas such as the manufacture of sulfuric acid, alum, and ferrous sulfate, distillation of carbon, the conversion of heat to work in heat engines, the use of the of steam power on the hydraulic ram, conservation of grains, and illumination with carbon gas (Clément-Desormes, 1819; 1820; 1834).
The scientific work of Clément alone and in collaboration with Desormes, was numerous and quite varied. In the period 1801-1802 they determined the exact composition of carbon monoxide and carbon disulfide (Clément, 1801; Clément & Desormes, 1802; Desormes & Clément, 1802). The first observations about carbon monoxide were made in 1776 by Joseph Marie François de Lassone (1717-1778) during his studies on the reduction of zinc oxide by carbon, and the gas assumed to be a mixture of hydrogen and hydrocarbons because it burned with a blue flame. In 1796 Joseph Priestley treated iron oxide scales with carbon and obtained the same gas, concluding that the scales contained water and that his gas was dephlogisticated flammable gas. In the same year William Cruikshank (1745-1800) recognized that the combustion of carbon oxide did not generate water but carbon dioxide, and concluded that it actually was an oxide of carbon. Further experiments done in Holland also repeated the claim that carbon oxide was a hydrocarbon. Guyton de Morveau suggested to Desormes and Clément to repeat the experiences to try to clarify the discrepancies. Their results indicated that distillation of ordinary coal yielded first a mixture of hydrogen, burning blue, and carbon dioxide; afterwards no more gas was released and, particularly, no water was observed to be produced at any time. No gas was released when heating zinc oxide alone but zinc oxide mixed with carbon produced carbon dioxide and carbon oxide. Chemical analysis indicated that the latter contained 47% wt oxygen and 53% wt carbon. Desormes and Clément established the correct composition of the gas by detonating it with oxygen in a eudiometer, and concluded that the essential condition for the formation of carbon monoxide from carbon and carbon dioxide was their contact at high temperature (Clément, 1801; Clément & Desormes, 1802; Desormes & Clément, 1802).
Another important contribution was the determination of the correct composition of carbon disulfide. This compound had been discovered by Wilhelm August Lampadius (1772-1842) in 1796 during his studies on the roasting of a mixture of iron pyrite with sulfur with the purpose of eliminating the maximum possible amount of sulfur from the mineral. He obtained a liquid product which he named liquid sulfur or alcohol sulfur. Lampadius obtained the same liquid when distilling fossilized wood mixed with iron pyrite. He reported that the new compound had a penetrating smell, high volatility, flammability, and solubility in alcohol, and a density of 1.300. He believed that it was composed of hydrogen and sulfur and for this reason he also named it hydrogenated sulfur. In 1802 Clément and Desormes prepared carbon disulfide by passing sulfur vapors over a bed of red-hot carbon (Clément & Desormes, 1802). Their chemical analysis indicated that the compound was actually a mixture of carbon and sulfur; hence they named it carburated sulfur. Amédée Berthollet (1780-1810), repeated the experiments and claimed that the correct composition was that reported by Lampadius because carbon could not exist without containing hydrogen and carbon disulfide was identical to hydrogen sulfide (Berthollet, 1807ab). The question of the composition was examined again in 1812 by Cluzet, who took Berthollet's position (Cluzet, 1812). The discrepancy was settled by a series of experiences done by a committee formed by Louis Nicolas Vauquelin (1763-1829), Claude-Louis Berthollet (1748-1822, the father of Amédée Berthollet), and Thenard, who confirmed the correctness of Clément and Desormes conclusion that carbon disulfide contained about 14-15% wt of carbon and 85-86% wt of sulfur (Vauquelin, et al., 1812).
AlumAlum was employed principally as a mordant in the dyeing and printing of textiles and in minor applications such as the preparation of leathers and manufacture of paper. In the mideighteenth century France's own production of this material was negligible and therefore it was imported in significant amounts. Alum of the finest quality, for use in high grade dyeing, came from the famous papal works at Tolfa, near Rome, while poorer alum for common use was brought from Britain, Liège, Swede, and the eastern Mediterranean. The minerals used for manufacturing alum were generally pyrite schists, which sometimes contained bitumen. In the first case it was enough to calcine the material over a bed of fuel to form alum; in the second, bitumen provided the necessary fuel. In either case, the calcined material was left weathering for months in heaps (Chaptal, 1806).
Synthetic alum was obtained in France from the hitherto rejected residues resulting from copperas (ferrous sulfate) production in the departments of the Aisne and the Oise. In 1804, Clément, Desormes, and Joseph Michael Montgolfier (1740-1810) established a large factory for this purpose at Verberie in the Oise. Initially the French product brought a lower price than the Italian one because the consumers believed it to be of lower quality. This preference was not based on actual testing, but solely on prejudice for certain purely exterior characteristics; for example, the color resulting from inert impurities present in the imported alum was considered essential; consequently, alum that was too pure was not accepted. The research work of Clément and Desormes established clearly that there was very little difference in quality between synthetic alums and the one coming from Italy (Clément & Desormes, 1806ab). The only difference was one of appearance, the Italian alum crystals were usually covered by a white rose layer, which Clément and Desormes found to be a mixed salt of aluminum and potassium sulfate. This mixed salt seemed to originate from the fact that during the low temperature lixiviation and evaporation of the Italian raw material, alum dissolved an excessive amount of base, which afterwards precipitated on top of the alum crystals. By a brilliant marketing stroke Clément and Desormes decided to overcome consumer resistance by adding the missing salt to their alum to achieve the appearance of the well-regarded Roman variety, and then sell it under that name. By the beginning of 1806, when they disclosed their practice to the Institute (Clément & Desormes, 1806b), they had successfully sold 153,000 pounds of ‘Roman’ alum in eighteen months, and Clément later recalled that by this subterfuge, conducted in a plant near Jouy, they made profits of 100,000 francs in a year with a capital of 1000 ècus (3000 francs), and could scarcely keep up with the demand. By the final years of the Empire, France had not only become self-sufficient in copperas but also virtually so in alum as well.
