The main objectives of this study are 1) to develop a perspective based on history and philosophy of science (HPS) considerations in order to understand the postulation of the covalent (shared pair) bond by Lewis; 2) to formulate criteria based on the HPS perspective that could be useful in the evaluation of general chemistry textbooks; and 3) to evaluate 27 general chemistry textbooks (published in Turkey) utilizing the criteria based on the HPS perspective. Results obtained showed that most of the general chemistry textbooks did not present the origin of the covalent bond based on a HPS perspective. Also, the textbooks mostly follow an inductivist interpretation of the origin of the covalent bond. It is plausible to suggest that textbook presentations based on a HPS perspective can perhaps facilitate students’ interest in the subject and hence lead to sound conceptual understanding.
Los principales objetivos de este estudio son: 1) Desarrollar una perspectiva basada en consideraciones sobre la historia y la filosofía de la ciencia (HFC), con el propósito de entender la postulación del enlace covalente por Lewis; 2) Formular criterios basados en la perspectiva HFC que puedan ser de utilidad para evaluar libros de texto de Química General y 3) Evaluar 27 libros de texto de Química General (publicados en Turquía) empleando criterios basados en l0a perspectiva HFC. Asimismo, los libros de texto siguen mayormente una interpretación inductivista del origen del enlace covalente.
Chemical bonding and the introduction of covalent bonds is considered to be a difficult topic for most high school and freshman students (Gillespie et al., 1996; Ünal et al., 2006; Ünal et al., 2010). Ionic bonds are formed by the actual transfer of electrons, which produces positively and negatively charged electrons. Formation of the ionic bond leads to the lowering of energy because of electrostatic attraction between ions of opposite charge. In this context, how can we explain the lowering of energy when two electrons are shared to form a covalent bond? Apparently, the approach of two electrons having the same charge should produce repulsive forces and hence produce destabilization. Most students if given an opportunity to think and reflect can be perplexed by this dilemma. It is not surprising that when first proposed the idea of a covalent bond was considered by some leading scientists to be not only untenable but even ‘absurd’ and ‘bizarre’. Niaz (2001) has presented a historical reconstruction of the events that led to the postulation of the covalent bond by G.N. Lewis. Based on criteria derived from this reconstruction, Niaz (2001) evaluated general chemistry textbooks (published in U.S.A.) and found that most textbooks lacked a history and philosophy of science (HPS) perspective and thus did not deal adequately with the dilemma faced by the students. Furthermore, in an attempt to simplify the topic most textbooks present rules (algorithms, 5-10 pages) for writing Lewis diagrams for covalent bonds, which are memorized by the students and do not facilitate conceptual understanding (Nurrenbern & Pickering, 1987). Research in science education has recognized not only the importance of history and philosophy of science but also its implications for textbooks (Abd-El-Khalick et al., 2008; Duschl, 1994; Hodson, 1988; Matthews, 1994; McComas et al., 1998; Niaz, 2008; Solomon, 1991; Stinner, 1992). Project 2061 (AAAS, 1989) and the National Science Education Standards (NRC, 1996) in the U.S.A., Science in the National Curriculum (NCC, 1988) in the UK and several other countries have also recognized the importance of history and philosophy of science.
The main objectives of this study are: 1) development of a perspective based on history and philosophy of science (HPS) considerations in order to understand the postulation of the covalent (shared pair) bond by Lewis; 2) formulation of criteria based on the HPS perspective that could be useful in the evaluation of general chemistry textbooks; and 3) evaluation of general chemistry textbooks (published in Turkey) utilizing the criteria based on the HPS perspective. This study is based on the same criteria that were used by Niaz (2001) to evaluate general chemistry textbooks published in U.S.A.
