Multiple chemical sensitivity (MCS) is a complex, acquired, chronic syndrome of multifactorial etiology with multiple symptoms. The aim of the study was to assess the nutritional habits, dietary characteristics and physical activity, as well as their determinants, of a population diagnosed with MCS, which may allow for a more precise approach to nutritional improvement.
Patients and methodA descriptive, cross-sectional study in patients diagnosed with MCS. Information was collected using adapted questionnaires. Data included presence of comorbidities, nutritional (use of supplements, types of diet) and food purchasing habits. Dietary intake, food intolerances, and physical activity were also recorded.
ResultsThe study included of 52 patients (48 female) aged 50.9±10.3 years. Diagnosis of MCS was commonly associated to chronic fatigue syndrome (70.1%), fibromyalgia (65.4%), or electrosensitivity (51.9%). The most common comorbidities were irritable bowel, gastroesophageal reflux, and depression/anxiety-depressive disorder. Exclusion diets were followed by 57.7%, 52.1% commonly used supplements (6.4±5.2 per person), and 16.0% took more than 10 daily. A high proportion of volunteers did not take the recommended amounts of dairy products (84.3%), fruit (82.3%), and cereals (64.7%), the foods to which intolerance was greatest. As regards physical activity, active subjects only represented 12.5%.
ConclusionsThe data collected support the need to improve food pattern and to perform physical activity according to individual characteristics. Nutritional education and diet personalization could prevent incomplete, monotonous, and unbalanced diets which impair quality of life and physiological status.
La sensibilidad química múltiple (SQM) es un síndrome complejo, adquirido, crónico y multifactorial, con amplia sintomatología. El objetivo del presente estudio fue conocer los hábitos alimentarios, las características dietéticas y la actividad física, así como sus condicionantes en un colectivo afectado de SQM, lo que permitirá un abordaje más preciso para la mejora de su estado nutricional.
Pacientes y métodoEstudio descriptivo y transversal en pacientes con SQM. Se recogió información mediante cuestionarios adaptados sobre presencia de comorbilidades, hábitos dietéticos (consumo de complementos/suplementos, tipos de dietas) y de compra, así como registro de ingesta dietética, intolerancias alimentarias y actividad física.
ResultadosSe incluyó a 52 pacientes (48 mujeres) de 50,9 ± 10,3 años de edad media. Fue habitual el diagnóstico conjunto de SQM con síndrome de fatiga crónica (70,1%), fibromialgia (65,4%) o electrosensibilidad (51,9%). Las comorbilidades más frecuentes fueron colon irritable, reflujo gastroesofágico y depresión/trastorno ansioso-depresivo. El 57,7% seguía regímenes de exclusión. El 52,1% consumía complementos/suplementos habitualmente (6,4 ± 5,2 por persona) y el 16,0% tomaba más de 10 diarios. Fue elevado el porcentaje de voluntarios que no alcanzó las raciones aconsejadas de lácteos (84,3%), frutas (82,3%) y cereales (64,7%), coincidiendo con los alimentos con mayor intolerancia. Con respecto a la actividad física, los sujetos activos solo representaban el 12,5%.
ConclusionesLos datos obtenidos confirman la necesidad de mejora del patrón alimentario y rehalización de actividad física según características individuales. La educación nutricional y personalización de las pautas podrían evitar dietas incompletas, monótonas y desequilibradas que empeoren la calidad de vida y situación fisiológica.