UltramarineUltramarine blue is the inorganic pigment derived from the naturally occurring mineral, lapis lazuli, and consisting primarily of a double silicate of aluminum and sodium, with some sulfides and sulfates. This semiprecious stone was used as a pigment in illumination of manuscripts, in murals, and in painting in general. The pigment was extracted from the mineral by a very laborious process. Up to the end of the eighteenth century some chemists believed that the blue color was due to copper oxide, and others to iron, until in 1804 Clément and Desormes succeeded in doing a careful analysis, which proved that the blue color was not due to iron (Clément & Desormes, 1804; 1806ab). They found that the iron oxide found in the analysis originated from iron sulfides com-monly present in lapis lazuli, but these iron compounds did not belong to the primary constituents of ultramarine because they disappeared during the extraction of ultramarine from the mineral. The essential components were 35.8% sili-ca, 34.8% alumina, 23.2% soda, 3.1% and sulfur. Clément and Desormes ended their paper with the hope that their analyti-cal results would be used to synthesize the pigment.
This discovery went without application until 1824, when the Société d’Encouragement pour l’Industrie Nationale offered a prize of six thousand francs to anyone who could suggest a process for making artificial ultramarine with all the properties of the substance prepared from lapis lazuli, and at a cost not exceeding three hundred francs per kilo. The prize was not awarded for four years because all the submissions were imitations based on cobalt or Prussian blue without regard for the analysis of the gem published by Desormes and Clément. On February 4, 1828, the prize was awarded to Jean Baptiste Guimet (1795-1871), who described a process he had secretly developed in 1826. A few years later Guimet resigned his official position of commissaire in the gunpowder (Poudres et Salpêtres) administration in order to devote himself to the commercial production of pigment in a factory he established at Fleurieu-sur-Saône. His product sold for four hundred francs per pound, about 10 percent the price of lapis lazuli. An interesting fact is that, independent of Guimet, Christian Leopold Gmelin (1788-1853) discovered a slightly different method based on the analytical results of Desormes and Clément, which he published only one month after Guimet. Although Gmelin claimed priority to the discovery to the Société d’Encouragement pour l’Industrie Nationale, the Société stood by its decision.
IodineIodine was discovered in 1811 by Bernard Courtois (1777-1838), a Parisian manufacturer of saltpetre from the ashes of calcined algae (vareck). These ashes, rich in potassium and sodium salts, were lixiviated with water to dissolve the soluble components, and the solution concentrated and evaporated. The concentrated solution contained the iodides of sodium and potassium, sodium chloride, sulfate and carbonate, cyanides, polysulfides, as well as the sulfites and hyposulfites that originated during the increased reduction of the sulfates during the calcination process. Courtois, like all later manufacturers, used sulfuric acid to destroy the sulfur derivatives. Towards the end of 1811 Courtois observed that when the copper vessels he used for concentrating the potassium carbonate were washed with hot sulfuric acid they became pitted and a violet vapor appeared that condensed forming bright crystalline scales, having the color and luster of graphite. A more detailed check verified his assumption that corrosion was due to a new body contained in the ashes. The violet vapors were easily produced just by adding excess sulfuric acid to the mother liquors.
Courtois's curiosity was stimulated by this discovery and led him to perform some preliminary tests on the new substance. He found that it melted at about 70°C generating violet vapors and was unaffected by high temperature, carbon, and oxygen, but reacted with hydrogen forming something like an acid; phosphorus yielded the same result. He tried its action on several metals and found that it attacked them without effervescence. It also combined with oxides yielding products that were soluble in water.
Courtois was very busy with his commercial activities and did not have the time or the facilities to investigate the matter further. Thus towards 1813 he requested from his two Dijon compatriots, Desormes and Clément, to continue his research and communicate his findings to the scientific community. On November 29, 1813 Clément read a memoir to the Académie des Sciences, France, in Courtois's name, announcing the discovery of the substance (Anonymous, 1813) and describing the procedure used to prepare it. According to Clément, the mother liquor of the lyes of vareck contained a large amount of a singular and curious substance that could be easily separated by adding sulfuric acid and heating. The substance (named iodine afterwards) precipitated as a bright black powder and generated a violet vapor, which condensed in very brilliant crystalline laminar sheets having brightness equal to that of crystallized lead sulfide. It looked like a metal, had a specific weight about four times that of water, a smell similar to that of oxymuriatic acid (chlorine), and it stained paper and the hands red-brown. It was neither acid nor alkaline and volatilized at about 75°C. It sublimated easily, producing very bright laminar sheets that were a little soluble in water, much more in alcohol, and very much in ether. Heated alone, or in the presence of air or carbon, did not affect it, but hydrogen gave place to a substantial change. When a mixture of iodine vapors (wet or dry) and hydrogen flowed through a tube heated red, the violet color disappeared almost completely and a colorless gas was recovered, part of which dissolved in water. The aqueous solution was acid and colored dark red.
Clément considered that the reaction of hydrogen with iodine was probably the most remarkable of the observed properties and would probably shed light over its nature. He indicated that Gay-Lussac considered the acid to be the direct combination of hydrogen and iodine (HI).
The action of phosphorus on iodine was very violent, it took place instantly at room temperature, was very exothermic and generated a large quantity of gas, which proved to be very acid and very flammable (HI). If the iodine was wet, the reaction of phosphorus and iodine produced a red substance (PI3). A similar behavior, although less energetic, was observed with sulfur.
The new substance was strongly active with metals (mercury, iron zinc, tin, and antimony); it combined with them and their oxides at room temperature and the resulting product was soluble in water. Iodine combined very easily with sodium and potassium hydroxide. The action of ammonia gave place to an explosive powder (NI3) that yielded the violet color at the time of the explosion.
Clément finished his remarks reporting that Gay-Lussac believed that iodine was a simple substance analog to chlorine, and like the latter, it gave place to particular acids.
