In patients receiving total parenteral nutrition (TPN), the frequency of hyponatraemia is high. However, the causes of hyponatraemia in TPN have not been elucidated, although diagnosis is required for appropriate therapy. The aim of this study is to describe the aetiology of hyponatraemia in non-critical hospitalised patients receiving TPN.
MethodsProspective multicentre study in 19 Spanish hospitals. Non-critically hyponatraemic patients receiving TPN and presenting hyponatraemia over a 9-month period were studied. Data collected included sex, age, previous comorbidities, and serum sodium levels (SNa) before and following TPN initiation. Parameters for study of hyponatraemia were also included: clinical volaemia, the presence of pain, nausea, gastrointestinal losses, diuretic use, oedema, renal function, plasma and urine osmolality, urinary electrolytes, cortisolaemia, and thyroid stimulating hormone.
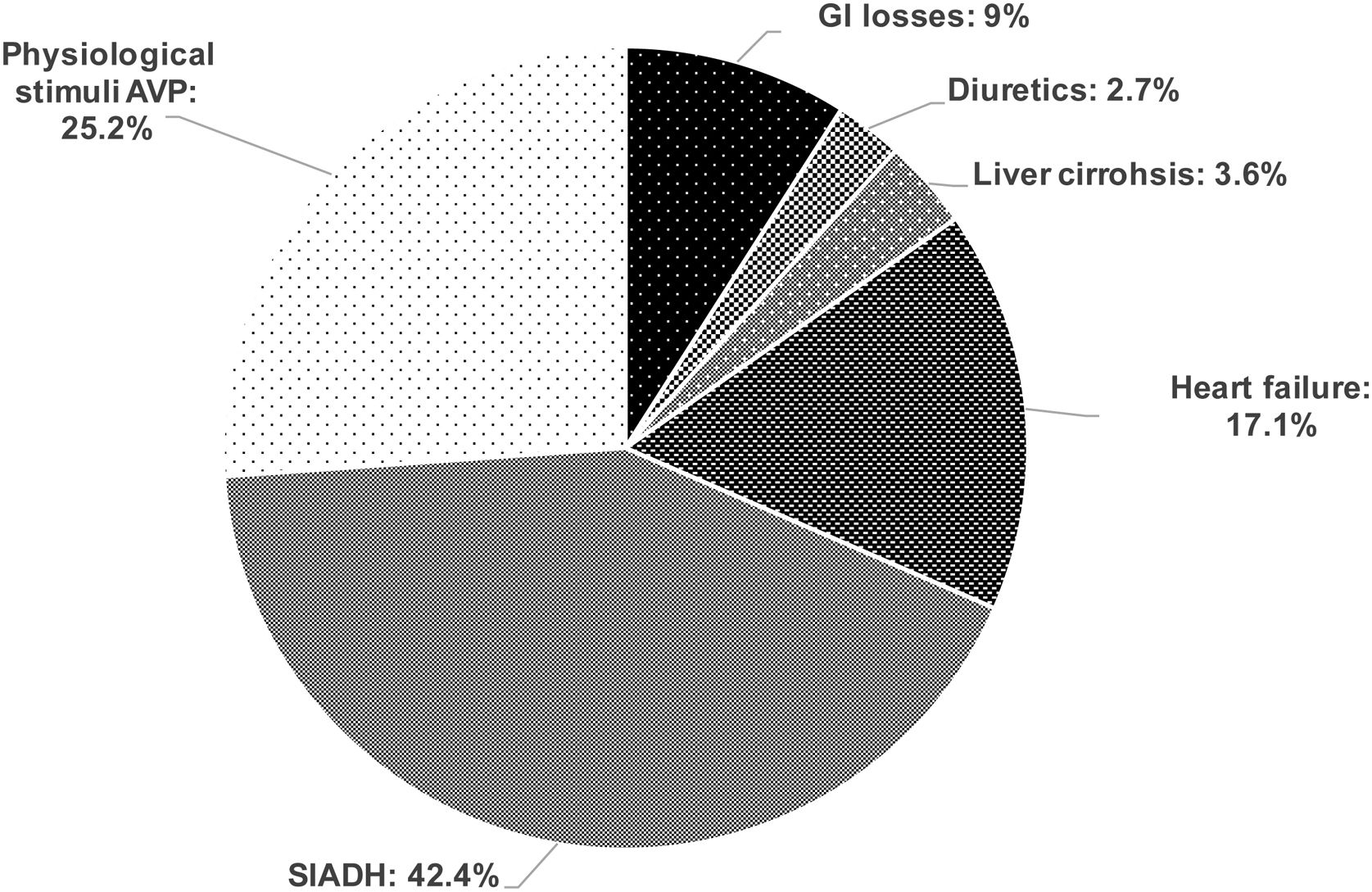
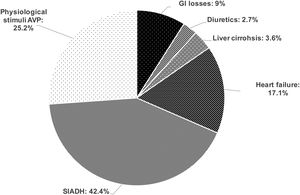
Results162 patients were included, 53.7% males, age 66.4 (SD13.8) years. Volume status was evaluated in 142 (88%): 21 (14.8%) were hypovolaemic, 96 (67.6%) euvolaemic and 25 (17.6%) hypervolaemic. In 111/142 patients the analytical assessment of hyponatraemia was completed. Hypovolaemic hyponatraemia was secondary to GI losses in 10/111 (9%), and to diuretics in 3/111 (2.7%). Euvolaemic hyponatraemia was due to Syndrome of Inappropriate Antidiuretic Hormone secretion (SIADH) in 47/111 (42.4%), and to physiological stimuli of Arginine Vasopressin (AVP) secretion in 28/111 (25.2%). Hypervolaemic hyponatraemia was induced by heart failure in 19/111 (17.1%), cirrhosis of the liver in 4/111 (3.6%).
ConclusionsSIADH was the most frequent cause of hyponatraemia in patients receiving TPN. The second most frequent cause was physiological stimuli of AVP secretion induced by pain/nausea.