Multiple chemical sensitivity (MCS) or idiopathic environmental intolerance is a complex, acquired and chronic syndrome characterized by a broad range of systemic and recurrent symptoms in certain subjects1–3 as a result of exposure to low levels of certain chemical agents and components commonly found in the environment, and which are tolerated by most people.4
The complexity of the medical diagnosis of MCS, attributable in part to interindividual variability of the degree of severity and related symptoms (respiratory, gastrointestinal, neurological, endocrine, cutaneous, etc.), makes it difficult to establish the true worldwide prevalence of the disorder, which is currently considered to be increasing. However, some authors estimate that between 0.1% and 5% of the world population is affected by MCS, and that the rate increases with age.2,5,6 Following the initiatives of other countries,6 MCS was recognized in 2011 by the Spanish Ministry of Health and Social Policy, and in 2014 it was included in the International Classification of Diseases (ICD).7 Although there is no census of affected individuals in Spain, the figures reported by toxicological units are somewhat lower than those estimated globally.8
The etiology of MCS remains unknown, and is defined as multifactorial. However, the recently proposed physiopathological hypothesis describing MCS as a central sensitization syndrome would explain why 30–50% of all patients with MCS present other disorders sharing similar clinical features and physiopathological mechanisms, such as fibromyalgia (FM), chronic fatigue syndrome (CFS)1,5,8 or electrosensitivity (ES),2 since hyperreactivity of the central nervous system has been postulated, with excessive sensitivity to stimuli due to structural, functional and molecular modifications occurring after peripheral tissue excitation or injury.9 Loria-Kohen et al. identified differences in the frequencies of certain single nucleotide polymorphisms (SNPs) (MTFHFR rs1801133, FADS1 rs174546 and PPARγ rs1801282) between individuals with MCS and controls,10 and other studies have described various SNPs associated with the diagnosis of MCS, FM and CFS.3
The limited scientific research on the nutritional status of these patients, together with the disinformation they have and the adverse food reactions they commonly experience, could condition the choice of foods or diets, and thus adversely affect patient health and quality of life.
The purpose of this study was to determine the eating habits and characteristics referring to food intake and physical activity in a group of individuals with MCS, as well as the factors influencing them, with the aim of defining those aspects amenable to improvement from the nutritional point of view.
Patients and methodsThis descriptive cross-sectional trial was conducted at the Unit of Nutrition and Clinical Trials of the Madrid Institute for Advanced Food Studies (Instituto Madrileño de Estudios Avanzados en Alimentación [IMDEA-Alimentación]) (Madrid, Spain). The study abided by the ethical standards of the Declaration of Helsinki and was approved by the Research Ethics Committee of the IMDEA-Alimentación Foundation.
Participants were recruited and screened by the Association of Patients with Chronic Fatigue Syndrome and Multiple Chemical Sensitivity Syndrome of the Community of Madrid (Asociación de Afectados por Síndrome de Fatiga Crónica y por Síndrome de Sensibilidad Química Múltiple de la Comunidad de Madrid [SFC-SQM Madrid]) from among their 120 associates. The inclusion criteria were: adult males and females with an adequate cultural level and understanding of the objectives and methods of the trial, a documented medical diagnosis established at least 1 year previously of MCS, either isolatedly or associated with CFS, FM or ES, and the signing of the informed consent form. Multiple chemical sensitivity grades were defined based on an adaptation of the Quick Environmental Exposure and Sensitivity Inventory (QEESI), a self-administered questionnaire exploring 50 items divided into 5 scales.4 The medical reports were conclusive in establishing the diagnosis of FM and CFS, while cases of ES were mostly reported by the volunteers in person. The exclusion parameters included mental illness, an inability to travel to the study center due to the severity of symptoms, and pregnancy and breast-feeding.
Before the face-to-face visit, an electronic case report form (eCRF) was provided to be completed by the patient. Among other elements, the form included validated questionnaires on diet (a 72-h consumption record and frequency of intake) and physical activity (a 24-h record).
The volunteers attended an evaluation visit at the facilities of IMDEA-Alimentación in which a case history was compiled, including personal data, the presence of comorbidities, food intolerances perceived by the patient, the use of food supplements/complements, and types of diet and food purchasing habits. Adequate completion of the supplied questionnaires was also checked. Likewise, we recorded anthropometric parameters (height, weight, body composition with electrical bioimpedance, waist and arm circumference, and tricipital fold thickness), dynamometric parameters and vital signs (blood pressure and heart rate). A blood sample was also obtained for the study of the genetic parameters. The methodological procedures and results associated with these parameters can be consulted in the article published by Loria-Kohen et al.10
The 72-h consumption record was required to include all food and drink consumed over 3 days, with 1 day corresponding to a weekend or holiday. The compiled data were tabulated using the DIAL v. 2.16 application (ALCE Ingeniería, S.L., 2012, www.alceingenieria.net/nutricion.htm) for qualitative and quantitative analysis regarding both macro- and micronutrients, nutritional adequacy and the number of daily servings. The quantification of servings corresponding to the different food groups was carried out using a validated questionnaire on consumption frequency in the Spanish population, adapted to MCS.