Gay-Lussac investigated this new substance (which he named “X”) and brought to the attention the analogy that existed between its chemical behavior and that of chlorine. Gay-Lussac declared that the body was a new chemical element, which he named iode (from the Greek ιoειδηζ) because of the violet color of its vapor (Gay-Lussac, 1813ab). Almost simultaneously, other chemists such as Humphry Davy (1778-1819) and Louis Nicolas Vauquelin (1763-1829), added information about the new element, reporting new and easier procedures for detecting its presence, and preparing derivatives.
Courtois is also considered to be the first industrial manufacturer of iodine. During the British blockade of France (Napoleonic wars) he used his facilities not only to produce the saltpetre required for gunpowder, but also for manufacturing iodine from the residues. With the help of Clément and Desormes he developed a small industrial process to extract iodine by treating the mother liquors of calcined brown algae with chlorine, by the reaction
The iodine thus produced, somewhat wet, was crude iodine. It was sublimated and then transformed into iodides.
In 1815, after the demise of Napoleon, French ports were opened to the import of many products, among them, saltpetre from India, fact that meant the ruin of the French nitrières artificielles. Courtois, who had invested all his fortune in this industry, was completely ruined. He tried to improve his lot by manufacturing larger amounts of iodine, but the market was far from enough to offer the appropriate benefit. Courtois was able to manufacture iodine only in small amounts, selling it for 600 francs per kilogram. The growing interest on iodine as a therapeutic agent led many to think about constructing an industry for large-scale production. Thus, in 1824, the baron d’Aigremont, under the advice of Clément-Desormes, established the first factory and put Tissier, his future son-in-law, to manage it. Within a few months Tissier had produced 120 kg of iodine prized at 200 francs per kilogram. Shortly afterwards, Tissier became associated with Couturier, a manufacturer of refined soda in Cherbourg, and started manufacturing potassium chloride and sulfate, and other salts contained in the vareck ashes. In this new facility they were able to produce 400 kg iodine per year, at a price of 100 francs per kilo. These low prices meant the economical ruin of Courtois and forced him to sell his premises to Coutorier. Anyhow, his discovery did not go unnoticed: In 1831 he received the Montyon prize, awarded to those who had improved the art of curing.
CatalysisDesormes and Clément were the first to propose a rational theory for the homogeneous catalytic effect of nitrogen oxides in the lead chamber process for the manufacture of sulfuric acid (Desormes & Clément, 1806). They did so by establishing for the first time a quantitative relation between the sulfur dioxide, oxygen, and oxides of nitrogen that participated in the process. There were different opinions on the advantages of using potassium nitrate in the fabrication of sulfuric acid. Some believed that the high temperature produced by its deflagration resulted in the formation of sulfuric acid; others thought that the nitrate contributed the complementary amount of oxygen for the combustion started initially by air; while others believed that it could decompose water, etc. Desormes and Clément decided to look only at the first two hypotheses, which seemed the most plausible. The first one was discarded immediately because the addition of potassium nitrate and sulfur was followed by the addition of clay and water, which resulted in a diminution of the temperature, clay by making the combustion slower, and water by generating vapor. It was also known that sulfur, which burned alone at temperatures above 1000°C, did not generate sulfuric acid at all. The second hypothesis was also unacceptable because it presupposed that the oxygen disengaged from potassium nitrate was sufficient for converting all the sulfur dioxide produced into sulfuric acid, but a simple mass balance proved that this was not true. Although the precise amounts in which every substance participated in the reaction were unknown, it was easy to see that the potassium nitrate employed could hardly provide more than 10% of the oxygen required for converting sulfur dioxide into sulfur trioxide. In addition, a visual observation of the burning of a mixture of sulfur, potassium nitrate, and wet clay, indicated that the nitric acid did not decompose completely and that an important part of NO gas went into the lead chamber with the sulfur dioxide; its typical color was clearly visible. They wrote: “This is the observation, which affords the key to the true theory, and it is in following up the consequences that we find the clear explanation of the production of sulfuric acid”. They were certain that the combustion generated a mixture of NO, SO2, water vapor, and nitrogen from the air, and probably the part of the oxygen that had escaped the action of sulfur. Their experiences indicated that NO and SO2 could not coexist together without reacting. The lower temperature existing downstream the lead chamber resulted in condensation of part of the vapor; the resulting mist contained the sulfuric acid generated, and offered a very large number of contact points that increased the conversion to acid (Desormes & Clément, 1806).
After production of the first amount of sulfur trioxide, the remaining gas mixture was composed of NO, sulfur dioxide, and residual air containing less oxygen. The NO would necessarily convert into nitrogen dioxide, which would then de-compose again reacting with a second portion of sulfur dioxide, until all of this oxide or the atmospheric oxygen became exhausted. After the sulfur dioxide had been converted to-tally into trioxide, the remaining gas was composed mainly of a large amount of nitrogen and nitrogen oxides. Hence, it was clear that nitric oxide was only the instrument for the total oxidation of sulfur; it was its base, NO, that took the oxygen from the atmospheric air and transferred it to SO2, time after time (Desormes & Clément, 1806).
Clément and Desormes's experiments revealed that the reaction between NO2 and SO2 was a series reaction, the two gases first forming a compound, observed as crystals, which was then decomposed by water. Their total theory can thus be represented as follows:
This explanation may be considered the first time that an intermediate compound is assumed to play a part in a catalytic reaction.
To prove their point, Desormes and Clément simulated the process taking place in the lead chamber by feeding into a glass vessel a mixture of sulfur dioxide, air, and a small quantity of nitric oxide (about 5% of the weight of SO2) and noting that “the oxide grows red (forming NO2), and spread itself throughout the space; then clouds of white fumes roll across the vessel, and deposit themselves on the walls in shinning, stellated crystals. These thick whirls of sulfuric acid were succeeded by clearness; and if at this moment a little water was added, the crystals of acid dissolved with great heat, and the nitrous oxide gas, again becoming free, changed once more into the red acid, and the same phenomena began anew until all the atmospheric oxygen was used up, or all the sulfurous acid burned. The remaining gases are just as we indicated in our conjectures; for the color of the nitrous acid appears with almost all its initial intensity; after the complete operation there is no more odor of sulfurous acid, but much nitrogen, and only sulfuric acid on the walls of the vessel. If during the combustion of SO2 there is a large contact between the added water and the gas, due to intensive mixing or the presence of a large amount of water, then the reaction becomes slower and incomplete because liquid nitric acid is formed; the latter has little action upon the gas being burned”.