La hiponatremia es una complicación frecuente en pacientes con nutrición parenteral total (NPT). Aunque su diagnóstico es esencial para indicar el tratamiento apropiado, sus causas no han sido evaluadas. El objetivo del presente estudio es describir la etiología de la hiponatremia en pacientes no críticos que reciben NPT.
Material y métodosEstudio multicéntrico prospectivo en 19 hospitales españoles. Se incluyeron pacientes no críticos que recibieron NPT y presentaron hiponatremia durante un periodo de 9meses. Se recogieron datos demográficos, comorbilidades previas, natremia sérica antes y durante la administración de NPT, y parámetros del estudio de hiponatremia: volemia clínica, presencia de dolor/náuseas, pérdidas gastrointestinales, uso de diuréticos, edema, función renal, osmolalidad plasmática y urinaria, iones en orina, cortisolemia y hormona estimulante del tiroides.
ResultadosSe incluyeron 162 pacientes, 53,7% varones, con una media de edad de 66,4 (DE 13,8) años. La volemia clínica se evaluó en 142 (88%): 21 (14,8%) hipovolémicos, 96 (67,6%) euvolémicos y 25 (17,6%) hipervolémicos. En 111/142 pacientes se completó el estudio bioquímico de hiponatremia. La hiponatremia hipovolémica fue secundaria a pérdidas gastrointestinales en 10/111 (9%) y diuréticos en 3/111 (2,7%). La hiponatremia euvolémica fue por síndrome de secreción inadecuada de la hormona antidiurética (SIADH) en 47/111 (42,4%) y por estímulo fisiológico de arginina-vasopresina (AVP) en 28/111 (25,2%). La hiponatremia hipervolémica fue inducida por insuficiencia cardiaca en 19/111 (17,1%) y por cirrosis hepática en 4/11 (3,6%).
ConclusionesEl SIADH fue la causa más frecuente de hiponatremia en pacientes que reciben NPT, seguida del estímulo fisiológico de AVP por dolor/náuseas.
