To analyze the clinical impact of the Flash glucose monitoring system in patients with type 1 diabetes mellitus (T1DM) treated with continuous subcutaneous insulin infusion (CSII).
MethodsA 24-week retrospective cohort study in CSII-treated T1DM patients exposed (1:1) to the Flash glucose monitoring system vs. self-monitoring of capillary blood glucose (SMBG). The primary outcome was the difference in hemoglobin A1c (HbA1c) levels between both groups at the end of the study.
ResultsThirty-six patients with a mean age of 38.2 years (range 22–55) and a mean T1DM duration of 20.9±7.8 years, treated with CSII for 7.1±5.4 years, were enrolled into the study. At the end of the study, mean HbA1c levels improved in patients in the Flash group (7.1±0.7 vs. 7.8±1.0, p=0.04). Only the Flash group showed a significant decrease in HbA1c levels of −0.4% (95% CI, −0.6, −0.2; p=0.004) during follow-up. Flash patients captured 93.9% of data through 17.8±9.9 scans daily. In fact, the Flash cohort showed a three-fold increase in daily self-monitoring of glucose, while daily frequency of SMBG decreased during the study (−1.8 tests/24h (95% CI −3, −0.7; p=0.01). No safety issues related to Flash use were recorded.
ConclusionsThe Flash glucose monitoring system is a novel approach to improve blood glucose control in CSII-treated T1DM patients. Randomized controlled trials are needed to assess the effectiveness of this system in CSII-treated T1DM patients.
Analizar el efecto clínico de la monitorización Flash de glucosa intersticial en pacientes con diabetes mellitus tipo 1 (DM1) tratados con infusión subcutánea continúa de insulina (ISCI).
MétodoEstudio de cohortes de 24 semanas de duración de sujetos con DM1 tratados con ISCI expuestos (1:1) a monitorización Flash de glucosa intersticial vs. autodeterminación de la glucemia capilar (AGC). El objetivo principal fueron las diferencias en los valores de hemoglobina glucosilada (HbA1c) entre ambos grupos.
ResultadosTreinta y seis pacientes fueron incluidos con edad media de 38,2 años (rango: 22-55 años), duración media de la DM1 de 20,9±7,8 años y tratados con ISCI durante 7,1±5,4 años. Detectamos una mejora al final del estudio en las cifras de HbA1c entre los pacientes del grupo Flash (7,1±0,7 vs. 7,8±1,0; p=0,04). Solo los pacientes del grupo Flash mostraron durante el seguimiento un descenso significativo en los valores de HbA1c de −0,4% (IC 95%: −0,6, −0,2; p=0,004). Los pacientes que usaron Flash capturaron el 93,9% de los datos mediante 17,8±9,9 escaneos diarios. De hecho, los pacientes de la cohorte Flash triplicaron la frecuencia de comprobación de su glucosa aunque la frecuencia diaria de AGC descendió a lo largo del estudio (−1,8 test/24h; IC 95%: −3, −0,7; p=0,01). No se detectaron eventos de seguridad relacionados con el uso de Flash.
ConclusionesLa monitorización Flash de glucosa supone un abordaje novedoso para mejorar el control glucémico en pacientes con DM1 tratados con ISCI. Resultan necesarios ensayos clínicos randomizados en el futuro para valorar con mayor consistencia la efectividad de esta terapia en este subgrupo de pacientes.
Intensive therapy with the goal of maintaining tight glycemic control reduces diabetes chronic complications.1,2 Continuous subcutaneous insulin infusion (CSII) is an effective tool to improve type 1 diabetes mellitus (T1DM) control, although many patients remain with hemoglobin A1c (HbA1c) levels >7%.3–6 Real-time continuous glucose monitoring (RT-CGM) can be added to CSII in order to improve glycemic control although significant reductions in HbA1c levels are usually achieved with at least 60–70% RT-CGM frequency of use.7–16
Flash glucose monitoring (FreeStyle Libre®, Abbott Diabetes Care Inc., Alameda, California, USA) is a factory calibrated system designed to replace capillary blood glucose. Flash glucose monitoring provides real-time interstitial glucose levels and trends of glucose levels, however, these systems do not alarm and do not connect to insulin pumps.17 Two randomized clinical studies have shown that Flash system reduce risk of hypoglycemia in type 2 and T1DM patients without HbA1c improvement.18,19 Recently, a few prospective descriptive studies reported HbA1c reduction associated to Flash use in intensive treated T1DM patients.20–22
Here, we have evaluated the efficacy and safety of Flash glucose monitoring focused in CSII-treated T1DM patients.