Desormes and Clément closed their paper with the statement that “this experience leaves no doubt regarding the theory of fabrication of sulfuric acid, which is only a simple exposition of the facts… This theory offers us the means to improve our knowledge about the proportion of the elements of sulfur dioxide and sulfuric acid; it gives us the hope of finding the same mode of action in other chemical operations ill-understood…” (Desormes & Clément, 1806).
Desormes and Clément's paper received a wide circulation. It was refereed by Berthollet and Guyton de Morveau and presented to the First Class of the Académie with the recommendation that it be published in the Journal des Savans Étrangers. It was published in the Annales de Chimie and in the Journal des Mines, and translated in the most important British and German scientific journals.
According to Smith (Smith, 1979) Clément and Desormes plainly identify the chamber process for the production of sulfuric acid as a catalytic one, stressing their finding that the nitrous gases were not consumed but served rather as ‘an ‘instrument’, and they predicted that further such reactions perhaps awaited discovery. The correctness of this prediction was proven shortly thereafter, as will be discussed now.
In 1821 Thenard, after a series of detailed experiments on the decomposition of hydrogen peroxide, also recognized clearly the generality of catalytic phenomena: “Whichever be the cause of the phenomena we have reported and their way of acting, is it not very probable that it is the same that produces so many other?” He mentions as examples, the detonation of silver ammonia, of the chloride and iodide of nitrogen, fulminating powders, decomposition of ammonia gas by metals, the transformation of starch into sugar by minute amounts of diastase, and the transformation of sugar into alcohol and carbon dioxide by a small amount of ferment (Thenard, 1821). In the sixth edition of his Traité de Chimie, published in 1834, Thenard repeated, without change, the summary of his researches on the decomposition of water peroxide, which he had exposed in the 1821 edition. He continued to be baffled about the reason for the phenomena: “After having exposed all the phenomena presented by water peroxide in its contact with most materials, we have failed in searching for their reason; unfortunately we can only present some conjectures about it”. He then discusses all the possible reasons for the catalytic action and selects again electricity by elimination and not by conviction: “The bodies that decompose do not take the place of any of the bodies that they generate, no new combination is released; it acts in a certain way like by repulsion. Similar results cannot be explained by the affinity, at least in the manner we usually understand it, and it cannot be produced by a physical cause. Also, it cannot be attributed to heat or light, or, apparently, to the magnetic fluid. We are thus directed (for lack of alternative) to attributing them to the electric fluid” (Kilani, et al., 2001).
It was left to Jöns Jakob Berzelius (1779-1848) to put all the information available in an ordered form. The term catalysis, which he proposed in 1835, comes from the Greek words kata meaning down and lyein meaning loosen. Berzelius employed the expression “catalytic strength” by observing that an element, such as platinum, in small quantities activates chemical reactions while remaining unchanged. He also wrote that by the term catalysis he meant “the property of exerting on other bodies an action which is very different from chemical affinity. By means of this action, they produce decomposition in bodies, and form new compounds into the composition of which they do not enter” (Berzelius, 1823-1825; 1835).
In the section of vegetable chemistry of his 1835 Jahres-Bericht (Annual Survey) (Berzelius, 1835), Berzelius summarized the findings of different scientists on the formation of ether from alcohols; on the enhanced conversion of starch to sugar by acids; the hastening of gas combustion by platinum, of the stability of hydrogen peroxide in acid solution but its decomposition in the presence of alkali and such metals as manganese, silver, platinum, and gold, and the observation that the oxidation of alcohol to acetic acid was accomplished in the presence of finely divided platinum. He repeated this report in French, in a paper published a year later (Berzelius, 1836). He wrote: “Until 1800 no one suspected that any factor other than the degree of affinity could be of importance, with the exceptions of heat and light in certain cases. Then the influence of electricity was discovered, and it was soon found that electrical and chemical relationships are the same and that preferential affinity is nothing other than the result of strong opposite electrical forces that are intensified by heat or light. Thus we still had no way of explaining the genesis of new compounds other than by the fact that the substances which tend to combine are those in which the electrical forces can be better neutralized by the interchange of component parts… Then Kirchhoff discovered that starch, dissolved in dilute acids at a certain temperature, first turns into gum and then into grape sugar... nothing had evaporated into gas, nothing had united with the acid, the bases of which were recovered in the same amount as that in which they had been used, and in the liquid only sugar was found, weighing slightly more than the starch”. Thenard then discovered hydrogen peroxide, “the component parts of which were rather weakly joined to each other. Under the influence of acids, they remained in unaltered combination; under the influence of alkalis, they showed a tendency to separate, a kind of slow fermentation occurred… Not only soluble substances were found to hasten this type of decomposition, but also solid inorganic and organic substances, e.g. manganese dioxide, silver, platinum, gold, and the fibrous element from animal blood. The foreign agent causing the conversion of the substances did not itself participate in the new compounds formed but remained unchanged, thus operating by means of an internal power, the nature of which is still unknown, although it was in this way that it revealed its existence… Edmund Davy observed that wetting platinum finely divided with alcohol rendered the platinum incandescent and the alcohol converted into acetic acid and water Now came the discovery that crowned the foregoing, namely Döbereiner's finding that platinum sponge is able to ignite a stream of hydrogen gas as it escapes into the air… This discovery was soon followed by the mutual investigation of Dulong and Thenard that showed that several single and compound substances have this property… Thus this power was extended from an exceptional property to a more general one possessed by substances to different degrees... Thus it is certain that substances, both simple and compound, in solid form as well as in solution, have the property of exerting an effect on compound bodies which is quite different from ordinary chemical affinity, in that they promote the conversion of the component parts of the body they influence into other states, without necessarily participating in the process with their own component parts, even if this should occasionally occur… This is a new power to produce chemical activity belonging to both inorganic and organic nature… and the nature of which is still concealed from us. When I call it new power, I do not mean to imply that it is a capacity independent of the electrochemical properties of the substance... I am unable to suppose that this is anything other than a special kind of special manifestation of these, but as long as we are unable to discover their mutual relationship... it will also make it easier for us to refer to it if it possesses a name of its own. I shall therefore… call it the catalytic power of the substances and decomposition by means of this power catalysis... Catalytic power actually means that substances are able to awaken affinities, which are asleep at this temperature by their mere presence and not by their own affinity. By means of these, the elements in a compound body rearrange themselves in another manner so as to achieve a greater degree of electro-chemical neutrality. Thus their overall effect resembles that of heat, and here the question may arise as to whether the different degrees of catalytic power possessed by different substances can produce the same dissimilarity in the products of catalysis as is often caused by heat or different temperatures, and thus whether different catalytic substances are able to produce dissimilar products from a given compound body… On this occasion it is sufficient that the existence of catalytic power has been demonstrated with a sufficient number of examples… Turning with this idea to the chemical processes in living nature…we find that the insoluble starch in the tuber is changed to gum and sugar by catalytic power… living tissues” (Berzelius, 1835).