For the collection of data on physical activity we used a 24-h activity questionnaire indicating the time taken to perform 19 items during one working day and one holiday. Of these items, 15 corresponded to closed activities (sleep, personal hygiene, watching television, eating, etc.) while four involved open-ended answers referring to simple domestic chores, work, other tasks, and sports. The activities were subsequently categorized according to intensity and multiplied by their coefficients (1 for rest, 1.5 for very light, 2.5 for light, 5 for moderate, and 7 for intense). The sum of these values was divided by 24h to obtain the daily coefficients according to the type of day. By means of this questionnaire we were able to calculate the individualized physical activity coefficient (IPAC), which in turn was used to calculate the total energy expenditure (TEE) of each participant, multiplied by the basal metabolic rate calculated according to the criteria of the World Health Organization (WHO).11 The comparison between TEE and total calorie value (TCV) obtained from the dietary survey allowed us to evaluate the individual energy balance.
The data were analyzed using R Statistical Software v. 2.15 (www.r_project.org). Qualitative data were reported as absolute frequencies and percentages, and quantitative data were reported as the mean and standard deviation (SD). Analysis of variance (ANOVA) was used for the comparison of several categories. Statistical significance was considered for p<0.05.
ResultsA total of 52 patients (48 females) with a mean age of 50.9±10.3 years were included in the study. Seventy-three percent of the volunteers resided in the Community of Madrid. The mean time from the diagnosis of MCS was 3.9±4.1 years (minimum: 1 year, maximum: 26 years). A combined diagnosis of MCS with CFS (70.1%), FM (65.4%) or ES (51.9%) was common. The cumulative presence of MCS, CFS and FM was recorded in 61.5% of the patients, while the cumulative presence of MCS, CFS, FM and ES was observed in 35.0%.
The most common comorbidities included irritable bowel syndrome (46.5%), gastroesophageal reflux (42.3%), depression or anxiety-depressive disorder (38.5%), hypercholesterolemia (34.6%), non-food intolerances (32.7%), hypothyroidism (28.8%), bronchial asthma (23.1%) and autoimmune thyroiditis (11.5%). Lower prevalences corresponding to other conditions such as food allergies (7.7%) and celiac disease (5.8%) were also recorded.
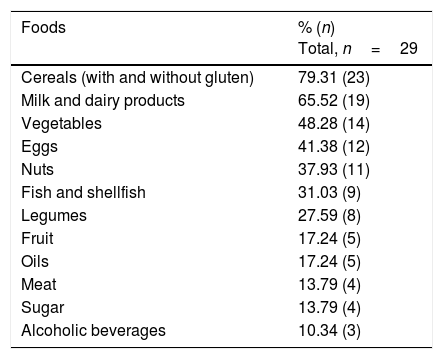
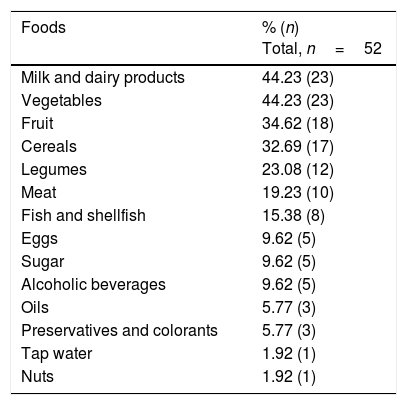
A total of 55.8% of the participants claimed to have undergone food intolerance tests in private laboratories, involving the determination of specific IgG against food, and cytotoxic studies involving cell activation and sensitization tests. Table 1 shows the foods most commonly associated with positive results. On the other hand, the patients were asked about foods that triggered some type of discomfort (urticaria, abdominal bloating, reflux, respiratory problems, headache, etc.). Table 2 reports the answers obtained.