MethodsPatientsThirty-six CSII-treated T1DM patients were enrolled at Ciudad Real University Hospital (Castilla-La Mancha Public Health Institute, SESCAM, Ciudad Real, Spain) and Hospital Complex Santiago de Compostela (Galicia Public Health Institute, SERGAS, Santiago de Compostela, Spain). Both centers are tertiary reference clinics for T1DM technological treatment including CSII, RT-CGM and Flash therapies.
Inclusion criteria required age between 18 and 65 years, T1DM diagnosed for >6 months, followed-up by the investigators for at least last 6 months, naïve to Flash glucose monitoring system, and received treatment with CSII for at least the previous six months before inclusion. Exclusion criteria were less than 70% of time Flash wear (Flash cohort), use of a different interstitial glucose monitoring system in the previous six months or simultaneous to study follow-up, current or planned pregnancy and breast-feeding. There were no exclusions for hypoglycemia unawareness, thyroid disease, or Addison's disease.
The protocol was approved by Castilla-La Mancha Public Health Institute Ethic Committee. The study was conducted according to the Declaration of Helsinki. All participants provided written informed consent.
Study designA retrospective two-cohort study was designed. First cohort included all CSII-treated T1DM patients starting and maintaining for at least six months Flash glucose monitoring system between November 2014 (launched Spanish date) and December 2016. Second cohort was obtained through random center sampling (1:1) from all CSII-treated T1DM patients who had never been exposed to Flash system and continued exclusively with self-monitoring of capillary blood glucose (SMBG) during the study period.
Efficacy and security assessmentsThe primary end-point was HbA1c difference between both cohorts at the end of the study. Secondary outcomes included: (1) average change in capillary glucose levels between both groups; (2) Flash use: captured data and daily scans; (3) daily SMBG frequency; (4) insulin requirements: basal and boluses; (5) security: serious device-related events, severe hypoglycemia, and diabetic ketoacidosis (DKA).
Glycated hemoglobin was measured at Hospital Analysis Departments with the use of methods certified by National Glycohemoglobin Standarization Program. Hypoglycemia events and severe hypoglycemia were defined in order to standardized concepts: an event of measured glucose concentration ≤70mg/dl and any hypoglycemia requiring assistance of another person to actively administer carbohydrates, glucagon, or take other corrective actions were used respectively.23 Severe hypoglycemia frequency was calculated from 6-month previous period to each data collection date.
FreeStyle Libre® was the glucose interstitial system used by Flash group. This factory calibrated interstitial glucose monitoring system requires SMBG confirmation in the following circumstances: Flash result and glycemic symptoms mismatch, high glycemic variability, diabetes therapeutic interventions.24 Accu-Chek Aviva Combo® (Roche Diabetes Care Inc., Indianapolis, Indiana, USA), Contour Next Link® and Contour Next Link 2.4® (Ascensia Diabetes Care Holdings AG, Basel, Switzerland) were the glucometers used in the study. Flash cohort data were gathered from visits before Flash start and after six months of treatment with these devices. SMBG cohort data were obtained from first two six-month consecutive visits carried out in the clinical follow-up of these patients during 2016. SMBG and Flash data were obtained from the previous 4 weeks to each visit. Data collection was conducted between June and July 2017 through chart reviews completed by a site investigator for each center and included the following data: date of birth, date of onset of diabetes, the age at which pump therapy began, pump model, Flash start date, Flash captured data percentage and daily scans, body mass index, insulin requirement (UI/kg/24h), basal and bolus insulin percentages, number of boluses, frequency of SMBG, carbohydrate daily intake, serious adverse effect related with Flash, severe hypoglycemia and DKA. This information was obtained from electronic medical records, Emminens eConecta® (Emminens Healthcare Service Inc., San Cugat del Valles, Barcelona, Spain) internet webpage (https://www.emminens-econecta.com/eConecta/Inicio/InicioAction?idioma=es) and two different specialized softwares: Medtronic Carelink Pro v4.0C® (Medtronic Inc., Dublin, Ireland) and FreeStyle Libre v1.0® (Abbott Diabetes Care Inc., Alameda, California, USA).