According to Pierre Lemay (Lemay, 1949), it is Clément the one who should be recognized as the first to have done the synthesis of all the information available and teach it in the course on industrial chemistry he gave at the Conservatoire des Arts et Métiers. Lemay quotes the following information from the sixth lesson, dedicated to the law of definite proportions, which Clément gave on January 11, 1821 (during the academic year 1820-1821): “It is found sometimes, that foreign substances determine or break combination by their sole presence, without incorporating or joining to any of the elements. Thus, the formation of certain esters (benzoates, citrates, oxalates, etc.) demands the presence of a strong acid, which does not contribute its mass to the composition of these esters. Sulfuric acid acts in the same manner during the transformation of aqueous starch into sugar. Iron or copper is necessary for the decomposition of ammonia at red heat. A stream of ammonia gas is not decomposed when flowing through a porcelain tube heated to red heat, but if this tube contains an iron wire, ammonia is decomposed completely into its elements, none of which combine with iron. The latter has broken substantially but its weight has not changed, hence it has acted only by its presence. We still do not know the cause of this type of influence. Perhaps it can be assumed that the foreign body modifies the electric state of the elements.”
“There is another way of action of the foreign substance upon the combination of elements, which in its absence would not combine. In this situation, the foreign substance remains intact after the combination; it has not become a part of it, but a true chemical reaction has taken place. This is the way nitrous oxide acts during the combination of sulfur dioxide with oxygen. The nitrous oxide, which is the true way of action, remains unchanged after the combination, although it has been engaged and liberated many times. This mode of action is present in other situations, for example, during the dissolution of certain metals in acids. For copper in hydrogen in muriatic acid (HCl), it is the protomuriate form, which absorbs oxygen from the atmosphere, to transport it to the coppers, oxidize it, and start a new cycle” (Clément, 1821).
According to Smith (Smith, 1979) the old hopes of saving saltpetre costs by using air currents, oxidizing agents, or heat instead, were shown by Clément and Desormes's theory to have been misguided. Their explanation of the course of the reaction suggested possible directions for improving the process. For example, since the nitrogen gases played a catalytic effect, then other substances which produced them could be employed instead of saltpetre, and also, finding ways to recycle these gases. Clément opted for the second alternative: “There are few practicable means known for economically obtaining the nitrous gas, but since it is not destroyed after the service it renders, one can recover it, something which has not yet been carried out, so that our theory has not really been utilized up to the present”. The other chief feature was the use of pyrites in place of sulfur, since they were very readily available and their roasting was known to disengage sulfur dioxide. A minor development was the idea of fitting chambers with internal partitions in order to increase the time of residence of the gases in the chamber. Clément calculated that existing chambers were ten times larger than theoretically necessary and thus their efficiency could be increased by dividing up the space to give the form of folded pipe (Smith, 1979).
ThermodynamicsThe caloric theory (Wisniak, 2004) came into general use in the eighteenth century and indeed proved to be much more helpful in explaining the thermal phenomena known that was the rival view that heat was something associated with the motions of the particles of ordinary matter. The theory reached the height of its popularity around 1825, and its supporters included many of the leading scientists, especially in Paris. One of the arguments for the materiality of heat at the beginning of the nineteenth century was the fact that heat can apparently travel through empty space without any accompanying movement of matter; hence it could not be simply molecular motion. Attempts to detect the weight ascribed to heat were made at various times during the seventeenth and eighteenth century by several scientists.
During his lectures of physics at the Paris Faculty of Sciences, Gay-Lussac made use of the concept of caloric, explaining to his students that the term had been introduced in order to distinguish the effect (heat, what is felt) from the cause. Caloric was usually treated as a substance or more precisely as a subtle fluid, which was material and yet did not have weight.
At the beginning of the nineteenth century it was felt strongly that to answer a series of theoretical questions (including the nature of caloric); it was necessary to calculate very precisely the amount of heat that corresponded to a given increase in temperature. This meant measuring the specific heat of different substances in different states of aggregation. It was believed that simple or composite gases, at equal volumes, had the same heat capacity. Most of the experimental techniques were based on an observation of the time required to heat (or to cool) a gas a given number of degrees. On, 1812 the Institut de France (the body that temporarily replaced the Académie des Sciences after the Revolution) announced a competition for the 1812 Prix de Physique, to be awarded for the best essay to measure and describe the specific heat of gases. The judging committee was composed of Gay Lussac, François Arago (1785-1853), Joseph Fourier (1768-1830), Jacques Alexandre César Charles (1746-1823), and Pierre-Simon Laplace (1749-1827).