Foods most commonly related to positive food intolerance tests.
| Foods | % (n) Total, n=29 |
|---|---|
| Cereals (with and without gluten) | 79.31 (23) |
| Milk and dairy products | 65.52 (19) |
| Vegetables | 48.28 (14) |
| Eggs | 41.38 (12) |
| Nuts | 37.93 (11) |
| Fish and shellfish | 31.03 (9) |
| Legumes | 27.59 (8) |
| Fruit | 17.24 (5) |
| Oils | 17.24 (5) |
| Meat | 13.79 (4) |
| Sugar | 13.79 (4) |
| Alcoholic beverages | 10.34 (3) |
Foods commonly reported by the participants as causing intolerance.
| Foods | % (n) Total, n=52 |
|---|---|
| Milk and dairy products | 44.23 (23) |
| Vegetables | 44.23 (23) |
| Fruit | 34.62 (18) |
| Cereals | 32.69 (17) |
| Legumes | 23.08 (12) |
| Meat | 19.23 (10) |
| Fish and shellfish | 15.38 (8) |
| Eggs | 9.62 (5) |
| Sugar | 9.62 (5) |
| Alcoholic beverages | 9.62 (5) |
| Oils | 5.77 (3) |
| Preservatives and colorants | 5.77 (3) |
| Tap water | 1.92 (1) |
| Nuts | 1.92 (1) |
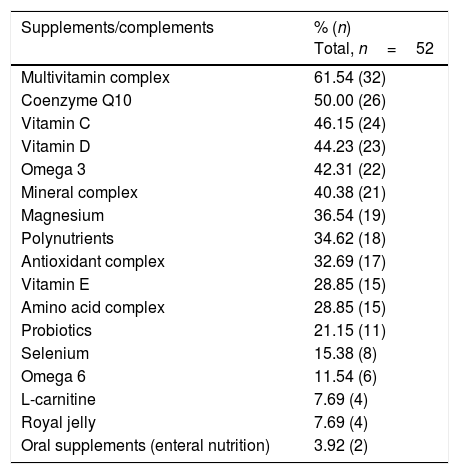
With regard to special diets, 67.3% of the subjects claimed to consume organic foods, 57.7% reported exclusion diets, 17.3% were vegetarians, 9.6% included food rotation, and 3.8% reported a macrobiotic diet. Over half of the patients (52.1%) claimed to consume food supplements or complements on a daily basis, 25.0% for periods of time, and 12.8% sporadically. The average number of supplements consumed over the previous year was 6.4±5.2 supplements per person, and 16.0% claimed to consume more than 10 daily. Table 3 shows the most commonly used food supplements/complements. The reported cost exclusively attributable to the purchase of special foods and nutritional supplements was 350±230 €/month.
List of commonly used food supplements/complements.
| Supplements/complements | % (n) Total, n=52 |
|---|---|
| Multivitamin complex | 61.54 (32) |
| Coenzyme Q10 | 50.00 (26) |
| Vitamin C | 46.15 (24) |
| Vitamin D | 44.23 (23) |
| Omega 3 | 42.31 (22) |
| Mineral complex | 40.38 (21) |
| Magnesium | 36.54 (19) |
| Polynutrients | 34.62 (18) |
| Antioxidant complex | 32.69 (17) |
| Vitamin E | 28.85 (15) |
| Amino acid complex | 28.85 (15) |
| Probiotics | 21.15 (11) |
| Selenium | 15.38 (8) |
| Omega 6 | 11.54 (6) |
| L-carnitine | 7.69 (4) |
| Royal jelly | 7.69 (4) |
| Oral supplements (enteral nutrition) | 3.92 (2) |
When purchasing food products, 50.0% of the participants claimed to have to resort to someone else or to online purchasing, due to the physical problems involved in acquiring the products personally. In relation to the choice of food, 84.0% claimed their choice was conditioned by tolerance, 44.0% considered the price, and 42.0% chose food according to its nutritional value. Thirty percent of the volunteers had to leave cooking to another person, and over 90.0% claimed to be conditioned by individual tolerance.
