Part II of this two-part article on the history of the teaching of qualitative analysis covers the hydrogen sulfide problem, the debates over why and how qualitative analysis should be taught to undergraduate chemistry majors, and comments on its eventual demise. It also contains the references for both parts.
La segunda parte de este artículo sobre la historia de la enseñanza del análisis cualitativo abarca el problema del sulfuro de hidrógeno, los debates sobre por qué y cómo se debe enseñar el análisis cualitativo a los estudiantes de licenciatura de química y comentarios sobre su eventual desaparición. También contiene las referencias para ambas partes.
The single most persistent problem to dog the teaching of qualitative analysis throughout its 175 years of existence was the problem of hydrogen sulfide, whether it be the question of the best method of generating and storing it in the laboratory, or – given its obnoxious odor and significant toxicity – the question of whether it should be dispensed with altogether.
For the first century or so this gas was usually generated using the reaction between aqueous hydrochloric acid and solid iron sulfide:
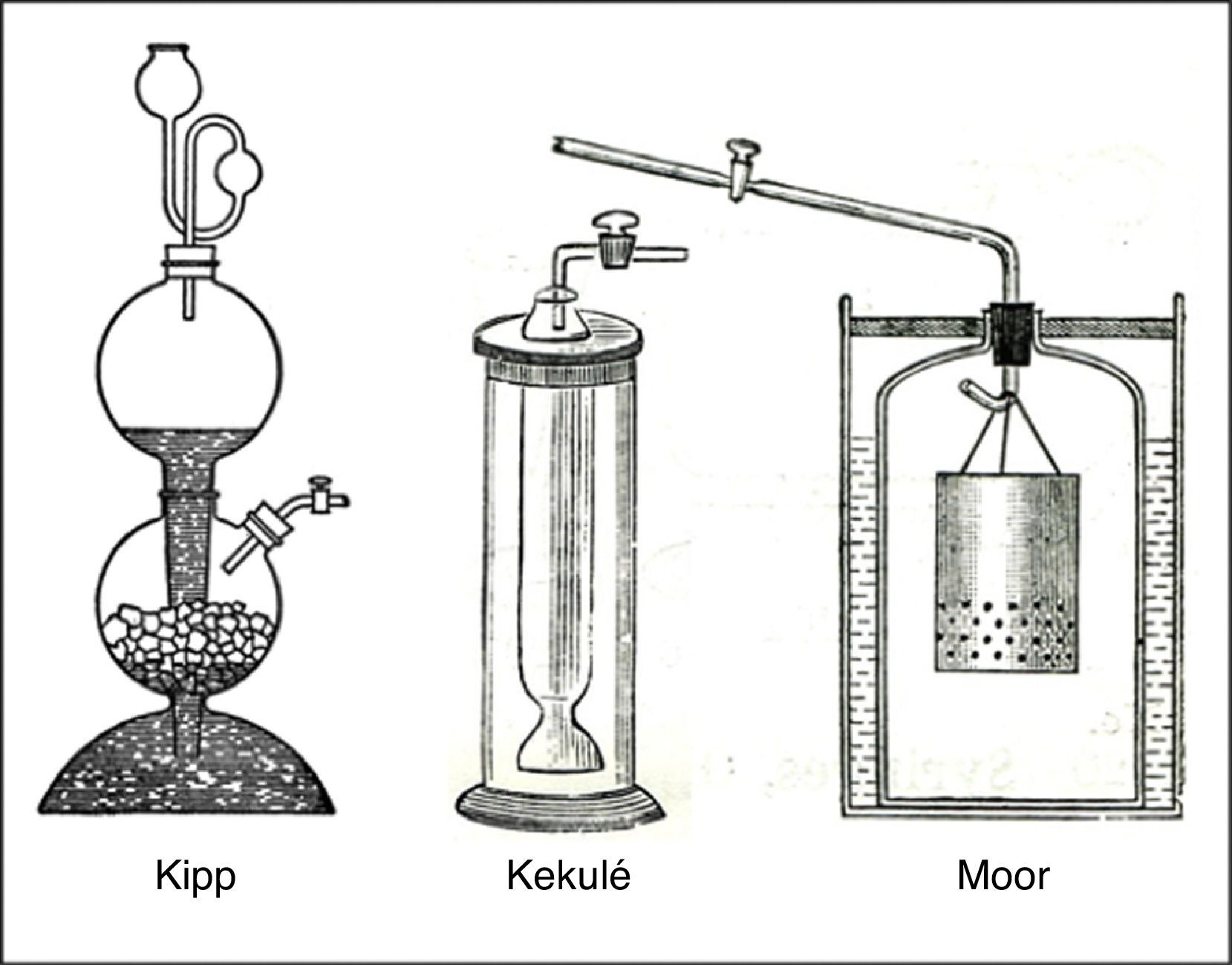
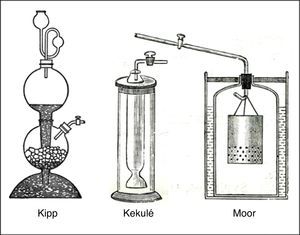
Though, like all gas-generating reactions involving solid and liquid reactants, this reaction could be carried out in a simple gas-generating bottle, this did not allow for stopping and starting the flow of gas as required. Consequently a variety of more elaborate gas generating devices were developed for this purpose, most of which made use of either hydrostatics or gravity to provide the desired control (Aynsley & Campbell, 1958).The most famous of the hydrostatic or gasometer devices was the gas generator introduced by the Dutch apothecary and instrument maker Petrus J. Kipp in 1844. This consisted of three bulbs or sections (Fig. 9). The top most section was removable and served as a funnel and reservoir for the HCl(aq). The middle and bottom bulbs were a single unit and were