To analyze the compliance with the Guide for home enteral nutrition (HEN) of the Spanish national health system of the prescriptions made in a specific area (Health Area I of the Region of Murcia) before and after implementation of a clinical pathway based on that guide, and to compare the changes in healthcare costs of diet therapy during the 2007–2014 period in the Regional and National Health system.
MethodA descriptive study to quantify compliance with the main criteria of the HEN guide before (2010) and after (2013–2014) implementation of the clinical pathway. Changes in health expenditure and consumption during the 2007–2014 period were also analyzed.
ResultsAll markers of compliance with the national HEN guide improved after implementation of the clinical pathway. In addition, Murcia has one of the Spanish lowest expenditures per population, below the national average.
ConclusionThe clinical pathway implemented improves compliance with the national guide of prescriptions to patients in the Region of Murcia while containing health resources expenditure and consumption, thus making diet therapy prescription more sustainable.
Analizar la adecuación a la Guía de Nutrición Enteral Domiciliaria (NED) en el Sistema Nacional de Salud (SNS), de las prescripciones realizadas en el Área 1 de Salud de la Región de Murcia, antes y después de implementar una Vía Clínica basada en dicha guía; así como examinar la evolución del coste sanitario en los años 2007-2014 en los Sistemas Murciano y Nacional de Salud en producto dietoterápico.
MétodoEstudio descriptivo de cuantificación de criterios de adecuación a la Guía NED en el SNS. Se analizaron los principales criterios de adecuación en el año 2010 (antes de la implementación de la Vía Clínica) y en 2013-2014 (después). Finalmente, se analizó la evolución del gasto y consumo sanitario durante el período 2007-2014.
ResultadosTras la implementación de la Vía Clínica se observa una mejora del cumplimiento de todos los indicadores de adecuación a la Guía NED nacional. Además, la Región de Murcia se coloca en uno de los últimos lugares en gasto y consumo de envases por habitantes, por debajo de la media española.
ConclusiónLa Vía Clínica implementada mejora la adecuación, a la Guía NED nacional, de la prescripción en los pacientes subsidiarios de NED en la Región de Murcia, al igual que contiene el gasto y el consumo, haciendo más sostenible la indicación de este recurso nutricional.
In Spain, the practice of home enteral nutrition (HEN), financed by the Spanish National Health System (Sistema Nacional de Salud [SNS]), is regulated by government legislation1,2 but also by corresponding regional regulations, with healthcare responsibilities in Spain having been transferred to the Autonomous Communities. As a result, a significant variability can be found in the evaluation, indication and monitoring of patients with HEN. This, along with a growing awareness of the considerable economic impact of artificial nutrition, led to the revision in 20083 of the 1998 Clinical Practice Guide on Home Enteral Nutrition of the Spanish Ministry of Health, Social Services and Equality.4 The recommendations regarding clinical practice guides in medicine allow clinicians to make decisions based on the available evidence, without imposing unnecessary costs in terms of either time or resources, and to take into consideration all relevant aspects before coming to a final decision.5 The above-mentioned Guide3 in general standardizes parameters such as who is responsible for deciding about the medical indication and patient requirements; how to select the type of formula; the suitability of the access route; and regimens and forms of administration and follow-up, among other aspects, as well as how to evaluate the Guide and keep it up to date. In 2007, the legislation of the Health Service of Murcia (Servicio Murciano de Salud [SMS])6 indicated that specialists assigned to hospital nutrition units (NUs), as well as specialists in endocrinology and nutrition, internal medicine, oncology, neurology, digestive diseases and nephrology were responsible for the provision of HEN in adult patients. This fact, among other factors, contributed to the aforementioned variability.
As a result, up until 2010 the practice of HEN in Murcia was characterized by two main aspects: a significant variability in the provision of care among patients receiving HEN, with no unified criteria regarding management, diagnosis, indication or follow-up; and a growing utilization of therapeutic dietetic products as demonstrated by the rise between 2007 and 20107 of the consumption of nonspecific, specific and modular diets. In addition, the HEN prescription and patient characteristics in Murcia were seen to differ from those found elsewhere in Spain and in other countries.7
In the first half of 2011, a “HEN Clinical Pathway” was introduced in Health Area 1 of the region of Murcia with the aim of following the indications of the SNS Guide.3 In 2012, the successful experience gained with this initiative led to regional legislation8 establishing the procedure to be followed in providing dietetic products, and gave NU staff members the exclusive right to prescribe HEN in adults within the context of the SMS.
The purpose of the present study was to analyze the adequacy of our clinical pathway, based on the criteria of the national Guide,3 as well as to examine the evolution of the health costs of such prescriptions in the period between 2007 and 2014 within Health Area 1 of the SMS, in the SMS in general, and within the Spanish SNS.