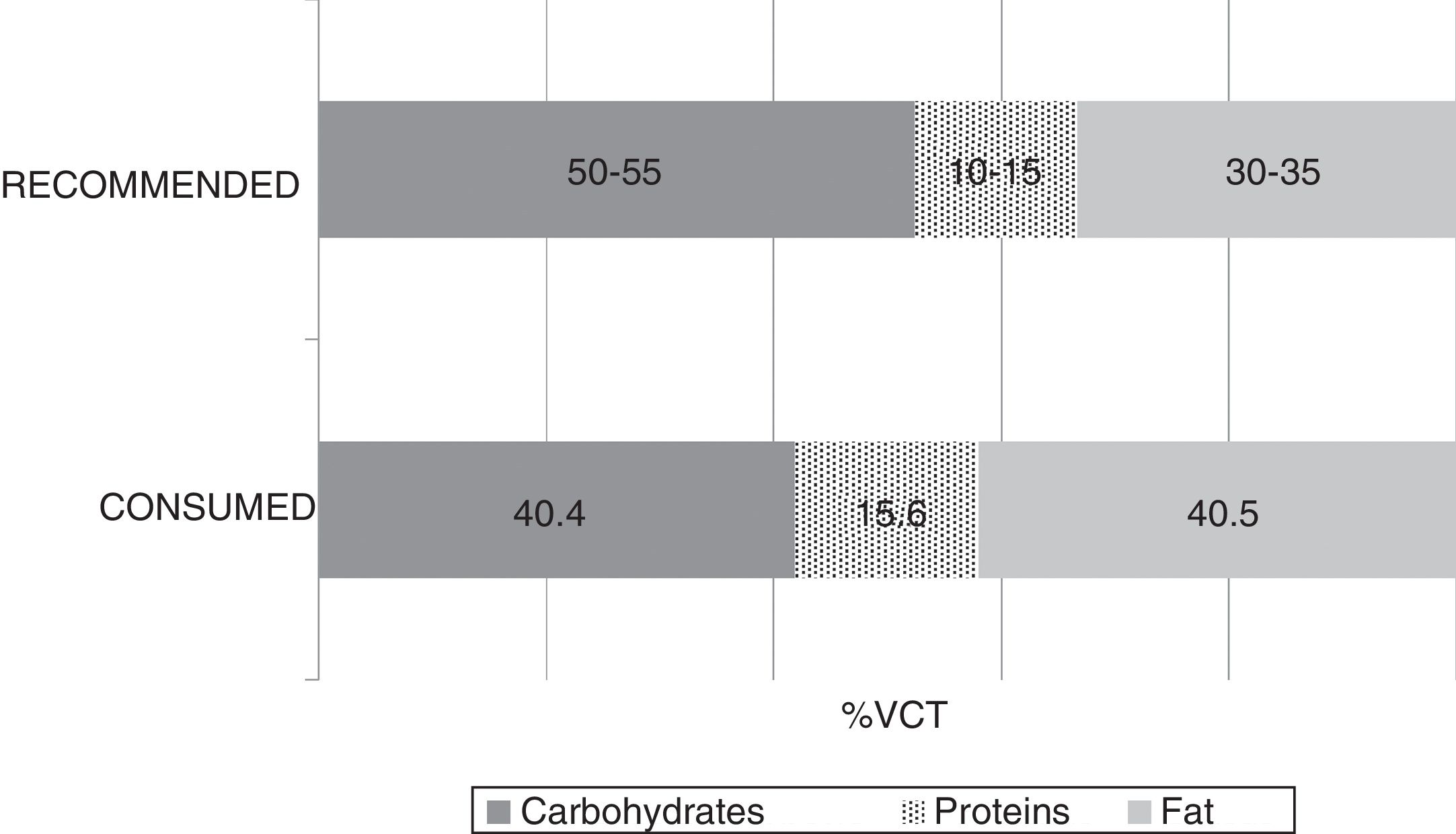
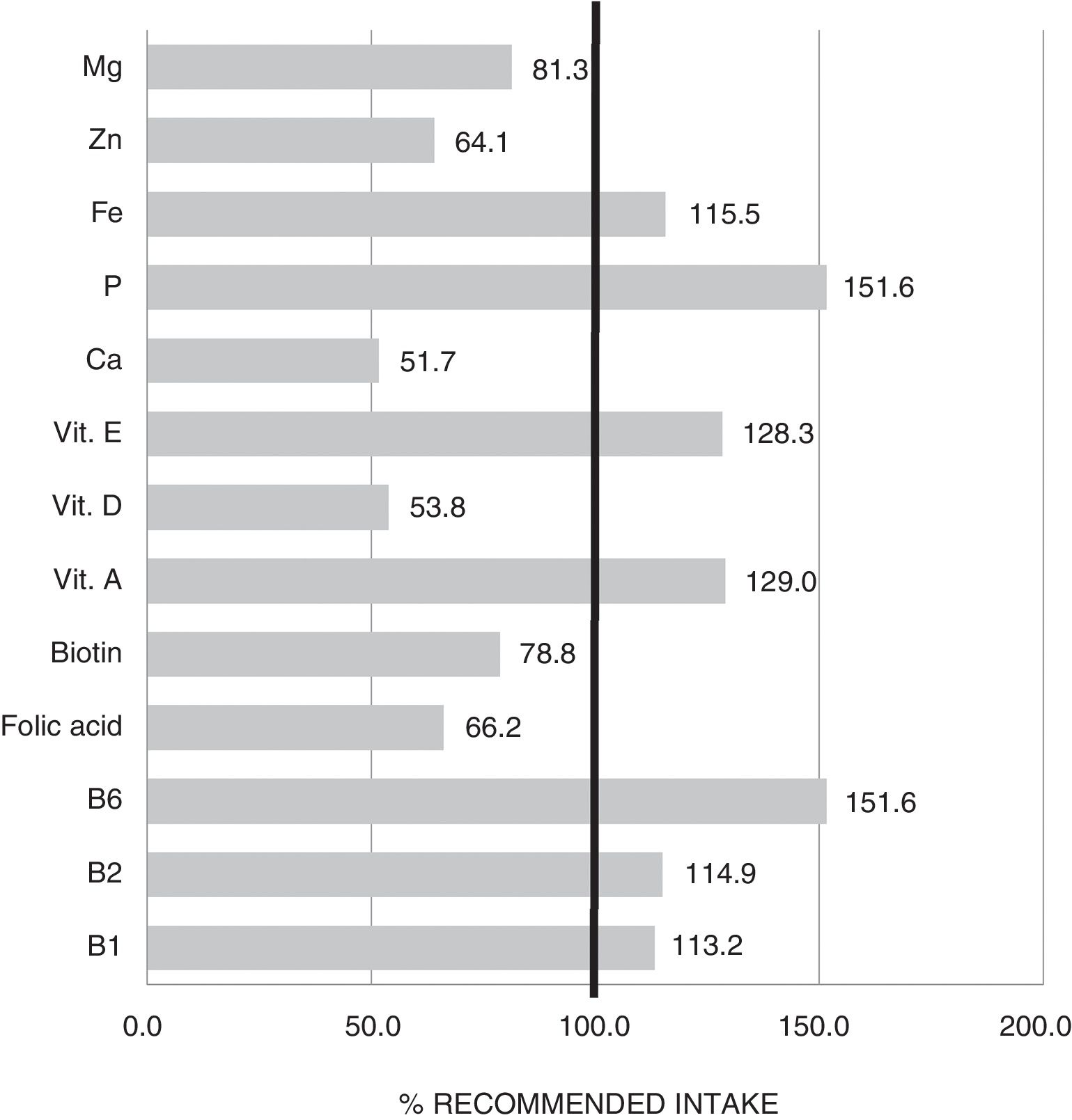
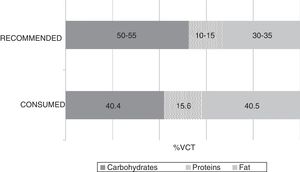
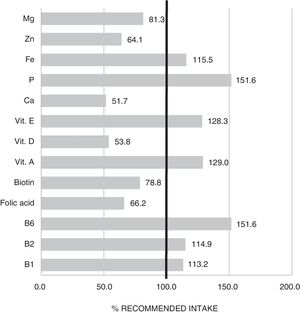
The mean TCV calculated from the 72-h consumption record was 1749.1±397.4kcal/day. Fig. 1 shows the distribution of macronutrients according to TCV. On the other hand, 10.2±3.2% of the TCV corresponded to saturated fats (saturated fatty acids [SFAs]), 18.8±4.3% to monounsaturated fats, and 6.9±2.4% to polyunsaturated fats (polyunsaturated fatty acids [PUFAs]). Fig. 2 shows the percentage of the recommended amount reached exclusively through dietetic sources, with regard to the main micronutrients.
Considering the recommendations regarding the consumption of the different food groups in the Spanish adult population,12 the percentage of individuals falling short of the recommended servings of milk and dairy products (84.3%), fruit (82.3%) and cereals (64.7%) is particularly alarming.