A significant discussion on the nature of caloric and its possible existence in vacuum took place between Gay-Lussac and Clément and Desormes. According to Gay-Lussac there was no truth in the argument that a vacuum contained more caloric than when air was present (…le vide ne contient pas de calorique a la manière des corps) (Gay-Lussac, 1807). Clément and Desormes believed that vacuum did contain caloric and in their entry for the 1812 Physics prize tried to prove the validity of their assumption. Not only their memoir did not win the prize, it was also rejected for publication by the journal of the Académie (Note 2). Their paper was published in 1819 by another journal, together with a follow-up work (Desormes & Clément, 1819ab), fact that brought a sharp reply from Gay-Lussac (Gay-Lussac, 1820). In it Gay-Lussac claimed that the idea that vacuum contained caloric was easily refuted by means of a simple experiment: Since caloric was assumed to be an elastic fluid then, similarly as with a gas, there should be a heat change when its volume was changed. Since an increase or decrease in the volume of a vacuum had no effect on the thermometer, Gay-Lussac considered that this negative result confirmed his view.
In their paper Desormes and Clément claimed that if instead of estimating the amount of caloric that constitutes the temperature of a body, attention was addressed to the caloric that is not included in any body, that is to say, to a space devoid of air, then all the experimental difficulties encountered in the past disappeared. It was true that their proposal also included experimental problems, but these were easier to solve that the ones encountered with other methods. The basic idea of their claim was based on the known experimental fact that if air was introduced suddenly (to make the process adiabatic; Desormes and Clément used the expression “impermeable to caloric”) into an empty vessel, the temperature increased, and vice versa, releasing the pressure of air enclosed in a given vessel led to a decrease in the temperature of the internal air. Gay-Lussac had proved that if two equal vessels, one under vacuum and the other full of air at atmospheric pressure, were connected, the cooling experimented by one of them was essentially identical to the heating that took place in the second (Gay-Lussac, 1807).
According to Desormes and Clément, the existence of caloric in a totally evacuated vessel explained the heating that took place in the air that would admitted: The air, with everything that formed its absolute temperature, would meet and mix with the caloric present in the vessel; the resulting accumulation of caloric would reflect in the heating of the incoming air.
A far reaching proposition by Desormes and Clément was that if a totally evacuated vessel was held at the ice temperature, 0°C, then the increase in temperature would provide an exact measurement of the absolute caloric of space (it should be noted that at the beginning of the nineteenth century the impossibility of building a perpetual mobile was an accepted fact, but the concept of the conservation of energy, the First law of thermodynamics, would be not be accepted until the second half of the century).
On the question of the absolute quantity of heat in a vacuum Gay-Lussac remarked that it could not be “resolved except with the help of numerous hypotheses which are all the less plausible as we do not know the nature of heat… there is a second hypothesis which has also been adopted for a long time and which begins to be held in increasing favour. This second hypothesis is that there exists an extremely subtle fluid spread out indefinitely in space, a fluid which was denoted not very long time ago by the name of ether or ethereal fluid, and it conceives all molecules of a heated body in a special state of vibration, of to and fro motion, undergoing a small oscillatory movement which motion produces the phenomena, the sensations and the effects of heat.”
Between 1812 and 1818 Gay-Lussac made a large number of observations of the cooling effect produced by the evaporation of liquids and remarked that under certain conditions the heat of vaporization would be equal to the heat transferred through the walls of the vessel (Gay-Lussac, 1812ab; 1818). However, if the liquid evaporated into a vacuum surrounded by a freezing mixture the cooling effect could be increased indefinitely as long as the liquid exerted an appreciable vapour pressure. Gay-Lussac reported that he had succeeded in freezing mercury by evaporating water in a vessel surrounded by a freezing mixture. Gay-Lussac had no doubt that with a volatile liquid it would be possible to obtain even lower temperatures. According to Gay-Lussac, if the liquid evaporated in a perfectly dry gas instead of a vacuum the cooling would not be so great because the gas pressing the liquid would retard the evaporation process. The cold achievable had a maximum value corresponding to the equilibrium between the caloric (heat) absorbed by the vapour and the caloric lost by the air (A reading of the arguments given by Gay-Lussac, in modern terms, would represent what today we define as the wet-bulb temperature of air) (Gay-Lussac, 1807).
In a second publication (Gay-Lussac, 1818) Gay-Lussac analyzed the production of cold by the expansion of a gas. He realized that cooling by evaporation was limited and pointed out that the minimum temperature achieved was only –80°C. He believed that it was possible to achieve lower temperatures by using the equivalence between cooling caused by the expansion of a gas and heating caused by compression. It was known that compressing the air to one-fifth of its original volume increased the temperature 300°C and Gay-Lussac thought that the temperature might be increased to 1000°C or even 2000°C, if the process was rapid enough. If air was first compressed to five atmospheres, then allowed to cool to atmospheric temperature, and finally allowed to expand, it should absorb as much heat as was given out in its compression and its temperature should be lowered by 300°C. From these results he believed that “if we take a mass of compressed air to 50, 100, etc., atmospheres, the cold produced by its instantaneous expansion will have not limit). In other words, it would be possible to achieve unlimited cold by the expansion of gases”.
Gay-Lussac concluded his paper stating: “If it is undisputable that expansion of a gas can produce an unlimited amount of cold, then the determination of the absolute zero of heat must seem a complete fantasy”, agreeing with the general ideas that Lavoisier had expressed earlier.
An interesting fact is that Desormes and Clément interpreted Gay-Lussac's results in a completely different manner, arguing that they could actually be used not only to demonstrate the existence of an absolute zero but also to calculate its location. According to Desormes and Clément, to support Gay-Lussac's ideas of unlimited cooling, that is, the temperature could be decreased infinitely, was the same as admitting that the quantity of heat that constitutes this temperature was infinite. If this were the case, then heat would be unlike any other measurable thing or quality.
As will be discussed below, although Desormes and Clément relied on a hypothesis that we know today to be faulty, they were able to arrive by several different methods to a value of the absolute zero quite close to the one accepted today (Wisniak, 2005).