A History and Philosophy of Science PerspectiveLewis (1916) is generally considered to have presented the first satisfactory model of the covalent (shared pair) bond based on the cubic atom in 1916. It is important to note that the genesis of the cubic atom can be traced to an unpublished memorandum written by Lewis in 1902 and recounted by him in the following terms: In the year 1902 (while I was attempting to explain to an elementary class in chemistry some of the ideas involved in the periodic law) becoming interested in the new theory of the electron (Thomson's discovery of the electron in 1897), and combining this idea with those which are implied in the periodic classification, I formed an idea of the inner structure of the atom (model of the cubic atom) which, although it contained crudities, I have ever since regarded as representing essentially the arrangement of the electrons in the atom. (Lewis, 1923, pp. 29-30, emphasis added)
Lewis (1916) reproduced the postulates of his 1902 theory of the cubical atom at length, of which the third postulate stated: “The atom tends to hold an even number of electrons in the shell, and especially to hold eight electrons which are normally arranged symmetrically at the eight corners of a cube” (p. 768). This postulate was the most striking and at the same time controversial feature of Lewis's theory, which led to the formulation of the ‘rule of eight’ or the ‘octet rule.’ Lewis postulated that the eight electrons of an octet formed the eight corners of a cube, as this provided, “… the most stable condition for the atomic shell” (p. 774). Thus the single bond was conceived of as two cubic atoms with a shared edge (pair of electrons) and the double bond as two cubes with a common face.
Lewis's model of the covalent bond in retrospectIn this section evidence is provided to show that Lewis's theory of sharing electrons (covalent bond) had to compete with a rival theory, viz. transfer of electrons (ionic bond). From a philosophy of science perspective the rivalry between competing theories (paradigms/research programs) is an integral part of scientific progress. According to Lakatos (1970): “… research programmes have achieved complete monopoly only rarely and then only for relatively short periods … The history of science has been and should be a history of competing research programmes …” (p. 155). According to Kohler (1971), who has presented a detailed account of the origin of Lewis's ideas: When it was first proposed, Lewis's theory was completely out of tune with established belief. For nearly 20 years it had been almost universally believed that all bonds were formed by the complete transfer of one electron from one atom to another. The paradigm was the ionic bond of Na+ Cl–, and even the bonds in compounds such as methane or hydrogen were believed to be polar, despite their lack of polar properties. From the standpoint of the polar theory the idea that two negative electrons could attract each other or that two atoms could share electrons was absurd (p. 344).
Rodebush (1928), a chemist reviewing the origin of the covalent bond in the late 1920s, shared the same concern: Since according to Coulomb's law two electrons should exert a repulsion for each other, the pairing of electrons seems at first glance to be a bizarre idea. In order to account for the peculiar behavior Lewis assumed the existence of a magnetic attraction between the electrons (pp. 513-514).
Lewis (1916) further clarified his attempt at building a theory of the atom: “In my original theory [1902] I considered the elements in the periodic table thus built up, as if block by block, forming concentric cubes” (p. 769). Later in the article Lewis recognizes that the cubic structure cannot represent the triple bond and suggests its replacement by the tetrahedral atom (p. 780). At this stage it is important to note that Thomson's (1897) discovery of the electron in 1897 and later publications (Thomson, 1907) provided powerful arguments for the polar theory of the ionic bond. According to Thomson (1907): “For each valency bond established between two atoms the transference of one corpuscle from the one atom to the other has taken place …” (p. 138). Although Thomson accepted that overlapping of corpuscles could produce a non-polar bond in theory, he believed that in reality all bonds were polar bonds (p. 131). Material presented in this section has been adapted from Niaz (2001).
More recently, Gavroglu and Simões (2012, pp. 47-55) have presented a historical reconstruction of the origin of the covalent bond, very similar to that presented by Niaz (2001).
Criteria for the Evaluation of General Chemistry Textbooks Published in TurkeyBased on the historical perspective (rational reconstruction) presented by Niaz (2001), here we present criteria for the evaluation of Turkish general chemistry textbooks (see Appendix 1).
Criterion 1.Lewis's cubic atom as a theoretical device for understanding the sharing of electrons: Lewis's cubic atom was based on his atomic theory based on postulates formulated in 1902. The cubic atom was thus a theoretical device that was later used for understanding the sharing of electrons (covalent bond) and provided the rationale for the octet rule. This criterion is based on the following references: Lewis (1916; 1923), Kohler (1971) and Jensen (1984). Following classifications were elaborated:
Satisfactory (S): treatment of the subject in the textbook is considered to be satisfactory if it is briefly explained that Lewis (1916) used his model of the cubic atom to explain the sharing of electrons and the octet rule.
Mention (M): a simple mention of Lewis's cubic atom.