The mean individualized physical activity coefficient (IPAC) was 1.46±0.3. A total of 27.1% of the subjects were sedentary and 60.4% were rated as barely active. The main reported causes of limited physical activity were a lack of energy (86.5%), contact with contaminating substances (82.6%), and pain (21.1%). Quality of sleep was described as “poor or insufficient” by 49.0% of the volunteers, while 38.0% rated sleep as being of “moderate” quality. The mean total energy expenditure (TEE) was 1991.4±312.5kcal/day.
The comparison between TEE and TCV yielded a mean value of −229.9±444.5kcal/day (minimum: −1323.4kcal/day, maximum: +499.7kcal/day). Sixty-eight percent of the patients with MCS presented a negative energy balance. Evaluation of the relationship between calorie imbalance and the different weight categories (underweight, normal weight, and overweight/obesity) yielded no statistically significant results.
DiscussionAlthough the Quick Environmental Exposure and Sensitivity Inventory (QEESI), used in the diagnosis of patients with MCS, addresses some aspects associated with eating and physical activity habits, the literature on these issues is scarce, and little is known about its true impact on patients of this kind.
A large number of patients followed special diets, and the choice of food was conditioned by the symptoms perceived as a result of its consumption. Food sensitivity reactions in patients with MCS have been addressed to a limited extent by other authors, and differences in prevalence have been assessed based on the presence of other comorbidities such as FM and CFS. In this regard, approximately 30% of all MCS patients are affected by problems of this kind.3 Although variability is great, the following foods have often been cited as triggering factors: gluten, lactose, corn, casein, soy, certain food additives (monosodium glutamate, artificial sweeteners and coloring agents), caffeine, nuts, solanaceous plant species, yeast, eggs, and alcoholic beverages.6 The above is in agreement with our own findings, since the great majority of the patients reported reactions to cereals in general and to lactose-containing foods. The decrease in the consumption of these foods implies that the mean recommended cereal and dairy product portions are not reached. This in turn results in an increased risk of macronutrient imbalances and micronutrient deficiencies, and favors the sarcopenic status seen in these individuals,10 thus aggravating the background physiological alterations.
Since over 60% of the patients with MCS also presented CFS and FM, a joint assessment of the dietary habits in these three syndromes could be of interest.1,5
Some authors have reported a higher frequency of food allergies and intolerance in patients with FM and CFS as compared to the rest of the population.13,14 In some cases the patients themselves described digestive problems similar to those seen in irritable bowel syndrome,14 and a substantial proportion considered that dietary changes were needed to control their gastrointestinal symptoms.13 The effects of dietary restriction or the exclusion of gluten and fermentable oligo-, di-, mono-saccharides and polyols (FODMAP) or dairy products in terms of the improvement of digestive symptoms and pain have been studied in these diseases, though the findings have not been included in the clinical guides due to insufficient scientific evidence.14–17 The tendency to avoid triggering foods contributes to the social isolation of these patients.6
The characteristic sensation of intolerance perceived by patients after eating certain foods causes concern and leads them to seek a clinical diagnosis, though often through procedures that are not accredited by medical bodies18 or that are based on information from the mass media lacking scientific support. This can be clearly seen when we consider the proportion of the participants who claimed to have undergone different food intolerance tests. The fact that patients with multiple sensitivities can show significant variations in serum IgG, IgG4 and IgA antibody response to specific antigens6 may lead to unwarranted food exclusion practices due to a mistaken diagnosis of food intolerance. This in turn can contribute to worsening the patient's condition.
In addition to the avoidance of those foods that cause symptoms, patients with MCS often turn to diets containing ecological products or “whole foods” as part of their treatment.8 This is consistent with our own observations, since two out of every three patients included such foods in their diet. The international clinical guides do not include specific dietary recommendations for individuals with central sensitivity disorders.19 We have found no literature reviews addressing vegan, vegetarian or macrobiotic diets in this field, while the effects of exclusion diets have not been examined,20 even though these are common practices among the affected population.21
Routine food supplementing is not advised in MCS or its comorbidities,14,20 due to the lack of scientific evidence concerning its capacity to afford clinical improvement.6 Nevertheless, some studies indicate that supplements are commonly prescribed once the disorder has been diagnosed.13 The present study found the great majority of patients to be taking nutritional supplements/complements on a regular basis. This considerably increased the amount of money spent on food. The consumption of products of this kind is common practice in the healthy population, though to a far lesser extent than in the population with MCS.22
The analysis of macronutrients in the diet of patients with MCS showed lipid and protein intake to exceed the recommendations, while carbohydrate intake fell short of the recommendations. As regards the food lipid profile, saturated fatty acid intake was seen to be higher than desired, while polyunsaturated fatty acid intake was within adequate ranges. These results agree with the latest surveys conducted in the adult Spanish population, in which a deviation from the Mediterranean diet has been confirmed.22 Despite the lack of knowledge of the specific impact which the abovementioned lipid profile could have upon these diseases, the adverse effects of saturated fats in relation to inflammation could be of particular interest in this group.