Hyponatraemia is the most frequent electrolyte disturbance in hospitalised patients.1 The rate of hyponatraemia is even higher in patients receiving total parenteral nutrition (TPN) than described in the general adult hospitalised population. In fact, a recent retrospective study found that 40% of patients receiving TPN developed hyponatraemia at some point during hospitalisation, versus 25.8% of control subjects who did not receive TPN.2
Hyponatraemia is associated with increased morbimortality.3,4 In fact hyponatraemic patients on TPN have been found to present a higher mortality rate than normonatraemic patients receiving TPN.2 The association between hyponatraemia and mortality was strongest in patients with sustained hyponatraemia – 75% of serum sodium (SNa) measurements below 135mmol/L. A recent meta-analysis found that improvement of hyponatraemia was associated with a reduction of the mortality rate of hospitalised patients.5 Furthermore, active treatment of hyponatraemia reduces hospital length-of-stay.6
Hyponatraemia reflects a relative excess of serum water with respect to serum sodium, even in the case of patients with a net loss of total body sodium and fluids. This relative fluid excess is induced by renal water retention, caused in 90% of cases by non-osmotic secretion of Arginine Vasopressin (AVP) - the human antidiuretic hormone.7 In other words, hypothalamic AVP secretion is not inhibited by a descent in plasma osmolality. Physiological non-osmotic AVP secretion occurs when there is a marked descent in effective circulating blood volume, as is the case in hyper- and hypovolaemia. Pain and nausea are potent stimuli of AVP secretion,8–10 and can induce marked euvolaemic hyponatraemia. Non-physiological AVP secretion inducing euvolaemic hyponatraemia is referred to as the Syndrome of Inappropriate ADH Secretion (SIADH), and is the most frequent cause of hyponatraemia.11
To correct non severe hyponatraemia, volume status must be evaluated, followed by diagnosis of the aetiology of the hyponatraemia. Thus, patients with hypovolaemic hyponatraemia are treated with intravenous (iv) isotonic saline. Patients with hypervolaemic hyponatraemia, characterised by fluid secretion into in a third space, are usually treated with fluid restriction and diuretics. Patients with euvolaemic hyponatraemia presenting pain are treated with analgesia, and when presenting nausea with antiemetic. Patients with SIADH can be treated with fluid restriction or tolvaptan.12,13 The application of these simple therapeutic rules has been previously applied in a series of 20 patients with hyponatraemia receiving TPN. Normonatraemia was reached within 72h in 15 of the patients.14
The aim of this prospective multicentre study was to determine the aetiologies of hyponatraemia in patients receiving TPN.
MethodsStudy designThis is a prospective, multicentre, observational study. Nineteen Spanish hospitals participated in the study (18/19 are teaching hospitals).
Between June of 2015 and February of 2016, all patients receiving TPN as the sole source of nutrition were included in the study, whether hyponatraemia was previous to TPN initiation or was developed during TPN. Patients were excluded if the TPN was started in the intensive care unit, if patients were receiving partial parenteral nutrition, if TPN duration was <48h, if subjects were pregnant, or <14 years of age. This study was approved by a Research Ethics Committee. All participants gave written informed consent. Patients were collected consecutively, and this was done by a single researcher from each centre.
The total parenteral nutrition protocolThe TPN formula, including the amounts of macronutrients, electrolytes and the total volume, was prescribed by a member of the hospital nutrition department according to usual clinical practice and the relevant guidelines.15–17 All patients receiving TPN were seen daily by this physician, who recorded the data of the study variables. Determinations of SNa, glycemia levels and renal function–serum creatinine and urea levels- were made before TPN and every 3–4 days following its initiation. In patients in whom hyponatraemia was detected, the medical research requested a specific analytical study at the same time, regardless of whether it was detected before or during TPN. The medical research also evaluated the presence of nausea, pain, vomiting and other data necessary to establish the aetiology of hyponatraemia.