joined by means of a narrow contraction or neck. The middle section also had an opening for an exit tube and stopcock and the bottom section an opening with a glass stopper, whereas the top section had a long stem and was joined to the top of the middle section by means of a ground-glass joint, such that the stem passed through this section into the lower most section. The thickness of this stem was just sufficient to barely clear, but not seal, the contraction between the middle and lower sections.
With the bulbs in place, the stopcock was removed from the middle section and chunks of FeS(s) added through the opening until this section was about a quarter full. Because of the partial blockage of the opening between the middle and lower sections by the reservoir stem, these solid chucks could not fall into the bottom section. The stopcock was then reattached and placed in the closed position, followed by addition of sufficient HCl(aq) to the top reservoir to fill the bottom bulb and, via passage through the narrow neck, to cover the FeS(s) in the middle bulb. As the ensuing reaction generated H2S(g), the increasing gas pressure in the middle bulb would force the HCl(aq) out of this bulb back into the lower bulb and up the stem of the reservoir, and thus terminate gas production. When the stopcock was opened to use the gas, the decreasing gas pressure in the middle bulb, in conjunction with the hydrostatic pressure in the reservoir, would force HCl(aq) back up into the middle bulb and thus once again allow generation of H2S(g). The net result was a replenishable supply of H2S(g) under moderate pressure that could be turned on and off at will.
By the second half of the century, laboratory supply houses were offering Kipp generators ranging in size from 250mL to 4L and literally dozens of variations were being proposed in the literature, some of which are shown in Fig. 9. Despite their great variation in shape and size, all of these generators worked on the same hydrostatic principle as Kipp's original device.