Material and methodsStudy design and participantsA descriptive study was made of the quantification of the evaluation criteria of a clinical guide (the HEN Guide3 of the SNS), through the implementation of a clinical pathway.9 We analyzed the main assessment criteria (adaptation to the national Guide) at two timepoints: in 2010 (before the implementation of the clinical pathway) and in 2013–2014 (after its implementation). Lastly, the evolution of health expenditure during the period 2007–2014 was analyzed.
The sample consisted of all patients prescribed with HEN (nonspecific, specific and modular diet) in Health Area 1 of the SMS corresponding to the years 2010 (HEN1) and 2013–2014 (HEN2). Further information on the study design and sample can be found in previous publications.7,10,11
The data corresponding to both samples – HEN1 and HEN2 – were compiled from the prescription requests received at our NU. In cases where information was missing, the healthcare electronic systems were used to access the medical records. In this regard, SELENE is the clinical network of the corporate hospital information system of the SMS, while AGORA PLUS provides a generic display of the data of each patient, and has now been reinforced by the interconnecting of all the Health Areas. It should be noted that in HEN2, if prescription information was still found to be missing, it was obtained from the requesting Department (primary care or specialized care), or the patient was consulted at the nutrition clinic.
Variables: evaluation of compliance with the HEN Guide of the SNSAll the criteria were dichotomic variables (Yes/No):
- •
Criterion 1 (C1) or indicated AII (annex II of the Home Enteral Nutrition guide) disease2
- •
Criterion 2 (C2) or adequate type of formula
- •
Criterion 3 (C3) or appropriate route
- •
Criterion 4 (C4) or prior education (oral route only)
- •
Criterion 5 (C5) or periodic reviews
- •
Criterion 6 (C6) or recording of height and weight
- •
Criterion 7 (C7) or registry of blood malnutrition parameters
- •
Criterion 8 (C8) or supplementing (where applicable) of meals/and non-substitute (oral route only and prescribed as a supplement)
- •
Criterion 9 (C9) or correct completion (≥50% of established prescription)
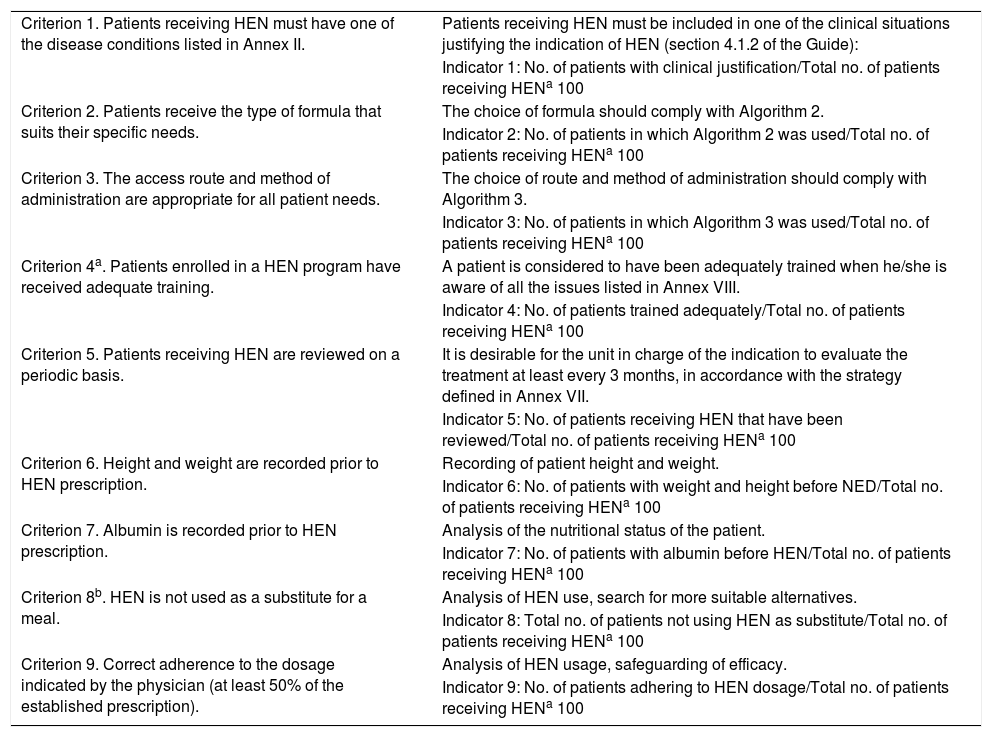
Criteria 1–5 are included in the national Guide,3 which contains the algorithms and annexes to be followed for adequate compliance (the descriptions of which appear in Table 1). The remaining criteria are considered in other studies and guides.12,13Table 1 defines these criteria and displays the formulas for calculating their indicators.
Table 1.Criteria for the evaluation and updating of the HEN Guide of the SNS (indicators 1–5) and other criteria established in other studies (indicators 6–9).