Statistical analysisMann–Whitney U and Wilcoxon signed-rank nonparametric tests were used to analyze statistical differences between groups and differences between baseline and study end, respectively. T-test and paired t-test was used to analyze continuous variables and their changes over time when both groups were considered together. We performed a multiple linear regression in order to examine the linear relationships between HbA1c response and predictors (age, gender, T1DM duration >10 years, CSII treatment longer than 5 years, HbA1c >8%). Significance was taken at p<0.05. Analyses were performed with IBM SPSS software version 12.0® for Windows® (SPSS Inc., Chicago, Illinois, USA).
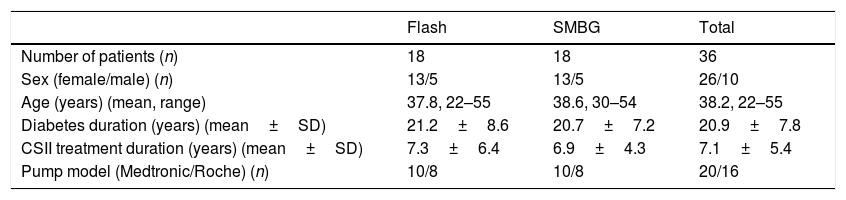
ResultsPatientsAmong the centers, we identified 23 patients on combined CSII and Flash treatment. After a careful evaluation of the data, 5 patients were excluded due to: pregnancy (one patient), planning pregnancy (two patients), less than 70% of the time Flash adherence (one patient), combined therapy (CSII+Flash) less than six months (one patient). The final analysis included thirty-six patients (18 in Flash cohort and 18 in SMBG cohort). Each hospital contributed equally (50%) to obtain the sample. Demographics and baseline characteristics by group are shown in Table 1. No statistically differences were found between them. All patients in Flash group started intermittent glucose monitoring according to a health professional advice. Flash glucose monitoring was self-financed by all patients.
Baseline characteristics of the patients.
| Flash | SMBG | Total | |
|---|---|---|---|
| Number of patients (n) | 18 | 18 | 36 |
| Sex (female/male) (n) | 13/5 | 13/5 | 26/10 |
| Age (years) (mean, range) | 37.8, 22–55 | 38.6, 30–54 | 38.2, 22–55 |
| Diabetes duration (years) (mean±SD) | 21.2±8.6 | 20.7±7.2 | 20.9±7.8 |
| CSII treatment duration (years) (mean±SD) | 7.3±6.4 | 6.9±4.3 | 7.1±5.4 |
| Pump model (Medtronic/Roche) (n) | 10/8 | 10/8 | 20/16 |
SMBG, self-monitoring of capillary blood glucose; CSII, continuous subcutaneous insulin infusion.
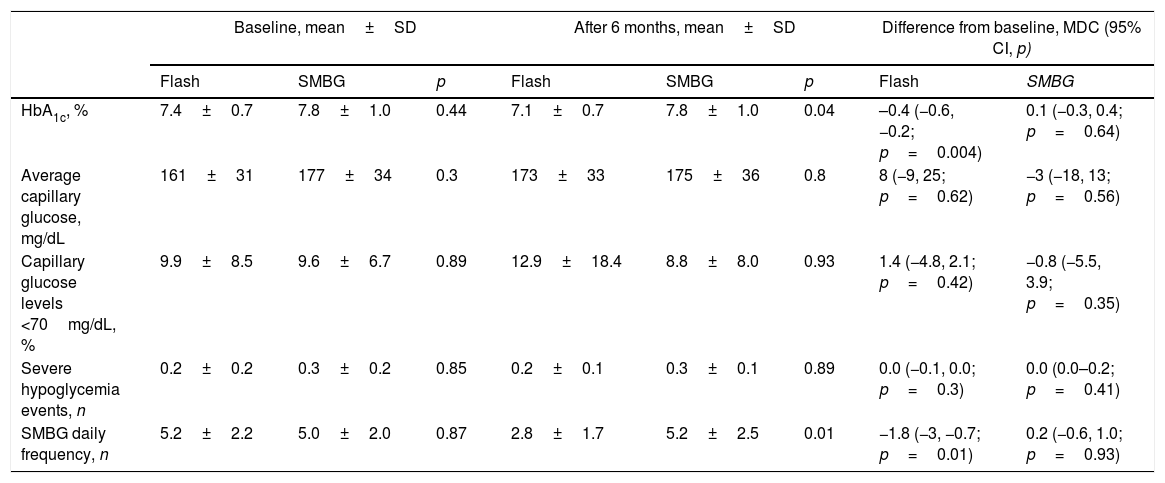
We detected an improvement in mean HbA1c among Flash patients at the study end (7.1±0.7 vs. 7.8±1.0, p=0.04). Only Flash group showed a significant HbA1c reduction of −0.4% (95% CI, −0.6, −0.2; p=0.004) during the follow-up, whereas SMBG patients did not show this clinical benefit (mean difference in change, 0.1%; 95% CI, −0.3, 0.4; p=0.64).