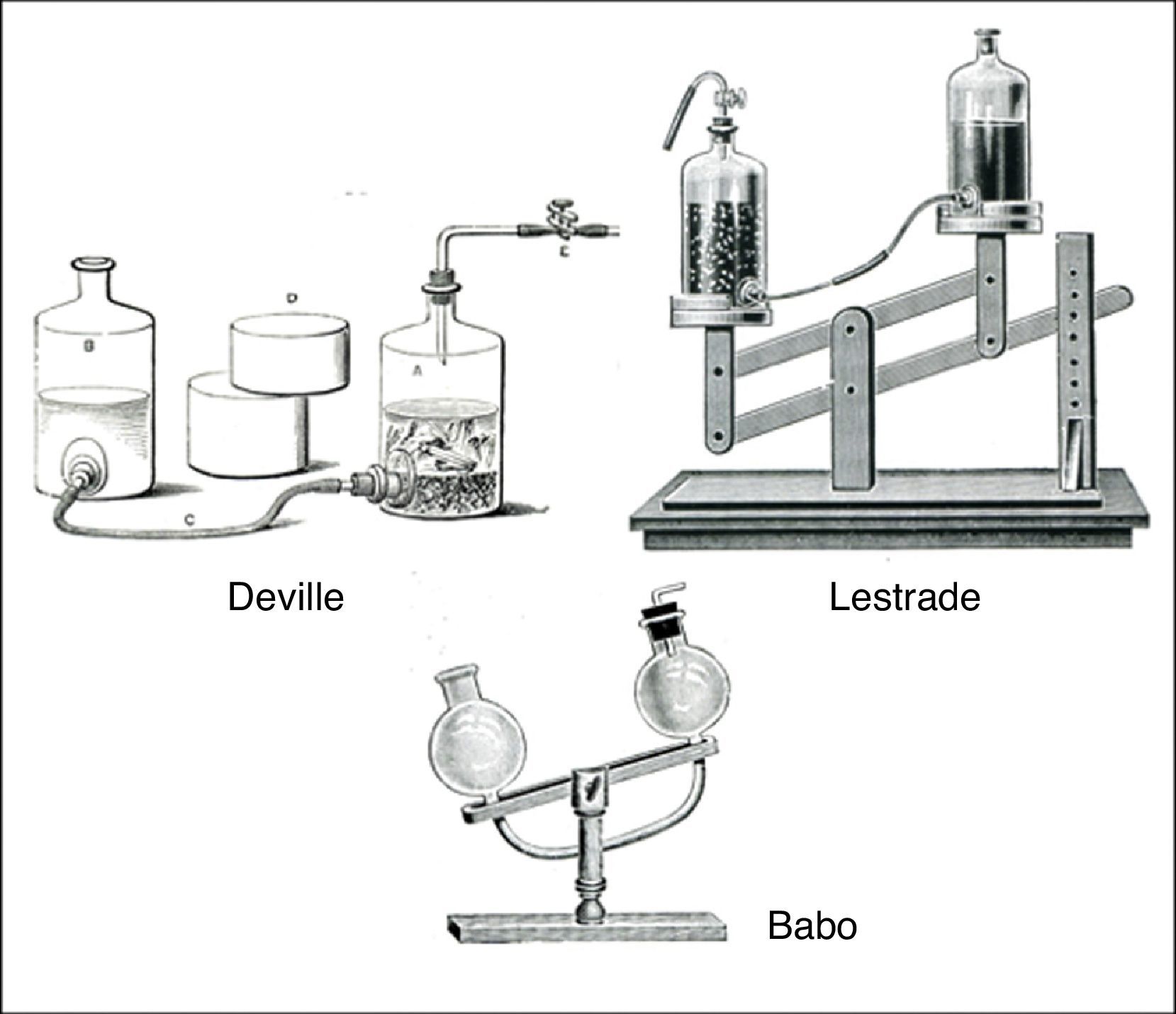
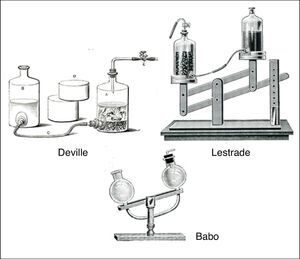
The most famous of the gravitational devices was the simple gas generator proposed by the German chemist Lambert von Babo. This consisted of two glass bulbs with openings connected by means of a curved glass tube and mounted in a stand that allowed them to be rocked back and forth (Fig. 10). The HCl(aq) was placed in one bulb and the FeS(s) in the other and the latter connected via its opening to a tube and stopcock. When the FeS(s) bulb was in the down position, the HCl(aq) would flow into the FeS(s) bulb and generate H2S(g). When the HCl(aq) bulb was in the down position, the acid was no longer in contact with the FeS(s) and H2S(g) generation would stop. Once again, many variations of this device were proposed, some of which are shown in Fig. 10. The only downside of these devices was, if the FeS(s) chunks became too small, they could be flushed into the HCl(aq) reservoir, with the result that lowering it would no longer terminate H2S(g) generation.