No-mention (N): no-mention of Lewis's cubic atom.
Criterion 2.Sharing of electrons (covalent bond) had to compete with the transfer of electrons (ionic bond): Lewis's idea of sharing electrons (covalent bond) had to compete with the transfer of electrons (polar/ionic bond). The origin of the polar bond as the dominant paradigm in chemical combination can be traced to Thomson's discovery of the electron in 1897. By 1913 the polar theory completely dominated chemistry, and it was in the early 1920s that Lewis's idea of sharing electrons became acceptable. This criterion is based on the following references: Thomson (1897; 1907 and 1914), Lewis (1916; 1923), Lakatos (1970) and Kohler (1971). Following classifications were elaborated:
Satisfactory (S): treatment of the subject is considered to be satisfactory if the role of competing frameworks (polar/ non-polar) is briefly described.
Mention (M): a simple mention of the competing frameworks.
No-mention (N): no-mention of the competing frameworks.
Criterion 3.Covalent bond: inductive generalization/derived from the cubical atom: the objective of this criterion (Rodebush, 1928; Lakatos, 1970; Kohler, 1971) is to evaluate if the textbooks follow one of the following interpretations with respect to the origin of the (shared pair) covalent bond:
Inductivist (I): Lewis's covalent bond was an inductive generalization based on: stability of the noble gases or formation of the hydrogen molecule leads to a lowering of the energy or Helium an inert gas has a pair of electrons or numbers of electrons in most compounds are even.
Lakatosian (L): Lewis's (shared pair) covalent bond was not induced from experimental evidence but derived from the cubic atom.
No-mention (N): textbook makes no-mention explicitly to either of the two interpretations, presented above.
Criterion 4.Pauli Exclusion Principle as an explanation of the sharing of electrons in covalent bonds: the objective of this criterion is to evaluate if textbooks consider Pauli's exclusion principle to provide an explanation of the sharing of electrons. According to Gavroglu and Simões (2012): “With the Pauli principle, it became possible to comprehend ‘valence’ saturation: It seemed reasonable to suppose that whenever two electrons of different atoms combine to form a symmetric Schrödinger vibration, a bond will result” (p. 18). This criterion is based on the following references: Pauli (1925), Rode-bush (1928), Lakatos (1970) and Kohler (1971). Following classifications were elaborated:
Satisfactory (S): treatment of the subject in the textbook is considered to be satisfactory if the role of Pauli Exclusion Principle is briefly described, in order to explain the covalent bond.
Mention (M): a simple mention of Pauli Exclusion Principle, in the context of the covalent bond.
No-mention (N): no-mention of Pauli Exclusion Principle.
For implementing the criteria, the following procedure was used to establish the reliability of the evaluation of textbooks:
First stage: The first author analyzed the following textbooks published in Turkey as translations of books originally published in U.S.A.: a) Chang, 1998 (Turkish translation: Soydan & Aroğ uz, 2000; b) Mortimer, 1983 (Turkish translation: Altınata, 1990). These textbooks had already been analyzed by Niaz (2001) and thus facilitated an understanding of the criteria. Furthermore, the first author re-analyzed two textbooks, namely, Bodner & Pardue (1989), and Segal (1989), which were previously analyzed by Niaz (2001), to provide an understanding of satisfactory, no mentioned explanation and interpretations (inductive generalization/derived from the cubic atom).
Second stage: The first author analyzed the following textbooks published in U.S.A.: a) Atkins & Jones, 1997 (Turkish translation: Kılıç et al., 1998; b) Petrucci & Harwood, 1993 (Turkish translation: Uyar, 1994). This provided further experience with respect to textbooks published in U.S.A.
Third stage: Two university chemistry professors with a Ph.D. in inorganic chemistry (with 14 and 15 years of teaching experience, respectively) and the first author of the paper, applied the criteria separately to evaluate four textbooks (selected randomly). It was found that each evaluator coincided on the evaluation of four criteria on three textbooks (Bekaroğ lu & Tan, 1986; Özcan, 1998; Soydan & Saraç, 1998; Yavuz, 1978). On the fourth book (Özcan, 1998) the evaluators coincided on two (criterion 1 and 2) of the four criteria. Each evaluator explained the points of disagreement for criteria (criterion 3 and 4). After some discussion it was decided that the textbook could be classified as (I) for criterion 3. As for criterion 4, although the textbook does not explicitly refer to the Pauli Exclusion Principle, it mentions that the two electrons in the covalent bond must have opposite spins; therefore, the textbook could be classified as Mention (M). With this procedure, all disagreements were resolved and consensus amongst evaluators was achieved. With this experience, rest of the textbooks (23), were then evaluated by the first author.