Data collectionPatient data collected included gender, age, previous comorbidity (history of kidney, liver, respiratory or cardiac disease), anthropometric data (weight, height, body mass index-BMI-) and nutritional assessment by subjective global assessment (SGA) before starting TPN. Other data recorded included gastrointestinal (GI) losses (diarrhoea, intestinal fistulae and GI obstruction), the presence or absence of pain, of nausea/vomiting and oedema/ascites, as well as concomitant use of diuretics. The indication and composition of TPN were also registered. Laboratory tests and biochemical study of hyponatraemia (serum/urinary electrolytes and osmolality, renal function, thyroid stimulating hormone-TSH- and cortisol) were performed at the laboratories of each hospital. Cortisol value was not assessed in patients treated with corticosteroids. SNa was measured using indirect ion-selective electrolyte methodology. Osmolalities were determined by osmometer. Effective plasma osmolality (tonicity) was calculated by the following formula: 2xNa+glycemia/18, where SNa is expressed in mmol/L and glycemia is expressed in mg/dl.18
Hyponatraemia: definition and detectionHyponatraemia was defined as a SNa below 135mmol/L, following correction for glycemia, in the presence of a plasma osmolality below 280mOsm/kg. SNa was corrected for glycemia by adding 1.6mmol/L of SNa for every 100mg/dl of glycemia over 100mg/dl up until a glycemia of 400mg/dl, from which point 4mmol/L were added for every 100mg/dl increment in glycemia.19
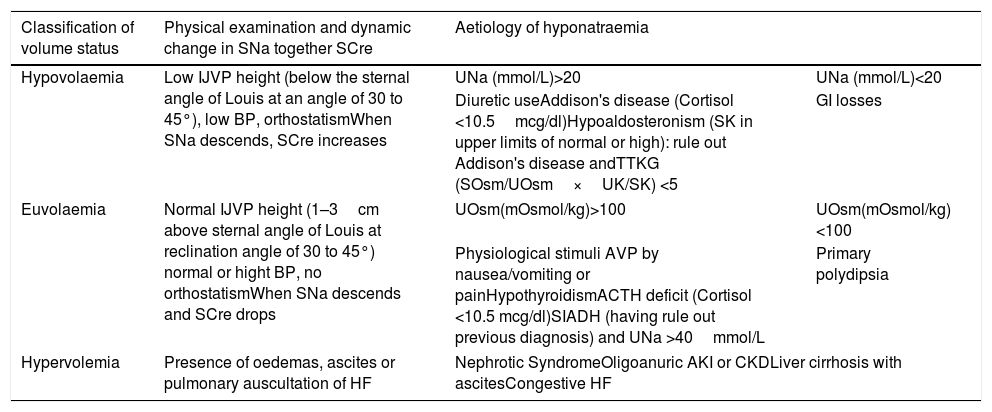
Hyponatraemia: classification of volume status and aetiological diagnosisOnce hyponatraemia was established, the next step was the evaluation of volume status by physical examination and the dynamic relationship between changes in SNa together with creatinine levels, as recommended by the guidelines on the diagnosis and treatment of hyponatraemia.12 Blood pressure, heart rate, the maximum height of the internal jugular vein pulse (can be identified as two descending waves, in close succession, between the cervical area behind the mandibular angle and the supraclavicular space),13 the presence/absence of oedemas, ascites, a pulmonary auscultation suggestive of heart failure were evaluated (Table 1). The aetiological diagnosis was then performed (Table 1). In patients with euvolaemic hyponatraemia presenting nausea/vomiting and/or pain, the diagnosis of euvolaemic hyponatraemia secondary to physiological stimuli of AVP secretion by nausea and/or pain was established when the relief of nausea and/or pain was followed by normalisation of SNa levels. SIADH was diagnosed in patients with euvolaemic hyponatraemia, in the absence of nausea/pain, hypothyroidism, adrenocoticotropic hormone (ACTH) deficit and diuretic use. Given the elevated urea levels observed sometimes in artificial nutrition,20 osmometer-measured plasma osmolality was higher than 275mOsm/kg. However, in all cases, calculated effective osmolality (tonicity) was <275mOsm/kg, with urine osmolality superior to 100mOsm/kg, and urine sodium levels >40mmol/L. Biochemical workup of hyponatraemia was considered complete when all of the following parameters had been measured: serum/urinary electrolytes and osmolality, renal function, TSH and cortisol.
Classification of volume status and aetiological diagnosis of hyponatraemia.
| Classification of volume status | Physical examination and dynamic change in SNa together SCre | Aetiology of hyponatraemia | |
|---|---|---|---|
| Hypovolaemia | Low IJVP height (below the sternal angle of Louis at an angle of 30 to 45°), low BP, orthostatismWhen SNa descends, SCre increases | UNa (mmol/L)>20 | UNa (mmol/L)<20 |
| Diuretic useAddison's disease (Cortisol <10.5mcg/dl)Hypoaldosteronism (SK in upper limits of normal or high): rule out Addison's disease andTTKG (SOsm/UOsm×UK/SK) <5 | GI losses | ||
| Euvolaemia | Normal IJVP height (1–3cm above sternal angle of Louis at reclination angle of 30 to 45°) normal or hight BP, no orthostatismWhen SNa descends and SCre drops | UOsm(mOsmol/kg)>100 | UOsm(mOsmol/kg)<100 |
| Physiological stimuli AVP by nausea/vomiting or painHypothyroidismACTH deficit (Cortisol <10.5 mcg/dl)SIADH (having rule out previous diagnosis) and UNa >40mmol/L | Primary polydipsia | ||
| Hypervolemia | Presence of oedemas, ascites or pulmonary auscultation of HF | Nephrotic SyndromeOligoanuric AKI or CKDLiver cirrhosis with ascitesCongestive HF | |
SNa (serum sodium), SCre (serum creatinine), JVP (Internal Jugular Vein Pulse), BP (Blood pressure), UNa (Urinary sodium), TTKG (transtubular potassium gradient), SOsm (serum osmolality), UOsm (Urine osmolality); SK (serum potassium),UK (urinary potassium), GI (gastrointestinal), HF (heart failure), AKI (Acute Kidney Injury), CKD (chronic kidney disease), AVP (arginine vasopressin), ACTH (adrenocoticotropic hormone), SIADH (syndrome of inappropriate antidiuretic hormone secretion).