Criterion 1. Patients receiving HEN must have one of the disease conditions listed in Annex II. Patients receiving HEN must be included in one of the clinical situations justifying the indication of HEN (section 4.1.2 of the Guide): Indicator 1: No. of patients with clinical justification/Total no. of patients receiving HENa 100 Criterion 2. Patients receive the type of formula that suits their specific needs. The choice of formula should comply with Algorithm 2. Indicator 2: No. of patients in which Algorithm 2 was used/Total no. of patients receiving HENa 100 Criterion 3. The access route and method of administration are appropriate for all patient needs. The choice of route and method of administration should comply with Algorithm 3. Indicator 3: No. of patients in which Algorithm 3 was used/Total no. of patients receiving HENa 100 Criterion 4a. Patients enrolled in a HEN program have received adequate training. A patient is considered to have been adequately trained when he/she is aware of all the issues listed in Annex VIII. Indicator 4: No. of patients trained adequately/Total no. of patients receiving HENa 100 Criterion 5. Patients receiving HEN are reviewed on a periodic basis. It is desirable for the unit in charge of the indication to evaluate the treatment at least every 3 months, in accordance with the strategy defined in Annex VII. Indicator 5: No. of patients receiving HEN that have been reviewed/Total no. of patients receiving HENa 100 Criterion 6. Height and weight are recorded prior to HEN prescription. Recording of patient height and weight. Indicator 6: No. of patients with weight and height before NED/Total no. of patients receiving HENa 100 Criterion 7. Albumin is recorded prior to HEN prescription. Analysis of the nutritional status of the patient. Indicator 7: No. of patients with albumin before HEN/Total no. of patients receiving HENa 100 Criterion 8b. HEN is not used as a substitute for a meal. Analysis of HEN use, search for more suitable alternatives. Indicator 8: Total no. of patients not using HEN as substitute/Total no. of patients receiving HENa 100 Criterion 9. Correct adherence to the dosage indicated by the physician (at least 50% of the established prescription). Analysis of HEN usage, safeguarding of efficacy. Indicator 9: No. of patients adhering to HEN dosage/Total no. of patients receiving HENa 100 HEN: Home Enteral Nutrition; SNS: Spanish National Health System.
In this case, we evaluated the total figures (the number of packs and the cost in euros) corresponding to both Health Area 1 and the global region of Murcia. Other indicators, such as packs per population (packs/100 inhabitants) or cost per population (euros/inhabitant), were obtained for both the SMS (regional) and the SNS (national).
Data analysisThe characteristics of the samples, clinical criteria, the suitability of the requests, and changes in the consumption of HEN were analyzed using the IBM SPSS® version 22.0 statistical package. Descriptive and statistical significance techniques were used to describe both samples and show any possible differences. Comparisons were made of the proportions of dichotomic and nominal variables using the chi-squared test with the corresponding significance level. The Student t-test or Mann–Whitney U-test was used in the application to quantitative variables. In the case of the measures referring to cost and the prevalence of HEN consumption, we used the prescription invoicing data system of the SMS, in combination with the official diet nomenclature of the Spanish Ministry of Health, Social Services and Equality.
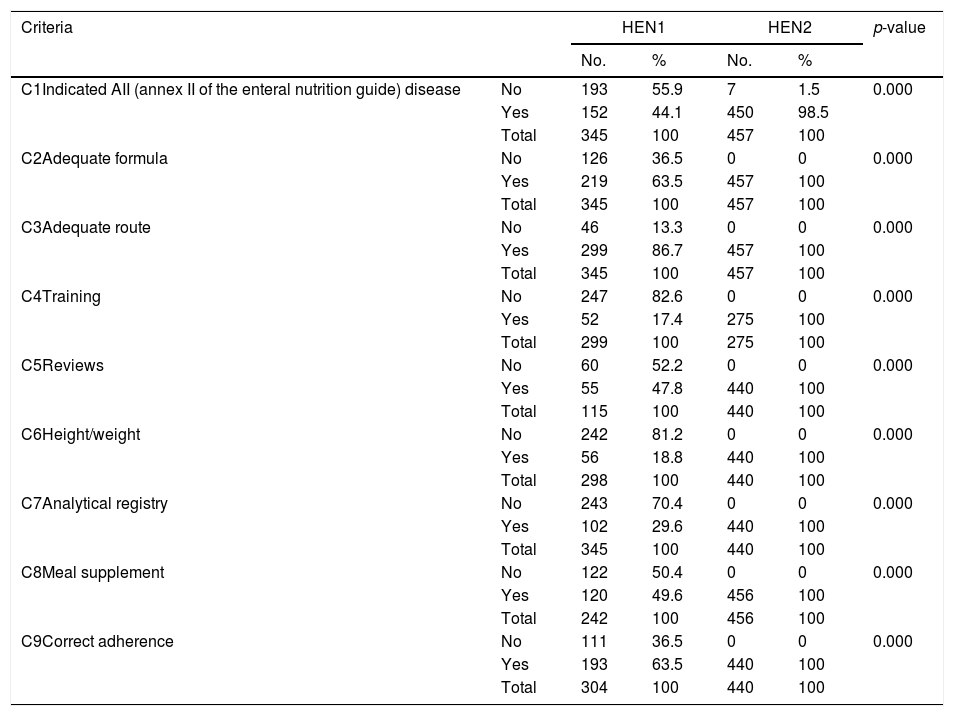
ResultsThe HEN1 sample consisted of 345 cases (51.9% females) with a mean age of 78.3±15.9 years. The HEN2 sample consisted of 457 cases (50.8% males) with a mean age of 76.3±16.9 years. There were no significant gender (p=0.72) or age differences between the two samples (p=0.92). Table 2 shows the differences in quality (compliance with the national Guide) of the indicators between groups HEN1 and HEN2. Table 3 shows the total number of packs consumed in Health Area 1 between 2010 and 2014, the difference (Δ) with regard to the previous year and to 2010, and the cumulative difference during the study period with regard to 2010 (the last year without the clinical pathway in Health Area 1). Table 4 with indicators similar to those of the previous table shows the total amounts in euros. Lastly, Figs. 1 and 2 show the development of the dietary product consumption indicators in the SMS after the introduction of the clinical pathway at regional level to have been a very positive one. This clearly distinguishes Murcia from the rest of the SNS, where the service is also provided through medical prescription practice.