However, average capillary glucose levels did not change during the study and were similar between both groups at 24-weeks (173±33mg/dL vs. 175±36mg/dL; p=0.8). We did not detect differences in capillary hypoglycemic frequency during the study duration (Table 2).
Glycemic outcomes.
| Baseline, mean±SD | After 6 months, mean±SD | Difference from baseline, MDC (95% CI, p) | ||||||
|---|---|---|---|---|---|---|---|---|
| Flash | SMBG | p | Flash | SMBG | p | Flash | SMBG | |
| HbA1c, % | 7.4±0.7 | 7.8±1.0 | 0.44 | 7.1±0.7 | 7.8±1.0 | 0.04 | –0.4 (−0.6, −0.2; p=0.004) | 0.1 (−0.3, 0.4; p=0.64) |
| Average capillary glucose, mg/dL | 161±31 | 177±34 | 0.3 | 173±33 | 175±36 | 0.8 | 8 (−9, 25; p=0.62) | −3 (−18, 13; p=0.56) |
| Capillary glucose levels <70mg/dL, % | 9.9±8.5 | 9.6±6.7 | 0.89 | 12.9±18.4 | 8.8±8.0 | 0.93 | 1.4 (−4.8, 2.1; p=0.42) | −0.8 (−5.5, 3.9; p=0.35) |
| Severe hypoglycemia events, n | 0.2±0.2 | 0.3±0.2 | 0.85 | 0.2±0.1 | 0.3±0.1 | 0.89 | 0.0 (−0.1, 0.0; p=0.3) | 0.0 (0.0–0.2; p=0.41) |
| SMBG daily frequency, n | 5.2±2.2 | 5.0±2.0 | 0.87 | 2.8±1.7 | 5.2±2.5 | 0.01 | −1.8 (−3, −0.7; p=0.01) | 0.2 (−0.6, 1.0; p=0.93) |
SMBG, self-monitoring of capillary blood glucose; MDC, mean difference in change; CI, confidence interval.
Predictors of response were not detected in both groups of patients.
Flash useAll Flash patients received an interstitial glucose monitoring educational program before starting FreeStyle Libre. Patients were encouraged to maximize its use during diabetes program.
Flash patients captured 93.9% of data through 17.8±9.9 scans per day. No correlation between Flash use and glycemic variables was detected.
We corroborated a reduction in daily SMBG frequency in Flash patients at study end (2.8±1.7 test/day vs. 5.2±2.5 test/day; p=0.01). Furthermore, only Flash group showed a reduction in SMBG frequency from baseline to the end of the study (mean difference in change, −1.8 test/day; 95% CI, −3.0, −0.7; p=0.01).
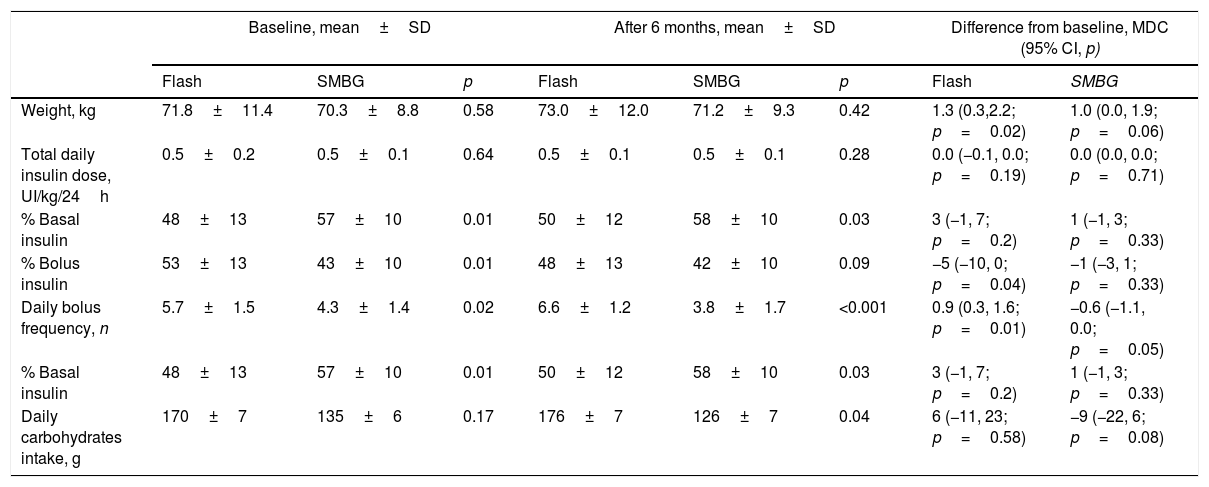
Insulin requirementInsulin requirement maintained stable during the study with no between-group difference. The initial amount of basal insulin was greater in SMBG patients compared to Flash group (57±10% vs. 48±13%, p=0.01). Flash patients showed higher initial bolus insulin proportion (53±13% vs. 43±10%, p=0.01) and higher daily bolus frequency (5.7±1.5 bolus/24h vs. 4.3±1.4 bolus/24h, p=0.02). These differences were stable at the study end.