Continuous variables are described as means with standard deviation (SD) for normally distributed variables or as medians with interquartile range (IR) in the case of non-normal distributions. The comparisons between qualitative variables were made using the Chi-squared test, with Fisher’ s correction when necessary. The distribution of the quantitative variables was examined with the Kolmogorov–Smirnov test. The differences between quantitative variables were analysed with Student's T test, using nonparametric tests (Mann–Whitney when the study variables did not follow a normal distribution. For all calculations, significance was set at p<0.05 for two tails. Statistical analyses were performed using SPSS 19.0 (SPSS Inc., Chicago IL, USA).
ResultsDemographic and clinical characteristics of patientsA total of 162 patients were studied, with a median of 10 patients per hospital [IR 4–19]. Hyponatraemia was present in 77 (47.5%) at the start of TPN. A further 85 (52.5%) developed it during TPN infusion. Those patients developing hyponatraemia did so within 4 [IR 2–7] days of starting TPN. Only 4 patients presented SNa <125mmol/L, with a minimum level of 123mmol/L. The mean age was 66 (SD 13.8) years, and 87 (53.7%) were males. Previous comorbidities were detected in 38/162 patients: in 5 (3.1%) chronic kidney disease, in 6 (3.7%) acute kidney injury, in 5 (3.1%) liver cirrhosis, in 10 (6.2%) acute liver failure, in 9 (5.6%) chronic respiratory failure, in 1 (0.6%) acute respiratory failure, in 6 (3.7%) chronic heart failure and in 5 (3.1%) acute heart failure. Nutritional status assessed by SGA was as follows: 75 (46.9%) presented severe malnutrition (SGA C), 55 (34.4%) moderate malnutrition (SGA B) and 30 (18.8%) were normally nourished (SGA A). Mean BMI was 23.6 (SD 5.0) kg/m2. Half of the patients were receiving diuretics (81 patients), 78 (97%) of whom received furosemide and 3 (3%) thiazides. Gastrointestinal losses occurred in 100 (61.7%) patients, ascites or oedema were present in 48 (29%) patients, with nausea or vomiting in 89 (54.9%). More than half of the patients presented pain (99 patients), and 67 (41.4%) of them received opiates.
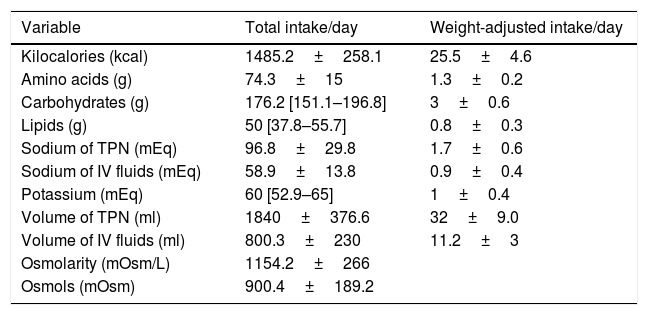
Characteristic of the total parenteral nutritionThe most frequent indications for TPN were bowel rest following surgery (24.7%), post-operative ileus (22.2%), bowel obstruction (17.9%), oral mucositis (6.8%), vomiting (9.9%), diarrhoea (3.7%) or various causes (14.8%). The median duration of TPN was 9 days [IR 5–14]. The osmolarity, total volume, energy, macronutrients, osmols and electrolytes in the TPN are presented in Table 2. The volume and sodium of the additional fluids to the TPN are also described in Table 2. Hyperglycaemia on TPN (fasting plasma glucose levels >126mg/dl) was present in 71 (43.8%) patients.