Home Enteral Nutrition quality criteria. Samples HEN1 and HEN2.
| Criteria | HEN1 | HEN2 | p-value | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| C1Indicated AII (annex II of the enteral nutrition guide) disease | No | 193 | 55.9 | 7 | 1.5 | 0.000 |
| Yes | 152 | 44.1 | 450 | 98.5 | ||
| Total | 345 | 100 | 457 | 100 | ||
| C2Adequate formula | No | 126 | 36.5 | 0 | 0 | 0.000 |
| Yes | 219 | 63.5 | 457 | 100 | ||
| Total | 345 | 100 | 457 | 100 | ||
| C3Adequate route | No | 46 | 13.3 | 0 | 0 | 0.000 |
| Yes | 299 | 86.7 | 457 | 100 | ||
| Total | 345 | 100 | 457 | 100 | ||
| C4Training | No | 247 | 82.6 | 0 | 0 | 0.000 |
| Yes | 52 | 17.4 | 275 | 100 | ||
| Total | 299 | 100 | 275 | 100 | ||
| C5Reviews | No | 60 | 52.2 | 0 | 0 | 0.000 |
| Yes | 55 | 47.8 | 440 | 100 | ||
| Total | 115 | 100 | 440 | 100 | ||
| C6Height/weight | No | 242 | 81.2 | 0 | 0 | 0.000 |
| Yes | 56 | 18.8 | 440 | 100 | ||
| Total | 298 | 100 | 440 | 100 | ||
| C7Analytical registry | No | 243 | 70.4 | 0 | 0 | 0.000 |
| Yes | 102 | 29.6 | 440 | 100 | ||
| Total | 345 | 100 | 440 | 100 | ||
| C8Meal supplement | No | 122 | 50.4 | 0 | 0 | 0.000 |
| Yes | 120 | 49.6 | 456 | 100 | ||
| Total | 242 | 100 | 456 | 100 | ||
| C9Correct adherence | No | 111 | 36.5 | 0 | 0 | 0.000 |
| Yes | 193 | 63.5 | 440 | 100 | ||
| Total | 304 | 100 | 440 | 100 | ||
AII: Annex II of the Home Enteral Nutrition guide of the Spanish National Health System, 2008; HEN: Home Enteral Nutrition.
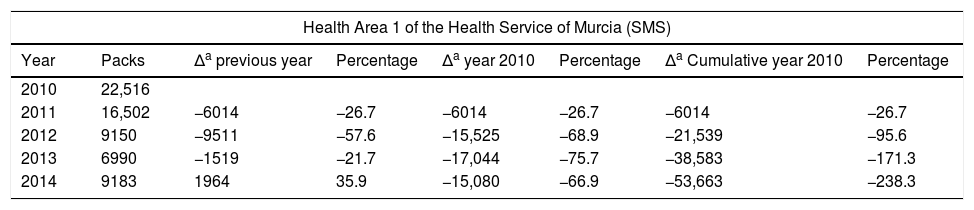
Number of Home Enteral Nutrition packs in Health Area 1 of the Health Service of Murcia (SMS) and total areas.