Comparing the cooling of a body to the flow of caloric and the temperature to the driving force (pressure drop), led to the result that for an elastic fluid the rate flow should be proportional to the active temperature, that is, to the temperature difference. This was exactly what Newton had stated, that the amount of caloric lost was proportional to the temperature difference. Another immediate consequence was that the capacity of dilated air for caloric was the mean between that of air at a high pressure and that of pure space (vacuum) (Desormes & Clément, 1819a).
Using these ideas, Desormes and Clément performed a series of experiments to determine the absolute caloric contained in space by expanding air at atmospheric pressure into an empty vessel maintained at 12.5°C. Their results indicated that “absolute vacuum at 12.5°C contains a quantity of absolute caloric able of raising by 99°C the temperature of a volume of air having the same volume at 766.8 mmHg.” This result was the same for dry or humid air. When the temperature was 18°C (or 98°C) the corresponding increase in temperature was 102°C (or 152°C) (the observant reader will realize that Desormes and Clément substituted readings of temperature by readings of pressure).
The basic equipment consisted of a large spherical glass vessel (28 L capacity), provided with a device for providing vacuum, a control valve, and a water manometer for measuring the pressure. The operation consisted in evacuating the vessel (after many experiences, Desormes and Clément concluded that the best initial pressure was about 14 mmHg), opening rapidly the valve to let air enter until the pressure was equal to the atmospheric, closing the valve, and letting the trapped air to reach the atmospheric temperature (The apparatus and the procedure constitute the so-called Desormes- Clément method to measure the ratio of the specific heats, cP/cV = γ — see below). According to Desormes and Clément, the size and shape of the vessel, the amounts of air before and after, and the operating pressures, assured that the heat losses be minimum.
Desormes and Clément realized that the actual heating effect was lower than the maximum one because their apparatus was non adiabatic. After estimating the heat losses they concluded that under adiabatic conditions the heating effect would be 132.62°C, instead of the 99°C observed. To the credit of Desormes and Clément we must say that their estimate was very good. Using modern thermodynamics we can show that when an ideal gas is suddenly expanded into empty space, the initial stage of the expansion is adiabatic and its final temperature, Tf, is given by Tf, = γTi, where Ti is the upstream temperature and g the ratio of the specific heats. For a diatomic gas like air γ = 1.4 so that Tf, = 1.4 (12.5 + 273.15) = 285.65 K = 126.76°C.
Desormes and Clément proceeded then to determine the specific caloric of space (vacuum) at constant volume by comparing it with that of air measured at two different pressures, and using the expression
where C and C’ are the capacity of air at two different pressures, c that of space, and N and n the volumes of the air and of space, respectively. Their final result was c = 400, compared with 1000 for air at 18°C and 762 mmHg pressure.It was now a question of simple proportions to determine the position of the absolute zero, since temperatures are in the inverse ratio of the capacities. Since air at 98°C had a capacity of only 870 the actual temperature increase should have been 0.870 ×152 = 132.24°C. The difference in temperature in the empty space (980 – 180 = 80°C) had resulted in a parallel increase of the air temperature of 132.240 –1020 = 30.24°C. Consequently the zero of the absolute temperature should be at
below the zero of the ordinary temperature.Desormes and Clément also calculated the position of the absolute zero by another procedure. Gay-Lussac had determined that the coefficient of thermal expansion of air at constant pressure was 1/266.66 at 0°C and that it remained constant up to a temperature of 500°C. The value of the expansion coefficient meant that for a given volume of air at atmospheric pressure and 0°C subtraction of the quantity of heat that changed the temperature by one degree would decrease the volume of the air by 1/266.66. If Gay-Lussac's law remained constant then the limit of the reduction in volume would be at 266.66°C below zero. Below this temperature it was impossible to reduce the temperature any further and hence no further cooling was possible. Hence, 266.66°C below zero represented the absolute zero of temperature.
In a second paper (Desormes & Clément, 1819b) Desormes and Clément presented the objections that had been raised against the doctrine of absolute temperature, and their refutations to the same: (a) Assumption of the existence of caloric in space free of matter (vacuum), (b) The possibility of measuring the caloric of space, and (c) The possibility of determining the absolute zero of temperature. In addition, they provided two additional procedures to determine the position of the absolute zero of temperature (Note 3). According to Costabel (Costabel, 1968) the set of the two papers clarify a third paper published shortly thereafter after the theory of heat engines) (Desormes & Clément, 1819).
The first one was based again on Gay-Lussac's results of the value of the thermal expansion coefficient, considering this time the influence of heat on the expansive force (pressure) of a constant volume of gas: Arbitrarily we can assign the value 266.66 to the pressure existing in a fixed volume of gas at 0°C. Again, each degree of decrease in the temperature will diminish the pressure by one unit, hence the pressure of the gas will reach zero when the temperature has decreased 266.66° below the original one (0°C).
The second procedure was based on the increase of the specific heat of ice upon fusion. Desormes and Clément indicated that at 0°C the specific heat of ice was 720 (0.72) and that of liquid water 1000 (1.00). Assuming that the absolute temperature of ice melting was 266.66°C if it were to pass spontaneously from solid to liquid without addition of caloric its capacity would increase from 720 to 1000 with the corresponding decrease in temperature. Again, the ratio of the new temperature to the initial one would be in an inverse ratio to their capacities, that is (266.66)(720/1000) = 192°C. The temperature had thus decreased by (266.66 – 192) = 74.66°C. In order to return the water to its original temperature would be necessary to add a quantity of heat equal to 74.66°. Since the heat of fusion has been determined experimentally to be 750, this calculation justified the assumption that the absolute temperature of fusing ice was 266.66°C.
The reader interested in a detailed analysis of the controversy between Gay-Lussac and Desormes-Clément regarding the question of “the temperature of vacuum” is referred to the paper by Costabel (Costabel, 1968).