Evaluation of Turkish General Chemistry Textbooks: Results and DiscussionCriterion 1
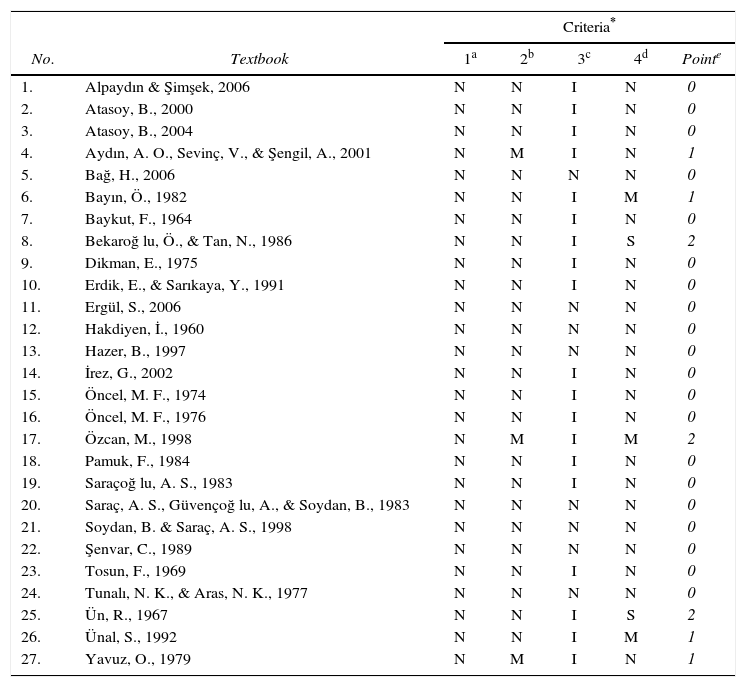
As seen from Table 1, none of the textbooks described satisfactorily (S) or mentioned (M) the Lewis's cubic atom. This shows that textbooks lack a history and philosophy of science (HPS) framework.
Evaluation of general chemistry textbooks (covalent bond) based on a history and philosophy of science framework.
| No. | Textbook | Criteria* | ||||
|---|---|---|---|---|---|---|
| 1a | 2b | 3c | 4d | Pointe | ||
| 1. | Alpaydın & Şimşek, 2006 | N | N | I | N | 0 |
| 2. | Atasoy, B., 2000 | N | N | I | N | 0 |
| 3. | Atasoy, B., 2004 | N | N | I | N | 0 |
| 4. | Aydın, A. O., Sevinç, V., & Şengil, A., 2001 | N | M | I | N | 1 |
| 5. | Bağ, H., 2006 | N | N | N | N | 0 |
| 6. | Bayın, Ö., 1982 | N | N | I | M | 1 |
| 7. | Baykut, F., 1964 | N | N | I | N | 0 |
| 8. | Bekaroğ lu, Ö., & Tan, N., 1986 | N | N | I | S | 2 |
| 9. | Dikman, E., 1975 | N | N | I | N | 0 |
| 10. | Erdik, E., & Sarıkaya, Y., 1991 | N | N | I | N | 0 |
| 11. | Ergül, S., 2006 | N | N | N | N | 0 |
| 12. | Hakdiyen, İ., 1960 | N | N | N | N | 0 |
| 13. | Hazer, B., 1997 | N | N | N | N | 0 |
| 14. | İrez, G., 2002 | N | N | I | N | 0 |
| 15. | Öncel, M. F., 1974 | N | N | I | N | 0 |
| 16. | Öncel, M. F., 1976 | N | N | I | N | 0 |
| 17. | Özcan, M., 1998 | N | M | I | M | 2 |
| 18. | Pamuk, F., 1984 | N | N | I | N | 0 |
| 19. | Saraçoğ lu, A. S., 1983 | N | N | I | N | 0 |
| 20. | Saraç, A. S., Güvençoğ lu, A., & Soydan, B., 1983 | N | N | N | N | 0 |
| 21. | Soydan, B. & Saraç, A. S., 1998 | N | N | N | N | 0 |
| 22. | Şenvar, C., 1989 | N | N | N | N | 0 |
| 23. | Tosun, F., 1969 | N | N | I | N | 0 |
| 24. | Tunalı, N. K., & Aras, N. K., 1977 | N | N | N | N | 0 |
| 25. | Ün, R., 1967 | N | N | I | S | 2 |
| 26. | Ünal, S., 1992 | N | N | I | M | 1 |
| 27. | Yavuz, O., 1979 | N | M | I | N | 1 |
(S = Satisfactory; M = Mention; N = No mention; I = Inductivist; L = Lakatosian).