Description of composition of the parenteral nutrition administered and the volume and sodium of the additional fluids to the total parenteral nutrition (n=162).
| Variable | Total intake/day | Weight-adjusted intake/day |
|---|---|---|
| Kilocalories (kcal) | 1485.2±258.1 | 25.5±4.6 |
| Amino acids (g) | 74.3±15 | 1.3±0.2 |
| Carbohydrates (g) | 176.2 [151.1–196.8] | 3±0.6 |
| Lipids (g) | 50 [37.8–55.7] | 0.8±0.3 |
| Sodium of TPN (mEq) | 96.8±29.8 | 1.7±0.6 |
| Sodium of IV fluids (mEq) | 58.9±13.8 | 0.9±0.4 |
| Potassium (mEq) | 60 [52.9–65] | 1±0.4 |
| Volume of TPN (ml) | 1840±376.6 | 32±9.0 |
| Volume of IV fluids (ml) | 800.3±230 | 11.2±3 |
| Osmolarity (mOsm/L) | 1154.2±266 | |
| Osmols (mOsm) | 900.4±189.2 |
Data are described as medians (interquartile range) or as means±standard deviation. Kcal, Kilocalories; g, grams; ml, millilitres; mEq, milliequivalent; mOsm, milliosmol; L, litre; kg, kilograms; IV (intravenous); TPN (total parenteral nutrition).
Volume status was evaluated in 142 (87.6%) of the hyponatraemic patients. Euvolaemia was found in 96 (67.6%), hypervolaemia in 25 (17.6%), hypovolaemia in 21 (14.8%).
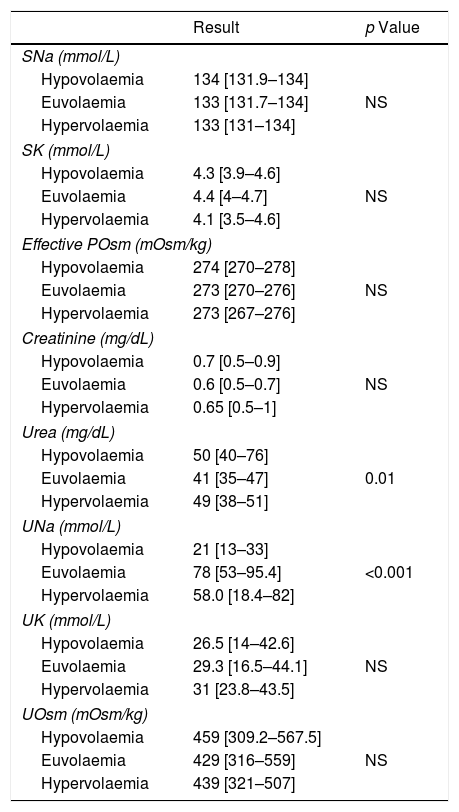
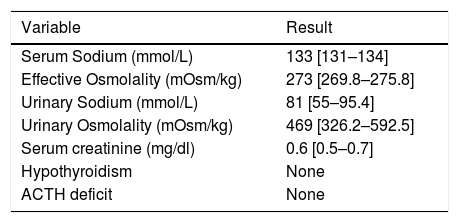
A complete biochemical investigation of hyponatraemia was performed in 111 (63.5%) patients. The results in these patients were as follows: serum sodium 133 [IR 132–134] mmol/L, serum potassium 4.3 [IR 3.9–4.6] mmol/L, creatinine 0.6 [IR 0.5–0.8] mg/dl (only 8 patients >1.2mg/dl), urea 50mg/dl [IR 40–60] mg/dl (15% of patients urea >80mg/dl), osmometer-determined plasma osmolality 279 [IR 274–285] mOsm/kg (70% of patients <280mOsm/kg), effective osmolality: 275 [IR 273–278] (100% of patients <280mOsm/kg), urine sodium 66 [IR 37–89] mmol/L, urine potassium 29.6 [IR 17.1–44] mmol/L, urine osmolality 434.0 [322–544] mOsm/kg. No patient presented adrenal insufficiency nor hypothyroidism. The results of the biochemical study as classified by volume status are shown in Table 3. Patients with hypovolaemic hyponatraemia presented significantly lower urinary Na levels than those who were euvolaemic. Serum urea levels were significantly higher in patients with hypovolaemic hyponatraemia than in euvolaemics. The results of the biochemical study of patients diagnosed with SIADH are shown in Table 4.