By the beginning of the 20th century, laboratory classes in qualitative analysis at many universities had become so large that several schools actually piped H2S(g), produced in large centralized cast-iron gasometric HCl-FeS generators, directly into the laboratory. At the University of Sydney, in Australia, the spigots for the H2S(g) were located at the individual lab benches (Fig. 11) and were each surrounded by a small glass fume cupboard (Tilden, 1916), whereas at the University of Wisconsin, in the United States, the gas spigots were located in special communal side hoods and had automatic shutoff valves to guard against careless students walking away after failing to turn off the H2S(g) supply (Ihde, 1990). At Sydney the central gasometer or generator was located outside under a lean-to, whereas at Wisconsin it was located in the basement. The author recalls historian Aaron Ihde telling him that this basement location led to the death of at least one janitor who was responsible for recharging the generator and who was found dead on the floor of the room after having been overwhelmed by the toxic gas.
A second approach to the hydrogen sulfide problem was to replace the gas with alternative and more easily manageable solid or liquid sources of sulfide ion, usually in the form of various organic thio compounds. The literature on this approach was summarized as early as 1909 in a small monograph published by Donath (1909). The alternatives sources discussed by him included ammonium thioacetate, ammonium dithiocarbonate, and ammonium dithiocarbamate, as well as such inorganic sources as disodium thiosulfate and disodium sulfide. Also included in the monograph were various alternative qual schemes based on the use of these reagents.
To the best of my knowledge, none of these alternatives were ever widely adopted in the teaching of qualitative analysis, and it was not until Barber and Grzeskowiak recommended the use of thioacetamide in 1949 that this approach finally had a significant impact (Barber & Grzeskowiak, 1949). This compound is stable in water at room temperature but above 80°C rapidly undergoes hydrolysis to give hydrogen sulfide and ammonium acetate:
Thus it was an ideal choice for the in situ generation of small quantities of hydrogen sulfide like those required by the semi-micro schemes that were rapidly displacing the more traditional macro approach by the late 1940s and early 1950s. Though articles exploring yet other organic thio compounds continued to appear (Clark & Neville, 1959; Page, Machel, & Ramsay, 1959), thioacetamide quickly became the reagent of choice and was soon the subject of articles in the educational literature recommending its use (Gunning, 1955; Lehrman & Schneider, 1959). Only when its potential carcinogenic properties were revealed in the 1980s was the initial enthusiasm moderated (Elo, 1987).As early as 1938 yet a third method for generating small quantities of hydrogen sulfide was brought to the attention of chemical educators by Jackson and Suhrer (1938). This was based on the dehydrogenation of long-chain hydrocarbon waxes upon heating them with elemental sulfur. Though the reaction is probably quite complex, the underlying idea can be summarized by means of the generalized equation:
and could be achieved by simply heating a mixture of paraffin, sulfur and an inert filler in a small test tube with an attached cork and delivery tube. Gas generation ceased when the heating was stopped and thus this arrangement could serve as a simple H2S(g) generator for a course in semi-micro analysis. Indeed, as the above authors noted, by 1938 ready-made pellets of this mixture were commercially available under the trade name of “Aitch Tu Ess” and this is the method that the present author used when first learning qual in the early 1960s (Jensen, 2013).Yet a fourth and final approach to the hydrogen sulfide problem was to eliminate the use of the gas altogether. As early as 1869 Zettnow published a small laboratory manual in which both hydrogen sulfide and ammonium sulfide were replaced by such group reagents as sulfuric acid, zinc metal, barium carbonate, ammonium carbonate, and sodium phosphate (Zettnow, 1867), and in his 1909 monograph Donath described a similar sulfide-free scheme based on the use of hydrazine and hydroxylamine salts (Donath, 1909). Since World War I both the research literature (Almkvist, 1918; Bascans, Olivers, Scoseria, & Serra, 1990; Lumme & Tummavuori, 1973) and the educational literature (Brockman, 1939; Cornog, 1938; Dobbins & Gilreath, 1945; Gaggero & Luaces, 1992; Munro, 1934) have featured numerous hydrogen sulfide-free qual schemes, several of which have relied on simple organic reagents, such as ammonium benzoate (West, Vick, & LeRosen, 1953) and phthalic acid (Lumme & Tummavuori, 1973), for group separations. Though several of these schemes were actually published as textbooks (Brockman, 1930; Cornog, 1948; West et al., 1953), they for the most part had no significant impact on the teaching of qual beyond the confines of the schools from whence they originated, thus illustrating once again the remarkable resiliency of the original sulfide-based qual scheme pioneered by Rose and Fresenius nearly 175 years ago.