Criterion 2
Table 1 shows that none of the textbooks described satisfactorily (S) that Lewis's idea of sharing of electrons (covalent bond) had to compete with the transfer of electrons (ionic bond). Only three textbooks (Aydın et al., 2001; Özcan, 1998; Yavuz, 1978) made a simple mention (M) and following are two examples: Previously, it was commonly accepted that all chemical bonds can form between ions through electrostatic attractions, that is, it was accepted that all chemical bonds were ionic bonds. However, in 1906, American chemist G. N. Lewis said that in some cases, the idea that electrons transfer entirely from one atom to another atom was illogical… [Comment: Textbook provides the example of formation of H2 to rebut ionic bond theory. However, it does not explicitly interpret the origin of the covalent bond as a rival research program based on an HPS perspective] (Aydın et al., 2001. pp. 73-74) Examining the ionic bond, we saw a bond formed by transfer of one or more electrons between two atoms, whose electron affinity and ionization energies were very different. In a wide variety of cases, a more stable state did not form with ionic bonding. On the contrary, a more stable state formed with covalent bonding between two atoms whose electron affinity and ionization energies were identical. As an example, consider the bond formed by two hydrogen atoms… [textbook explains formation of the hydrogen molecule in detail]… in the formation of this bond [H—H], electron transfer from one atom to the other is impossible [textbook gives detailed reasons, implying rebuttal of the ionic bond] … therefore, covalent bond is formed differently as compared to ionic bonding… [Comment: Textbook also provides detailed information in following paragraphs, implying rebuttal of ionic bonding. However, the textbook does not explicitly interpret the origin of the covalent bond as a rival research program based on an HPS perspective] (Özcan, 1998, pp. 184-185).
Most textbooks (24) made no mention (N) that Lewis's idea of sharing of electrons (covalent bond) had to compete with the transfer of electrons (ionic bond). The controversial origin of the covalent bond and its rivalry with the ionic bond provides a good opportunity to illustrate how progress in science is based on controversy and how established theories or ways of thinking are difficult to change. Following is an example of a textbook that was classified as (N), and shows the difference between textbooks classified as (M): Covalent bonds are bonds between two identical or different non-metals. Since the electro negativity of two atoms is close to each other, there is little difference in the abilities of two atoms to attract the bonding electrons to them. Therefore, electrontransfer between two atoms does not occur; instead, the electrons involved in such a bond are shared. A chemical bond formed by sharing electrons is called covalent bond… (Alpaydın & Şimşek, 2006, p. 93)
As seen from the evaluation of textbooks, they do not interpret the origin of the covalent bond as a rival research program, based on an HPS framework (Lakatos, 1970). Besides, the textbooks only provide students detailed information for writing the Lewis structures. Even a brief mention of the historical details can facilitate conceptual understanding of the difference between ionic and covalent bonds.