| Health Area 1 of the Health Service of Murcia (SMS) | |||||||
|---|---|---|---|---|---|---|---|
| Year | Packs | Δa previous year | Percentage | Δa year 2010 | Percentage | Δa Cumulative year 2010 | Percentage |
| 2010 | 22,516 | ||||||
| 2011 | 16,502 | −6014 | −26.7 | −6014 | −26.7 | −6014 | −26.7 |
| 2012 | 9150 | −9511 | −57.6 | −15,525 | −68.9 | −21,539 | −95.6 |
| 2013 | 6990 | −1519 | −21.7 | −17,044 | −75.7 | −38,583 | −171.3 |
| 2014 | 9183 | 1964 | 35.9 | −15,080 | −66.9 | −53,663 | −238.3 |
| Total Health Areas of the Health Service of Murcia (SMS) | |||||||
|---|---|---|---|---|---|---|---|
| Year | Packs | Δa previous year | Percentage | Δa year 2010 | Percentage | Δa Cumulative year 2010 | Percentage |
| 2010 | 192,838 | ||||||
| 2011 | 167,089 | −25,749.00 | −13.3 | −25,749 | −13.3 | −25,749 | −13.3 |
| 2012 | 129,536 | −37,553.00 | −22.4 | −63,302 | −32.8 | −89,051 | −46.2 |
| 2013 | 115,656 | −13,880.00 | −0.72 | −77,182 | −40.0 | −166,233 | −86.2 |
| 2014 | 114,975 | −681.00 | −0.6 | −77,863 | −40.4 | −244,096 | −126.6 |
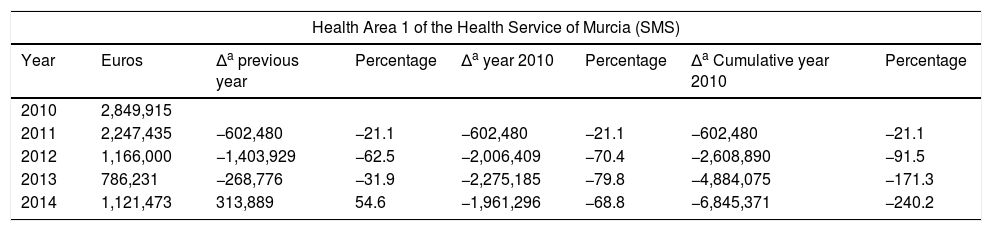
Cost (€) of Home Enteral Nutrition in Health Area 1 of the Health Service of Murcia (SMS) and total areas.
| Health Area 1 of the Health Service of Murcia (SMS) | |||||||
|---|---|---|---|---|---|---|---|
| Year | Euros | Δa previous year | Percentage | Δa year 2010 | Percentage | Δa Cumulative year 2010 | Percentage |
| 2010 | 2,849,915 | ||||||
| 2011 | 2,247,435 | −602,480 | −21.1 | −602,480 | −21.1 | −602,480 | −21.1 |
| 2012 | 1,166,000 | −1,403,929 | −62.5 | −2,006,409 | −70.4 | −2,608,890 | −91.5 |
| 2013 | 786,231 | −268,776 | −31.9 | −2,275,185 | −79.8 | −4,884,075 | −171.3 |
| 2014 | 1,121,473 | 313,889 | 54.6 | −1,961,296 | −68.8 | −6,845,371 | −240.2 |
| Total Health Areas of the Health Service of Murcia (SMS) | |||||||
|---|---|---|---|---|---|---|---|
| Year | Euros | Δa previous year | Percentage | Δa year 2010 | Percentage | Δa Cumulative year 2010 | Percentage |
| 2010 | 16,897,508 | ||||||
| 2011 | 15,405,585 | −1,491,923 | −8.8 | −1,491,923 | −8.8 | −1,491,923 | −8.8 |
| 2012 | 10,934,871 | −4,470,714 | −29.0 | −5,962,637 | −35.3 | −7,454,560 | −44.1 |
| 2013 | 8,623,552 | −2,311,319 | −21.1 | −8,273,956 | −48.9 | −15,728,516 | −93.1 |
| 2014 | 9,043,210 | 419,658 | 4.8 | −7,854,298 | −46.5 | −23,582,814 | −139.5 |
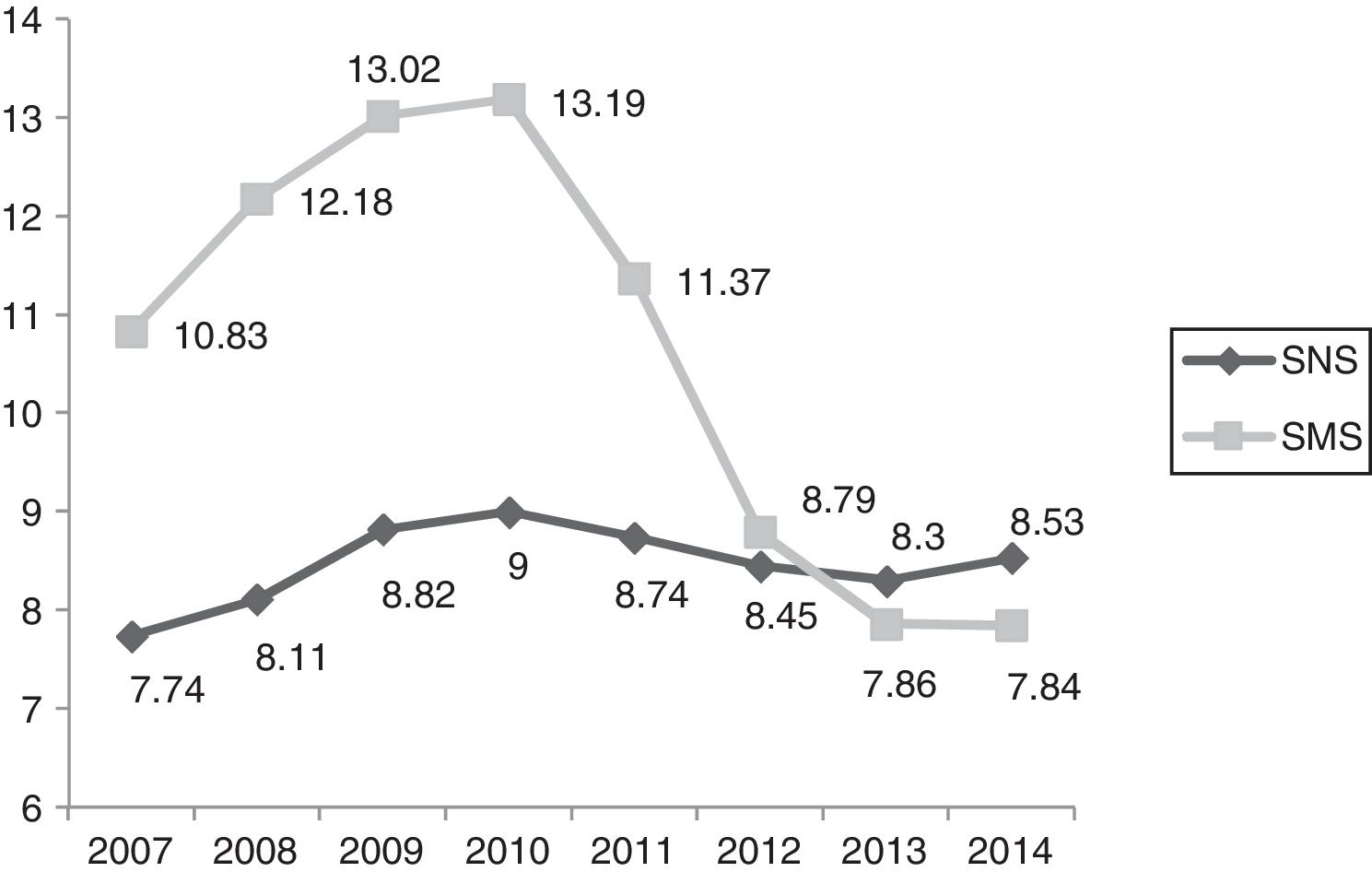
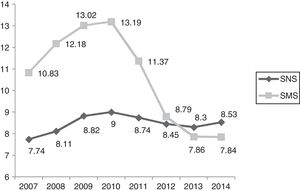