Measurement of the ratio cP/cV = γThe earliest and simplest method of measuring γ of an ideal gas is based on the procedure used by Clément and Desormes to measure the specific heat of a gas) (Desormes & Clément, 1819a).The gas is contained in a glass bottle, fitted with a large bore stopcock (to allow the fast release of the gas) and a manometer, at room temperature and at a pressure Pi slightly above atmospheric pressure P0. By rapidly opening and closing a stopcock, the gas is allowed to expand rapidly and almost adiabatically to atmospheric pressure, when the stopcock is closed again. The gas is allowed to stand for a few minutes at constant volume until the temperature comes back to the initial value, and the final pressure, Pf, is read in the manometer. The first stage is assumed to be an adiabatic g g quasistatic expansion, described by PiViγ = P0Vfγ the second stage is a non-adiabatic heating, described by PiVi = PfVf. Eliminating Vi and Vf from the two equations we get P1/P0 = (Pi/Pf)γ, so that
The pressures are usually measured with an open U-tube manometer containing a convenient light liquid. Denoting the height of the barometric column of this liquid by h0 we may write
where hi and hf are small compared with h0. Upon substitution and rearrangement we getSince the ratios hi/h0 and hf /h0 are small compared with unity, the equation simplifies to
An interesting description of the Clément-Desormes method and the experimental setup may be found in the class notes of Antoine Henri Becquerel (1852-1908; 1903) Nobel Prize in Physics), for the physics course he gave at the École Polytechnique (Becquerel, 1896-1897). Here the experiment is conducted with the initial air under vacuum (20 cm of water); the water manometer may be replaced by one of sulfuric acid for the case where it is desired to operate with dry gases. The manometric tube must be of such small dimensions that the ascension of the liquid in it will be negligible with the respect to the volume of the bottle. Becquerel remarks that the method is very delicate; the entrance of air into the bottle is accompanied by a series of very fast oscillations. The closing of the stopcock must not be closed to soon or too late, because otherwise the cooling will begin before total closure and the final reading of the manometer will be too small, leading to a value of γ, smaller than the real one, as reported by Cazin (Cazin, 1862).
Energy conversionIn their Mémoire sur la Théorie des Machines à Feu, read to the Académie des Sciences of August 16 and 23, 1819 (Anonymous, 1819), Desormes and Clément addressed the basic question of how to extract the maximum mechanical power from heat (what today we call the thermal efficiency of a heat engine). The answer to the question was fundamental at a time when steam engines were developing very fast and represented the tool for technological supremacy. In the first part of this memoir they described their experiments to determine the amount of caloric (heat content) present in steam at different temperatures and pressures, and their (wrong) conclusion that for saturated steam this amount was independent of the pressure and temperature. In other words, a given mass of steam could expand or condense without losing its elastic state, independently of the modifications that is volume might experiment. It is interesting to indicate that this “law” was accepted as true until Despretz proved it to be wrong. In his Traité Élémentaire de Physique (Despretz, 1829), Despretz stated that he had shown experimentally that Clément and Desormes's law for steam, could be also applied to the vapors of other liquids (alcohol, turpentine, and ether), but in later editions of his book he wrote that he had repeated his experiences at higher temperatures (up to 160°C) an found that the total heat content actually increased as the temperature was raised. This conclusion had already been presented in the pamphlet he published when being considered for membership in the Académie des Sciences (Despretz, 1837).
The second subject discussed by Desormes and Clément dealt with the calculation of the motive power, which could be extracted from a given amount of heat. They did it by means of the clever thought experiment: A bubble of gas or steam was imagined to be introduced at the bottom of a tall cylinder of water filled to the brim with water at a given temperature; the bubble grew while rising adiabatically, and as a consequence, water overflowed from the vessel and work was performed. The amount of work could be easily calculated by the product of the height of the cylinder and the amount of water discharged, Desormes and Clément assumed that during this process the pressure of the working substance in the bubble remained constant and equal to the pressure exerted by the column of water in the vessel. They also assumed that the same amount of work could be extracted in a heat engine.
Desormes and Clément's lecture on the theory of heat engines was never published in the journals of the Académie, although an extract of it was published by an outside journal (Desormes & Clément, 1819).
Coal gas (illumination gas)At the beginning of the 19th century, coal gas (illumination gas), produced from the partial combustion of coal, begun to be an important source of light; its success in England gave place to a strong discussion in France on the possibility of using this mean of illumination as a replacement of the one then used, based on the burning of oil. Although the French government began investing large amounts of money on implementing it, Clément came strongly against the project and published a detailed technical and economical study trying to prove that the new procedure was inferior from all points of view (Clément-Desormes, 1819). According to Clément, the enthusiasm was due more too subjective motives than to objective ones. It was fascinating to see a flame produced by an invisible substance (the gas); it was presented as a triumph of the new chemical discoveries, and some even try to protect it as if were a French invention.
Clément first analyzed the procedure from an economical aspect, looking at its cost in three important British cities, Manchester, Glasgow, and London. He based his evaluation assuming as unit of light as that produced by the burning of 30 grams of oil in an Argand beak, during one hour. His results indicated that the British success was due to their coal being of a superior quality and cheaper than the French one. The cost of operating an Argand beak for four hours cost in London about 140 francs when using oil (whale), and only 120 francs when using coal gas. The corresponding figures in France would be 100 against 278 francs. In addition, the cost of capital was much lower in England (3% per year) compared to that in France (9% per year). In the same manner, the better quality of British coal made the production process of gas cheaper and easier.
Clément concluded his study with the recommendation of that since coal was the best raw material for producing light, the procedure appropriate for France should be to burn carbon finally pulverized and dispersed in a stream of coal gas. This mixture would yield a more intense and more beautiful flame that the one produced by oil.
Notes- 1.
Desormes and Courtois were also born in Dijon.
- 2.
The prize was awarded to François Marcet Delaroche (1803-1883) and Jacques-Étienne Bérard (1789-1869).
- 3.
On a footnote on the first page of this paper, Desormes and Clément indicate that they have decided to use the word heat instead of caloric and specific heat instead of specific caloric.