PedagogyBut far more important than issues of scale, advances in separation techniques, the introduction of tailored organic reagents, or the use or nonuse of hydrogen sulfide was the pedagogical issue of why qualitative analysis should be taught in an introductory chemistry course in the first place, and it was largely the failure of chemistry teachers to reach a consensus on this issue that ultimately led to the subject's virtual demise.
This question was raised as early as 1928 by J. S. Guy in an article published in the recently founded Journal of Chemical Education (Guy, 1928). Guy felt that there were three possible reasons for teaching such a course:
- 1.
To teach students the practical laboratory art of qualitative inorganic chemical analysis.
- 2.
To serve as a training ground for the writing and balancing of chemical reactions.
- 3.
To teach the basic principles of chemistry as exemplified in the laboratory by the aqueous solution chemistry of the common metals.
Though the first of these objectives was most certainly the one adopted by all 19th-century courses in qualitative analysis, Guy felt that it was no longer appropriate for a 20th-century introductory college chemistry course, though he grudgingly acknowledged that it might still have some value for mining and engineering schools.
Indeed, this motive for teaching qual was rejected in far more specific terms by Louis P. Hammett of Columbia University (Fig. 12) in his manual of qualitative analysis, published the next year. As stated in his preface (Hammett, 1929): This book is not an attempt to teach an immediately useful practical art. A properly conducted course in qualitative analysis does teach much valuable analytical chemistry, in the sense of general principles, typical methods, and some experience in technique. But this is not a practical art, and cannot be so as long as we must exclude elements as common as tungsten and vanadium lest the course become too time consuming.
The second objective listed by Guy probably strikes the modern teacher as eccentric, but it was a reflection, as he noted, of what often happened on quizzes and exams in a typical qual course and so became, in practice, the actual objective of the course even when not considered to be so in theory.
It was the third objective that Guy fully endorsed, arguing that the laboratory course should be coupled with lectures dealing with such topics as the theory of ionic dissociation, the laws of equilibrium and mass action, the colloidal behavior of precipitates etc. This was in fact the essence of Ostwald's famous 1894 monograph on the foundations of analytical chemistry (Ostwald, 1894), but by 1928 this classic was largely unknown to the newer generation of chemistry teachers. However, its lessons had already been taken to heart by several authors of qualitative analysis manuals, among whom Guy singled out for special mention the pioneering 1911 textbook by Julius Stieglitz of the University of Chicago, noted above (Stieglitz, 1911).
An even better example of this approach, however, was Louis Hammett's 1929 manual, mentioned earlier, which bore the title Solutions of Electrolytes with Particular Application to Qualitative Analysis. The order in which the topics are listed in this title is of great significance since, as they suggest, Hammett viewed qualitative analysis as but one way of illustrating the more general topic of the physical and chemical behavior of aqueous solutions of electrolytes (Hammett, 1929): This book is based upon the belief that a course in qualitative analysis is an ideal method of presenting and of illustrating by copious examples the general principles relating to the behavior of solutions of electrolytes; and that this part of physical chemistry is an indispensable part of the preparation for advanced work in chemistry and for the study of medicine and engineering. It is an attempt to make the fullest use of qualitative analysis as a means of teaching chemistry.
It might be thought that the change in objectives advocated by Guy, Hammett and others finally resolved the pedagogical debate and that, with this turn of events, the standard course in qualitative analysis had successfully identified a new and worthwhile teaching objective that assured it a continuing place in the chemical curriculum. Yet this was not to be the case. A mere 25 years after the appearance of Hammett's book, we read the following comments by the British analytical chemist Cecil L. Wilson (Charlot, 1942): No teacher of inorganic qualitative analysis who has made any attempt to remain abreast of movements within the subject during the past few years can ignore the uncertainty that exists regarding its precise function in the training of chemists. The “solution” to the problem adopted by some teachers, particularly in the United States of America – to drop the teaching of qualitative analysis quietly out of the course – is no solution, but is rather an evasion of the issues involved.