Packs/100 inhabitants, years 2007–2014/SNS–SMS. SMS: Health Service of Murcia; SNS: Spanish National Health System. Source: Dietetic product consumption information service of the Spanish Ministry of Health, Social Services and Equality; Pharmacy information system of the SMS, census-based population on 1 January of each year (Spanish National Statistics Institute, INE). Those Autonomous Communities that acquire part of their dietetic products through health centres at PTR (price to retailer), INGESA and insurance associations are excluded.
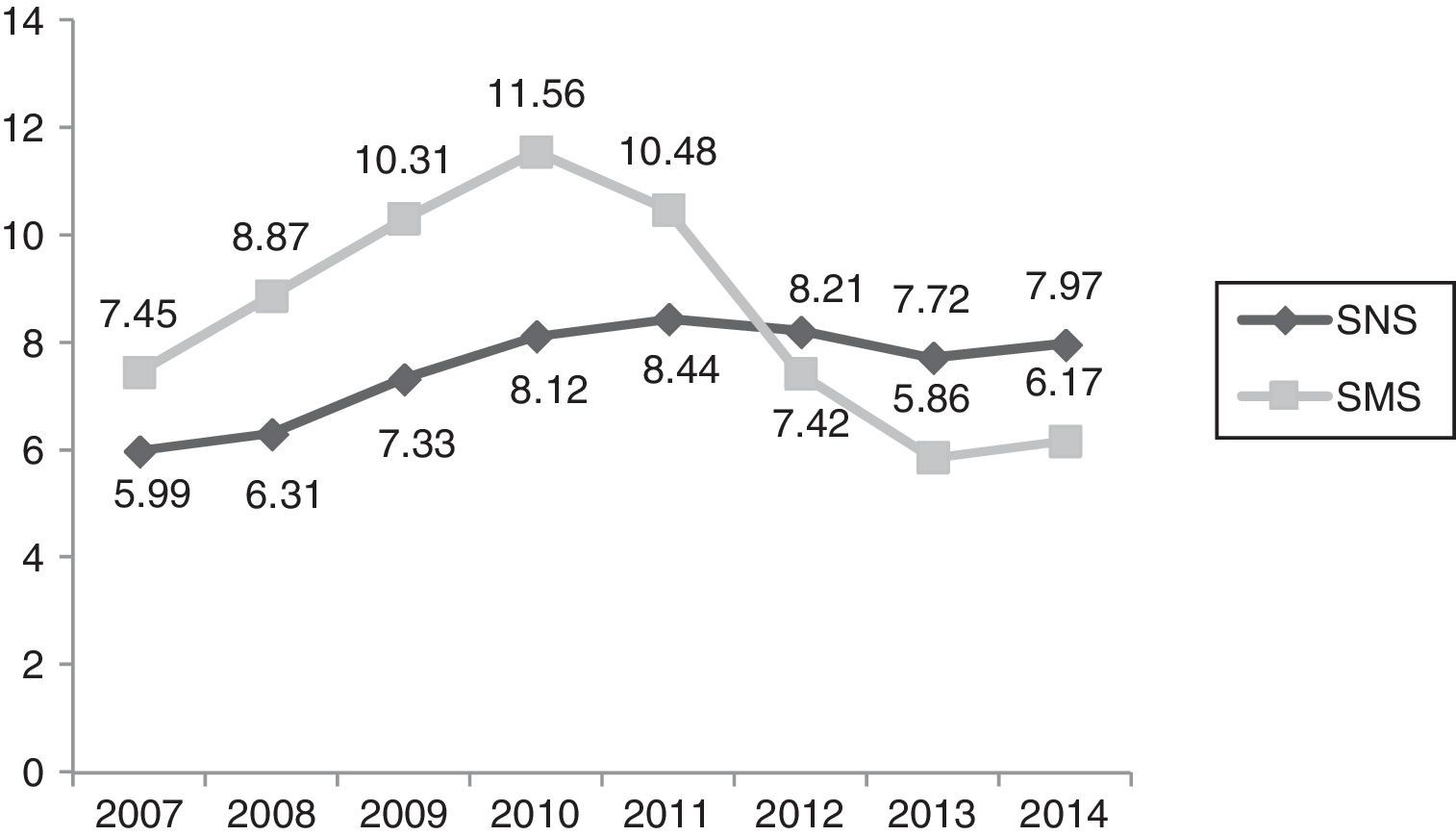
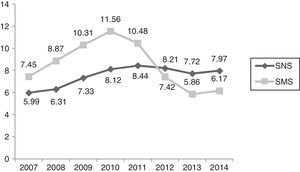
Cost ()/inhabitants, years 2007–2014/SNS–SMS. SMS: Health Service of Murcia; SNS: Spanish National Health System. Source: Dietetic product consumption information service of the Spanish Ministry of Health, Social Services and Equality; Pharmacy information system of the SMS, census-based population on 1 January of each year (Spanish National Statistics Institute, INE). Those Autonomous Communities that acquire part of their dietetic products through health centres at PTR (price to retailer), INGESA and insurance associations are excluded.
In the first decade of this century, Spanish legislation in general and that of the region of Murcia in particular led to a considerable variation in HEN prescriptions, with no unified criteria regarding their application to patient care. In this respect, the main regulations referring to artificial nutrition in Spain appeared in 1998,4 and were followed by subsequent guidelines1,2,14–16 and a revision of the HEN Guide3 by the SNS in 2008. Of particular relevance is Spanish Royal Decree 1030/2006,2 which establishes the common services portfolio of the SNS and the procedure for updating it. The concrete specifications referring to dietetic products are found in annex VII. In addition, the 2008 Guide establishes the criteria for the implementation of nutrition therapy, as well as those situations amenable to financing within our health system. The consumption of dietetic products in the SMS, through medical prescriptions and financed by the SNS, increased in the period 2007–2010 by 27.86% in packs and by 62.87% in cost. In addition, the region of Murcia ranked above the SNS average in the indicators referring to the number of packs and the cost per population.17 This trend is consistent with the observations of studies in other Spanish regions.18,19