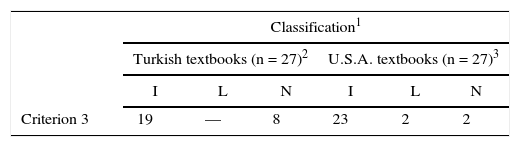
Criterion 3
Table 1 shows that none of the textbooks presented the Lakatosian interpretation (L), viz., tracing the origin of the stability of the covalent bond to the cubic atom and giving enough details to show that Lewis's ideas developed slowly based on conjectures. Most textbooks (19) consider the origin of the covalent bond to be an inductive (I) generalization and following are three examples: … between two identical atoms, ionic bonds can not be formed. Therefore, how is a bond formed between such atoms? The question was answered in 1916 by the American chemist Gilbert. N. Lewis…G. N. Lewis supposed that the bond [between two identical atoms] is a covalent bond…. [textbook gives additional information about covalent bonds and an example of H2 molecule]…as a result of filled in outer shell of the atom with shared electron, a bond between two atoms lead to stable molecules if they share electrons in such a way as to create a noble gas configuration for each atom as shown Figure… [one page after, textbook gives following explanation dealing with inductive generalization] …Helium does not form a molecule of He2, because repulsive forces exert on attractive forces as distance between the two helium atoms decreases. Therefore, the atoms do not come near enough to form a bond…” (Bayın, 1982, p. 226) Lewis and Langmiur explained formation of ionic and covalent bonds by using the octet rule (or duplet rule [for He]). According to this rule, atoms share or transfer electrons in the outermost shell to create a noble gas configuration… (Pamuk, 1984, p. 110) [textbook explains the covalent bond by giving an example of H2 molecule] … as the distance between two hydrogen atoms decreases, the electrostatic interactions between each electron and nuclei of the other atom, as well as between the two electrons and between the two nuclei, become increasingly important. When attractive forces exert on repulsive forces, atoms close up… [textbook gives extra information about attraction and repulsion forces]… since electrons revolving around the two nuclei were distributed in a greater region, repulsive forces between the two electrons were lower than attractive forces from the two nuclei. Therefore, as compared with hydrogen atoms, they were more stable and lower energies as hydrogen molecule (as seen Figure…)… (Tunalı & Aras, 1977, p. 254)
These presentations are quite representative of most textbooks and show explicitly that the octet rule is sustained by empirical evidence.
On the other hand, eight textbooks made no-mention explicitly to either of the two interpretations (Lakatosian or inductive generalization).
Criterion 4
Table 1 shows that three textbooks mentioned (M) and only two textbooks described satisfactorily (S) Pauli Exclusion Principle as an explanation of the sharing of electrons in covalent bonds. The following were considered to be two examples of a satisfactory description: [textbook explains the covalent bond and then gives an example of H2 molecule] … one hydrogen atom has only one electron that is symmetrically distributed around the nucleus in a 1s orbital. When two hydrogen atoms form a covalent bond, two atomic orbitals overlap in such a way that the electron clouds are in the region between the two nuclei, and there is an increased probability of finding an electron in this region. According to Pauli exclusion principle, the two electrons of the bond must have opposite spins. (Bekaroğ lu & Tan, 1986, pp. 74-75) …spins of two electrons in a [covalent] bond must have opposite directions (see page 211) [on page 211, textbook explains Pauli Exclusion Principle in detail] (Ün, 1967, p. 228).
Three textbooks made a simple mention (M) of Criterion 4 and following was considered to be an example: … a single covalent bond consists of a pair electrons, with opposite spin, shared by two atoms… (Ünal, 1992, p. 42)
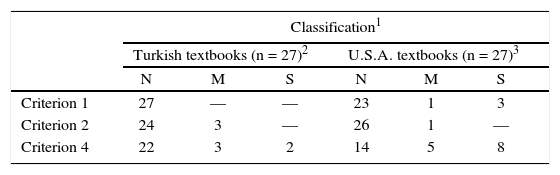
In order to compare general chemistry textbooks analyzed in a previous study (Niaz, 2001) and Turkish chemistry textbooks analyzed in this study, we used the following scale: Satisfactory = 2 points, Mention = 1 point, and No mention = 0 point (see Table 1 for points awarded to each chemistry textbook, published in Turkey). Table 2 provides a comparison of textbooks published in Turkey and U.S.A., with respect to the three criteria (criterion 1, 2 and 4) and classification as Satisfactory (S), Mention (M) or No mention (N).
It can be observed that none of the textbooks published in Turkey had a classification of Satisfactory (S) except for Criterion 4. As for criterion 2, none of the two groups of textbooks had a classification of Satisfactory (S). In general, textbooks published in U.S.A., performed better than those published in Turkey in terms of a history and philosophy of science perspective.