As regards micronutrient supply, the results indicated that a high proportion of patients failed to reach the recommended intake of magnesium, biotin, folic acid, zinc, vitamin D and calcium. Some of these deficiencies may be associated with the low intake of cereals and dairy products commented on above. Adequate replacement with foods belonging to the same food group but that are better tolerated is therefore advisable. This would help to avoid the nutritional deficiencies that largely condition the clinical course of those diagnosed with central sensitization diseases.20
The hypothesis that MCS is a chronic inflammatory disorder associated with an accumulation of or an excessive response to toxic substances could imply an increase in calorie and nutritional requirements in this group of individuals.6 The fact that we recorded a negative energy balance in over two-thirds of the study sample is a cause for concern, with numerous associated consequences that could have an important impact upon patients with MCS by increasing the risk of malnutrition. No association between body weight and energy balance was demonstrated in our sample. However, the results should be viewed with caution, for although the 72-h consumption record is often used due to its recognized capacity to assess dietary intake, overweight individuals may tend to underestimate the portions of food they eat, this circumstance having been highlighted in other studies as a limiting factor of such questionnaires.23
The typical involvement of the musculoskeletal system in patients with MCS, including generalized weakness, muscle pain and fatigue6,19 worsens if the patient also has FM, CFS or ES.24 This in turn may result in decreased activity, which would be consistent with our own observations, since the individualized physical activity coefficient (IPAC) assigned most of the patients to the sedentary and barely active categories. Some reviews point to the potential benefits of personalizing physical activity regimens in patients with chronic pain25 and depression, which are very common in individuals diagnosed with MCS, FM, CFS and ES.20,24
The chronic nature of MCS, the limited knowledge of its etiopathogenic mechanisms, the lack of therapeutic guidelines and knowledge on the part of the healthcare sector, and the typically highly diverse symptoms all make it necessary to adopt a multidisciplinary approach to these patients. The importance of nutrigenomic and nutritional strategies such as the creation of a personalized food plan is still underestimated. Addressing the disorder from these perspectives therefore may represent a new challenge for investigators and health professionals.14
To our knowledge, this is the first study in Spain to describe the eating patterns and certain nutritional weaknesses that require special attention in this population group. However, the sample size does not allow for the extrapolation of the results to the MCS population, and the cross-sectional design of the study does not allow us to assess the evolution of patient nutritional status.
Multiple chemical sensitivity is a disorder of growing magnitude that is still largely unknown to both society in general and in the healthcare setting in particular. The data obtained in the present study confirm the need for improved eating habits and increased physical activity. Only through comprehensive study of the role of nutrition in MCS can a personalized food plan be designed which takes into account the eating habits, food supplements and physical activity best suited to each individual situation. Nutritional education in this group of subjects may contribute to the avoidance of incomplete, monotonous and unbalanced diets that only worsen the physiological conditions and therefore the quality of life of the affected population.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Thanks are due to the Association of Patients with Chronic Fatigue Syndrome and Multiple Chemical Sensitivity Syndrome of the Community of Madrid (Asociación de Afectados por Síndrome de Fatiga Crónica y por Síndrome de Sensibilidad Química Múltiple de la Comunidad de Madrid [SFC-SQM Madrid]) for its invaluable help in the recruitment of volunteers. Thanks are also due to the latter for agreeing to participate in the study. The collaboration of all of them has helped to improve knowledge and research in the field of MCS.
Please cite this article as: Aguilar-Aguilar E, Marcos-Pasero H, de la Iglesia R, Espinosa-Salinas I, Ramírez de Molina A, Reglero G, et al. Características y condicionantes de la ingesta dietética y actividad física en un grupo de pacientes diagnosticados de sensibilidad química múltiple. Endocrinol Diabetes Nutr. 2018;65:564–570.