Our study is set within the context of the development and implementation of the HEN clinical pathway in SMS Health Area 1, generalized in 20128 through the regional guideline referring to financial access to dietetic products in the region of Murcia. The centralization of HEN indication capacity in NUs is the main modification introduced by the pathway. The pathway aims to change the situation as it existed in the region of Murcia in 2010, characterized not only by dispersion in the prescription of HEN, but also by being the Autonomous Community with the greatest expenditure per inhabitant.10
Two samples have been defined, HEN1 and HEN2, comprising those patients in SMS Health Area 1 who received HEN before and after implementation of the clinical pathway in 2010 and 2013–2014, respectively, with no differences in age and gender (balanced at around 50%). The average age was over 75 years, which is higher than in most published HEN registries, including both that of the Home and Ambulatory Enteral Nutrition (NADyA) group20,21 and those of international groups,22,23 with ages that usually range between 65 and 70 years. However, it coincides with the data obtained in a study of the University Hospital Complex in Santiago de Compostela (Spain), carried out between October 2009 and 2010.24 Both samples were characterized by a predominance of neurological disease, in coincidence with the data from other registries, while oncological disease appears underestimated in comparison with the latter.20,25 With regard to the route employed, oral administration was the most widely used approach in both samples. An increase in the use of nasogastric and ostomy tubes was noted in HEN2, with data more consistent with those of international registries.23 The comparison of these figures with those of certain registries is not possible, since they exclude the oral route from analysis,20 or the levels of the variable are not exactly coincident.22 Lastly, on considering the type of formula requested, we likewise found important differences between HEN1 and HEN2. In the latter sample a decrease was noted in the prescription of nonspecific formulas, but more especially in the prescription of specific formulas, with an important increase in the use of modules. These changes can be explained by the switch in patients with dysphagia from diets used as a supplement to thickening modular formulas, and the switch from specific diets to nonspecific diets in those cases where their use lacks scientific evidence (criterion 3: appropriate route).