With respect to interpretation of the origin of the covalent bond, Criterion 3 (Lakatosian/Inductive) most textbooks published in Turkey and U.S.A., consider the origin of the covalent bond to be an inductive (I) generalization (see Table 3). Only two textbooks published in U.S.A., presented the Lakatosian interpretation, while none of textbooks published in Turkey did so.
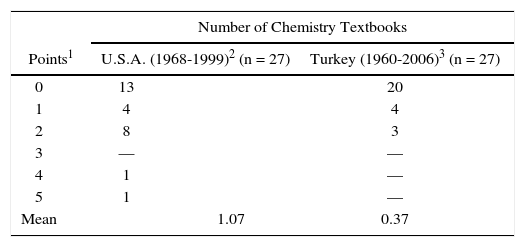
Table 4 provides an overall comparison of textbooks published in Turkey (n = 27, 1960-2006, this study) and those published in U.S.A. (n = 27, 1968-1999, Niaz, 2001).
Comparison of the covalent bond (based on Criteria 1, 2 & 4) in chemistry textbooks published in U.S.A. and Turkey.
| Points1 | Number of Chemistry Textbooks | ||
|---|---|---|---|
| U.S.A. (1968-1999)2 (n = 27) | Turkey (1960-2006)3 (n = 27) | ||
| 0 | 13 | 20 | |
| 1 | 4 | 4 | |
| 2 | 8 | 3 | |
| 3 | — | — | |
| 4 | 1 | — | |
| 5 | 1 | — | |
| Mean | 1.07 | 0.37 | |
All textbooks were evaluated on a scale of 0-6 points. For each of the 3 criteria (namely, criterion 1, 2 and 4) in Table 1, textbooks were awarded the following points: Satisfactory = 2 points, Mention = 1 point, No mention = 0 point.
It can be observed that the number of textbooks that scored from 0 point towards 5 points decreased in the two groups. While the highest score was 5 points (on a scale of 0-6) for textbooks published in U.S.A, the highest score was 2 points for those published in Turkey. Treatment of the covalent bond, based on an HPS perspective is better in textbooks published in U.S.A. (mean score = 1.07) as compared to those published in Turkey (mean score = 0.37). It can of course be argued that these are rough estimates and are not entirely representative of all textbooks published in these two countries.
ConclusionResults obtained show that Turkish general chemistry textbooks generally ignore the history and philosophy of science (HPS) framework for understanding the postulation of the covalent bond (shared pair). Very few textbooks satisfactorily presented the Pauli Exclusion Principle as an explanation of the sharing of electrons in covalent bonds within a historical perspective. In the same line, very similar results have been reported for general chemistry textbooks published in U.S.A.
A major finding of the study is that most of the general chemistry textbooks published in Turkey, follow an inductivist interpretation of the origin of the covalent bond, which construes Pauli's exclusion principle as the theoretical explanation and ignores the fact that Lewis's cubic atom was crucial for his later explanation of the sharing of electrons. Thus scientific progress is characterized by a series of theories or models (plausible explanations, from cubic atom to Pauli's principle), which vary in the degree to which they explain the experimental findings. In other words, science does not necessarily progress from experimental findings to scientific laws to theoretical explanations. According to Lakatos (1970, p. 129) the conflict is not between theories and laws but rather between an interpretative and an explanatory theory. Blanco and Niaz (1997) found that many chemistry teachers and students consider progress in science to be characterized by a ‘Baconian inductive ascent’, that is experimental findings → scientific laws → theoretical explanations. An alternative approach in the present case would be a textbook presentation emphasizing the origin of the covalent bond as a product of conflicting or rival theories (models) for the explanation of bond formation. This shows that appropriate historical reconstructions can benefit students both by providing them with models for alternative/rival approaches and by instilling in them a deeper conceptual understanding of the topic (cf.Chiappetta et al., 1991; Niaz, 2009). Furthermore, it is important to note that such approaches approximate a strategy based on ‘science as practiced by scientists’ (cf.Niaz, 2010).