Implicit in these comments is a reversion to the first of Guy's three objectives – namely that an introductory course in qualitative analysis should reflect current, rather than traditional and largely outdated practices, in analytical chemistry. A possible solution to this problem, in Wilson's opinion, was a complete revamping of the course based on an aggressive application of new specific organic reagents and drop analysis as outlined in the 1954 English translation of the 1942 book for which his comments served as a foreword – Qualitative Inorganic Analysis: A New Physico-Chemical Approach, by the French analytical chemist Gaston Charlot (Charlot, 1942).
The Charlot book was ambitious to say the least and went far beyond the simple use of drop reactions as confirmatory tests that had been advocated in the earlier schemes of van Nieuwenburg and Davis (Davis, 1940; van Nieuwenburg & Dulfer, 1933). Rather Charlot believed that he and his coworkers had identified a series of specific colorimetric tests, based on the use of new organic reagents, the use of masking agents, and the proper adjustment of pH and other reaction conditions, which allowed one to dispense with virtually all separations and to directly test an unknown for each cation individually (Charlot, Bezier, & Gauguin, 1950). As for the book's “new physico-chemical approach” this appears to have consisted of the qualitative use of a series of either concentration–pH or Eh–pH plots to determine optimal reaction conditions.
An objective look at the book quickly reveals that it lacks the organization and detailed laboratory directions required for an introductory course directed at freshmen and would in fact prove something of a challenge even for a first-year graduate student. More serious is the fact that, by dispensing with separations, it also eliminated one of the most important intellectual aspects of a traditional qual course – the logical application of a sequence of specific reactions to attain a predetermined goal. Rather surprisingly, none of the authors quoted above called attention to this aspect of a qual course, though it was the feature that the current author found most compelling when first learning about qualitative analysis back in the 1960s (Jensen, 2013).
Teaching students to select and logically apply a sequence of organic type reactions to achieve the synthesis of a specific compound from a given set of starting materials is considered to be an important part of intellectual training in organic chemistry, yet precisely the same kind of training was implicit in the sequential application of standard ionic reactions to achieve the separation and identification of a given set of cations. Indeed, application of this training formed the culmination of many qual courses in the form of the so-called nine-bottle problem in which students were given a list of nine solutes and nine unlabeled solutions and asked to formulate a sequence of reactions using only the solutions in question that would allow them to deduce which solute was in which bottle (Finholt, 1984; MacWood, Lassettre, & Breen, 1940). In sharp contrast, Charlot's collection of specific cation tests leaves one with the impression that they are dealing with a collection of magic recipes. However convenient and efficient for the practicing analytical chemist, they seem devoid of any larger intellectual lesson for the introductory student.
Yet a second problem with spot tests that rely on a heavy use of specially designed organic reagents is that they subvert the use of the qual scheme to teach the descriptive and theoretical solution chemistry of simple inorganic compounds, such as chlorides, nitrates, oxides, hydroxides, carbonates, and sulfides, as well as simple coordination compounds. The manner in which the qual scheme could be used for this purpose was illustrated in great detail by A. F. Clifford's 1961 text, Inorganic Chemistry of Qualitative Analysis. In his preface to this 500-page textbook, Clifford outlined the assumptions of such an approach (Clifford, 1961): It has been long recognized by instructors of undergraduate chemistry that there is little need to teach qualitative analysis for its own sake. Actual analyses are very seldom carried out in this manner any longer. It is nevertheless true that the classical analytical scheme is one of the best vehicles ever devised for teaching the systematics of inorganic chemistry. The purpose of this book, then, is not to teach methods of analysis, but rather to give as thorough a grounding as possible in the chemical relationships in the periodic table on which the classical analytical scheme is founded. This is done in terms of trends in solubility, trends in acidity and basicity, trends in oxidizing and reducing power, and the like. In order to accomplish this intelligibly, such topics as electronegativity, oxidation potentials, and the equilibrium principle are treated, the last extensively.