Regarding the suitability criteria reflected in the HEN Guide,3 and on comparing the samples HEN1 and HEN2, we found the former to have indicators significantly different from those of the latter sample. Thus, the background disease condition, the administration route, the type of formula, the suitability of training and the periodic revisions prior to the implementation of the clinical pathway did not comply with the indications of the HEN Guide of the SNS.3 This situation was seen to improve in HEN2. With regard to the periodic revisions, these were made by the NU with the information supplied by the primary care physician, or even through personal appointments where they were deemed opportune. Our pathway illustrates how the specialist must first establish the indication, but with the obligation to conduct a revision after no more than 6 months. Consequently, at the time of the indication, the prescription is issued with a concrete date. When this date draws near, primary care submits a renewal report to our NU, which the specialist must accept or not accept, or modify, depending on the current condition of the patient. Regarding the indicators proposed after a review of the literature,12,13 the differences between samples HEN1 and HEN2 were clear and in favour of the latter sample, particularly with regard to the collection of anthropometric (C6) and laboratory test data (C7). Criteria 8 and 9 have a decisive impact upon the effect of nutrition therapy, and are of particular interest when possible improvements through education programs are being considered, since they are entirely dependent upon the patients and their caregivers.
In relation to the number of packs and the total cost, we observed a increasing control over expenditure in Health Area 1 of the SMS between 2010 and 2014 of close to 240% in terms of packs and of 7,000,000 in terms of cost. In the last two years, a plateau has been reached in both expenditure measures. Taking into consideration the economic data of the total Health Areas of the SMS referring to the number of packs and total cost, we observed cumulative expenditure containment between 2010 and 2014 of close to 130% in terms of packs and of 24,000,000 in terms of cost. The national ranking of the region of Murcia in terms of HEN expenditure has changed drastically. In effect, while Murcia was among the highest ranking Spanish Autonomous Communities in terms of cost/population and packs/100 inhabitants, it has since dropped to almost the bottom of the list, well below the Spanish national average.
Study limitations: The suitability criteria in HEN1 (especially C4–C6 and C8–C9) may have been underestimated, since missing data in the issued requests (or in the consulted electronic data sources) does not necessarily imply that the suitability criteria were not met. Nevertheless, the fact that such data may have been missing does imply that compliance with national suitability criteria was not adequately ensured. In addition, criteria 8 and 9 are particularly subjective, since desirability on the part of the patient may be introduced. Another limitation of our study is the absence of the measurement of health outcomes, precluding the drawing of conclusions regarding the cost-effectiveness of the treatment.
In conclusion, the clinical pathway implemented in the SMS has improved compliance with the national Guide in the prescription of HEN in the region of Murcia, and at the same time has resulted in the control of consumption, making the indication of this nutritional resource more sustainable and viable for the public health system.
This study and previous publications seek to compile population-based data on the characteristics of this type of nutrition, reflecting the reality of HEN in our region. Following these first steps, and while much remains to be done in this regard, our future aim is to create a population-based registry with all the Health Areas of the SMS. In this way, we hope to obtain detailed information in our region with a view to improving the prescription of HEN.
AuthorshipMFG, MVGZ, AAG and VJRR conceived and designed the Home Enteral Nutrition clinical pathway in the region of Murcia. All the authors participated at different moments in the fieldwork, data analysis and interpretation, and in the development of reports. JFSR and MFG produced the first version of the manuscript. All the authors made a critical review with important intellectual contributions, and have approved the final version of the manuscript for publication.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Ferrer M, Sánchez-Romera JF, García-Zafra MV, Hernández-Cascales AB, Arráez M, Aranda A, et al. Análisis de la vía clínica de nutrición enteral domiciliaria de la Región de Murcia. Consumo y gastos asociados y adecuación a la Guía del Sistema Nacional de Salud. Endocrinol Diabetes Nutr. 2019;66:232–239.