Description of biochemical study of hyponatraemia according to the volume status (n=111).
| Result | p Value | |
|---|---|---|
| SNa (mmol/L) | ||
| Hypovolaemia | 134 [131.9–134] | |
| Euvolaemia | 133 [131.7–134] | NS |
| Hypervolaemia | 133 [131–134] | |
| SK (mmol/L) | ||
| Hypovolaemia | 4.3 [3.9–4.6] | |
| Euvolaemia | 4.4 [4–4.7] | NS |
| Hypervolaemia | 4.1 [3.5–4.6] | |
| Effective POsm (mOsm/kg) | ||
| Hypovolaemia | 274 [270–278] | |
| Euvolaemia | 273 [270–276] | NS |
| Hypervolaemia | 273 [267–276] | |
| Creatinine (mg/dL) | ||
| Hypovolaemia | 0.7 [0.5–0.9] | |
| Euvolaemia | 0.6 [0.5–0.7] | NS |
| Hypervolaemia | 0.65 [0.5–1] | |
| Urea (mg/dL) | ||
| Hypovolaemia | 50 [40–76] | |
| Euvolaemia | 41 [35–47] | 0.01 |
| Hypervolaemia | 49 [38–51] | |
| UNa (mmol/L) | ||
| Hypovolaemia | 21 [13–33] | |
| Euvolaemia | 78 [53–95.4] | <0.001 |
| Hypervolaemia | 58.0 [18.4–82] | |
| UK (mmol/L) | ||
| Hypovolaemia | 26.5 [14–42.6] | |
| Euvolaemia | 29.3 [16.5–44.1] | NS |
| Hypervolaemia | 31 [23.8–43.5] | |
| UOsm (mOsm/kg) | ||
| Hypovolaemia | 459 [309.2–567.5] | |
| Euvolaemia | 429 [316–559] | NS |
| Hypervolaemia | 439 [321–507] | |
NaS (serum sodium), SK (serum potassium), SOsm (serum osmolality), UNa (urinary sodium), UK (urinary potassium), UOsm (urine osmolality), NS (not significant).
Description of biochemical study of patients diagnosed with SIADH (n=47).
| Variable | Result |
|---|---|
| Serum Sodium (mmol/L) | 133 [131–134] |
| Effective Osmolality (mOsm/kg) | 273 [269.8–275.8] |
| Urinary Sodium (mmol/L) | 81 [55–95.4] |
| Urinary Osmolality (mOsm/kg) | 469 [326.2–592.5] |
| Serum creatinine (mg/dl) | 0.6 [0.5–0.7] |
| Hypothyroidism | None |
| ACTH deficit | None |
Data are described as medians (interquartile range). mEq, milliequivalent; mOsm, milliosmol; L, litre; dl, decilitres; kg, kilograms; mg, milligrams.
Evaluation of volume status, of clinical characteristics and complete biochemical workup of hyponatraemia were carried out in 111 patients, permitting aetiological diagnosis. The causes of hyponatraemia are shown in Fig. 1.
DiscussionIn our prospective multicentre study of patients receiving TPN, the majority of patients with hyponatraemia were euvolaemic. SIADH was the most frequent diagnosis. This finding coincides with what has been described in the general hospitalised population.21,22 However, our finding that physiological stimuli of AVP secretion such as pain and/or nausea were the second most frequent cause of hyponatraemia has not been reported in other studies of hyponatraemic hospitalised patients. The therapeutic implications of this finding are evident and indicate the need for aggressive analgesia/antiemetic therapy in euvolaemic hyponatraemic patients with pain or nausea.
A previous prospective study of hospitalised patients by Cuesta et al.21 found a similar prevalence of SIADH in hospitalised patients with hyponatraemia: 43.5%, as compared with our finding of 42.4%. However, the percent of hyponatraemic patients that were found to be euvolaemic was lower: 43.5% versus our finding of 67.9%. This discrepancy could be explained by the high rate of nausea/vomiting and pain (physiological stimuli of AVP secretion) found in our TPN patients. In fact, nausea and pain are often found in patients who have undergone gastrointestinal surgery, one of the primary indications for TPN. Thus, in TPN patients presenting hyponatraemia, aggressive therapy of nausea and pain are essential both to establish the cause of hyponatraemia as well as for its treatment. In turn, it is also essential to avoid the development of pain and nausea/vomiting with the application of protocols during the post-surgical period and in oncology patients.