Clifford also went on to make the points outlined above concerning the pedagogical consequences of the overuse of organic reagents and spot tests (Clifford, 1961): The laboratory procedures have been selected for their pedagogical worth rather than for their analytical utility. For example, the detection of strontium is accomplished with a saturated calcium sulfate solution in order to demonstrate the trend of solubilities of the alkaline-earth sulfates, rather than, for example, by complexing calcium with triethanolamine which, from the analytical point of view, is more satisfactory but which teaches very little. For the same reason, the use of organic spot test reagents has been reduced to a minimum to emphasize inorganic reagents which perhaps are less satisfactory analytically but which nevertheless illustrate fundamental inorganic chemistry better.
Though 32 years after its publication, Laing would attempt to revive the descriptive inorganic approach to qual advocated by Clifford in his textbook (Laing, 1993), the timing of the original book's publication could not have been worse. The 1960s saw the beginnings of a major change in the nature of the introductory university chemistry course whereby it was transformed from a traditional course in descriptive inorganic chemistry into a baby physical chemistry course. The newer generation of freshman chemistry teachers was less and less interested in teaching the details of basic descriptive inorganic chemistry, let alone in illustrating it in detail in the laboratory. The qual course had traditionally consumed an entire semester of freshman laboratory. Now more and more schools either eliminated it altogether or truncated it to a single experiment in which either the analysis of the silver group or some version of the nine-bottle experiment was used as a “representative example.”
The consequences of this change were brought home to the author in the early 1980s during his first teaching job. I was lecturing on the origin of Werner's coordination theory to a senior class in inorganic chemistry and observed that the difference in the behavior of chloride ligands in the first versus the second coordination sphere of a metal complex was easily demonstrated using the standard chloride test. I had naively assumed that every chemistry student knew that the standard analytical test for the chloride ion was to precipitate it as silver chloride, but to my horror I found myself instead facing a roomful of blank stares. Something in the chemical universe had changed and that something was directly traceable to the disappearance of the standard qual course.
At present very few schools teach an entire semester of qualitative analysis and the plethora of qual manuals characteristic of the late 19th century and the first half of the 20th century is no more. One of the very few American qual manuals to survive this upheaval is the volume by C. Harvey Sorum of the University of Wisconsin (Fig. 13). This volume can be traced back to a macro qual manual first published by Louis Kahlenberg and J. H. Walton in 1911 (Kahlenberg & Walton, 1911). By 1922 at least four editions of this text had appeared and, starting in 1937, it was continued under the authorship of Walton and C. H. Sorum for at least another 12 years (Walton & Sorum, 1937). Finally, in 1949, Sorum replaced it with a manual of semi-micro qualitative analysis which he saw through four editions before being joined by Joseph Lagowski of the University of Texas-Austin (Fig. 14) for the 5th edition in 1977 (Sorum, 1949; Sorum & Lagowski, 1977). Lagowski, in turn, has seen the book through three more editions, the last of which was published in 2005. Thus this book has a history spanning more than a century or almost as long as that of the classic text by Fresenius.
When interviewed in 2010, Lagowski touched on his reasons for continuing to teach qualitative inorganic analysis (Cardellini, 2010): I know that qualitative analysis is not very popular with many teaching chemists today, but I like the subject because it allows students to learn about descriptive chemistry in an interesting way. That is, students can be trained to do simple manipulation techniques in the laboratory – measuring, mixing, observing, estimating – in the context of a simple unknown. For example, given access to the substances hydrochloric acid, aqueous solutions of sodium carbonate, silver nitrate, and sodium hydroxide, all in unmarked containers, place the appropriate correct labels on the containers. You may recognize this “qual problem” as a version of the 10 solution experiment. The most valuable thing to be gained from a scientific education is the ability to find things out by experiment. Descriptive experiments whose results can be foretold by reference to the textbook are not good examples of scientific method, and it is precisely to the most intelligent students that they are most tiresome … It is the great virtue of analytical chemistry as a teaching instrument that it sets problems which can only be answered by experimentation.
The authors declare that they have no conflicts of interest.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.
Figures are numbered consecutively with those in Part I.