Study reported here can help to improve general chemistry textbooks published in Turkey and other countries. In taking into account the findings reported here, textbooks can also provide students a historical reconstruction based on the development of scientific theories involving controversies, conflicts and rivalries among scientist. Moreover, it may encourage some textbook authors who write chemistry textbooks to become interested in researches on history and philosophy of science. Finally, it could help in the design of studies that could use HPS related material to facilitate students and teachers’ understanding of historical context in which the origin of covalent bonding developed.
- 1.
Alpaydın, S., & Şimşek, A. (2006). Genel kimya (2. Baskı). Ankara: Nobel Yayın Dağ ıtım.
- 2.
Atasoy, B. (2000). Genel kimya. Ankara: Gündüz Eğ itim ve Yayıncılık.
- 3.
Atasoy, B. (2004). Temel kimya kavramları (2. Baskı). Ankara: Asil Yayın Dağ ıtım.
- 4.
Aydın, A. O., Sevinç, V., & Şengil, İ. A. (2001). Temel kimya (2. Baskı). Adapazarı: Aşiyan Yayınları.
- 5.
Bağ, H. (2006). Genel kimya-I (1. Baskı). Ankara: Pegem A Yayıncılık.
- 6.
Bayın, Ö. (1982). Modern kavramlar yaklaşımıyla kimya. İstanbul: Fil Yayınevi.
- 7.
Baykut F. (1964). Modern denel, genel kimya dersleri. İstanbul: İstanbul Üniversitesi Yayınları.
- 8.
Bekaroğ lu, Ö., & Tan, N. (1986). Genel kimya (teori ve problemler). İstanbul: Kipaş Dağ ıtımcılık.
- 9.
Dikman, E. (1975). Temel kimya (anorganik). İzmir: Ege Üniversitesi Fen Fakültesi Yayınları.
- 10.
Erdik, E., & Sarıkaya, Y. (1991). Temel üniversite kimyası (5. Baskı). Ankara: Hacettepe-Taş Kitapçılık Ltd. Şt.
- 11.
Ergül, S. (2006). Genel kimya. Ankara: Anı Yayıncılık.
- 12.
Hakdiyen, İ. (1960). Genel ve teknik kimya. İstanbul: Teknik Okulu Yayınları.
- 13.
Hazer, B. (1997). Genel kimya. Trabzon: Akademi Ltd. Şti.
- 14.
İrez, G. (2002). Temel kimya-1. Muğ la: Muğ la Üniversitesi Yayınları.
- 15.
Öncel, M. F. (1974). Genel kimya notları-1, Ankara.
- 16.
Öncel, M. F. (1976). Deney ve problemleri ile modern genel kimya-1. Ankara: Fen Yayınevi.
- 17.
Özcan, M. (1998). Modern temel kimya-I (Genişletilmiş 2. Baskı). Balıkesir: Vipaş Yayınları.
- 18.
Pamuk, F. (1984). Genel kimya. Ankara: Gazi Üniversitesi Yayınları.
- 19.
Saracoğ lu, A. S. (1983). Temel kimya (3. Baskı). İstanbul: Çağ layan Kitabevi.
- 20.
Saraç, A.S., Güvençoğ lu, A., & Soydan, A. B. (1983). Modern genel kimya ve çözümlü problemleri. İstanbul: Murat Matbaacılık.
- 21.
Soydan, B., & Saraç, A.S. (1998). Genel üniversite kimyası ve modern uygulamaları (2. Baskı). İstanbul: Seç Yayın Dağ ıtım.
- 22.
Şenvar, C. (1989). Temel kimya. Ankara: Hacettepe Üniversitesi Yayınları.
- 23.
Tosun, F. (1969). Genel kimya, prensipler. Trabzon: Karadeniz Teknik Üniversitesi Yayınları.
- 24.
Tunalı, N. K., & Aras, N. K. (1977). Kimya temel kavramlar (11. Baskı). Ankara: Başarı Yayınları.
- 25.
Ün, R. (1967). Genel kimya (Genel ve anorganik). Trabzon: Karadeniz Teknik Üniversitesi Yayınları.
- 26.
Ünal, S. (1992). Genel kimya. İstanbul: Marmara Üniversitesi Yayınları.
- 27.
Yavuz, O. (1978). Genel kimya. Erzurum: Atatürk Üniversitesi Basımevi.