The most frequent cause of hypovolaemic hyponatraemia was the presence of GI losses. However, not all patients presenting GI losses developed hyponatraemia. Baroreceptor–mediated AVP secretion is induced when effective circulating blood volume has fallen by at least 5–10%.11 Patients with minor GI losses receiving sufficient iv fluids would thus not develop hypovolaemic hyponatraemia. The same case applies to patients receiving diuretic therapy: only those administered insufficient IV fluids would develop hypovolaemic hyponatraemia.
This is the first study to evaluate volume status for the aetiological diagnosis of hyponatraemia in patients receiving TPN. Two key elements were used to distinguish hypo- from euvolaemia: the physical examination, and the dynamic changes of SNa together with serum creatinine levels. The physical examination consisted of inspection of the maximum height of internal jugular vein pulse. Euvolaemic patients presented a maximum height between 1–3cm above sternal angle of Louis at reclination angle of 30° to 45°, whereas hypovolaemic patients presented a jugular vein pulse below the sternal angle of Louis. Since this evaluation by the physician could have been subjective in clinical practice, the dynamic relationship between changes in SNa together with creatinine levels was also considered to be important for classification of volume status. Euvolaemic patients presented a parallel descent in creatinine and SNa values. However, in hypovolaemic patients, SNa would descend as serum creatinine rose, indicating reduced renal perfusion. On the other hand, in our biochemical workup of hyponatraemia we found statistically significant differences in the levels of plasma urea and urinary sodium among patients with euvolaemic and hypovolaemic hyponatraemia. However, neither of them are useful to differentiate both types of volume status. In the case of urea levels, these can be affected by artificial nutrition.20 Regarding urinary sodium, it only has value in the differential diagnosis of hypovolaemic hyponatraemia, low in GI losses and high in renal sodium losses.12
Classification of volume status, evaluation of clinical characteristics and performing the appropriate laboratory parameters are necessary to correctly diagnosis and therefore treat hyponatraemia. A complete diagnostic workup is particularly important to correctly detect SIADH, a diagnosis reached after ruling out other causes of euvolaemic hyponatraemia. However, laboratory parameters are often incomplete, as found in the “Hyponatraemia Registry” study22 in which only 21% of patients diagnosed as having SIADH fulfilled biochemical criteria. In the current study, both classification of volume status and a complete biochemical workup were available in 63% of all patients with hyponatraemia and in the 100% patients diagnosed SIADH.
In patients receiving TPN, interpretation of osmometer- measured plasma osmolality can be difficult. Artificial nutrition induces rises in serum urea levels,20 thus increasing measured osmolality. However, urea is not an effective osmol, and does not influence the osmotic gradient between a hypotonic intravascular medium and the intracellular compartment. In these patients, effective serum osmolality (tonicity) must be calculated, by eliminating urea from estimating of plasma osmolality.18
There are several methodological limitations of this study. First, the number of patients recruited by each hospital was different. Although the number of patients was proportional to the size of the hospital. Second, the aetiology of hyponatraemia could be analysed in only 111 of the 162 patients who had a complete hyponatraemia workup. However, this is the first research work that has studied the aetiology of hyponatraemia in patients with TPN. Therefore, we consider it important to report our data. Third, not all physicians have the same level of experience in the inspection of the internal jugular vein pulse. However, we counterweighted this subjective assessment of the volume status by analysing the dynamic relationship between serum sodium changes and plasma creatinine levels. Fourth, the measurement of biochemical parameters in each respective participating hospital laboratory, instead to a single centre.
ConclusionsHyponatraemia induced by SIADH is the most frequent cause of hyponatraemia in non-critical patients receiving TPN. The second most frequent cause is hyponatraemia induced by nausea/vomiting and/or pain. Volaemic classification of volume status, evaluation of clinical characteristics and performing a complete biochemical workup are necessary for diagnosis.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Dr. Isabelle Runkle and Dr. Emilia Gómez Hoyos have worked in an advisory capacity for Otsuka, and given talks sponsored by Otsuka.
Conflicts of interestThe authors declare no conflicts of interest.
The Spanish Society of Endocrinology and Nutrition provided help with the publicity of the study among Spanish Nutrition specialists.