A daily bolus frequency increase was observed from baseline to study end only in Flash group (mean difference in change, 0.9 bolus/24h; 95% CI, 0.3, 1.6; p=0.01). Complementary insulin outcomes are shown in Table 3.
Weight, insulin and carbohydrates intake outcomes.
| Baseline, mean±SD | After 6 months, mean±SD | Difference from baseline, MDC (95% CI, p) | ||||||
|---|---|---|---|---|---|---|---|---|
| Flash | SMBG | p | Flash | SMBG | p | Flash | SMBG | |
| Weight, kg | 71.8±11.4 | 70.3±8.8 | 0.58 | 73.0±12.0 | 71.2±9.3 | 0.42 | 1.3 (0.3,2.2; p=0.02) | 1.0 (0.0, 1.9; p=0.06) |
| Total daily insulin dose, UI/kg/24h | 0.5±0.2 | 0.5±0.1 | 0.64 | 0.5±0.1 | 0.5±0.1 | 0.28 | 0.0 (−0.1, 0.0; p=0.19) | 0.0 (0.0, 0.0; p=0.71) |
| % Basal insulin | 48±13 | 57±10 | 0.01 | 50±12 | 58±10 | 0.03 | 3 (−1, 7; p=0.2) | 1 (−1, 3; p=0.33) |
| % Bolus insulin | 53±13 | 43±10 | 0.01 | 48±13 | 42±10 | 0.09 | −5 (−10, 0; p=0.04) | −1 (−3, 1; p=0.33) |
| Daily bolus frequency, n | 5.7±1.5 | 4.3±1.4 | 0.02 | 6.6±1.2 | 3.8±1.7 | <0.001 | 0.9 (0.3, 1.6; p=0.01) | −0.6 (−1.1, 0.0; p=0.05) |
| % Basal insulin | 48±13 | 57±10 | 0.01 | 50±12 | 58±10 | 0.03 | 3 (−1, 7; p=0.2) | 1 (−1, 3; p=0.33) |
| Daily carbohydrates intake, g | 170±7 | 135±6 | 0.17 | 176±7 | 126±7 | 0.04 | 6 (−11, 23; p=0.58) | −9 (−22, 6; p=0.08) |
SMBG, self-monitoring of capillary blood glucose; MDC, mean difference in change; CI, confidence interval.
No episodes of serious device-related events occurred. No patient died during the follow-up. Severe hypoglycemia incidence was similar among both groups at study end (0.2±0.1 events vs. 0.3±0.1 events; p=0.89). No DKA episodes were detected during the study.
DiscussionThe evidence about beneficial impact of Flash technology in CSII treated T1DM patients is limited compared with RT-CGM.17,19–22 We demonstrated a significant HbA1c improvement associated with a reduction in daily SMBG frequency only among Flash patients. The novelty of our present results resides in HbA1c reduction associated with Flash use focused in CSII patients compared with those using only SMBG. For the first time, our results exclusively came from CSII-treated T1DM patients and not from mixed, CSII and multiple dose insulin injection, intensive-treated patient groups.
Flash interstitial glucose monitoring system reduced time, frequency and area under curve in hypoglycaemia in T1DM patients.19 Nevertheless, between-group HbA1c differences were not found in the IMPACT study. In our study, Flash group showed a significant HbA1c reduction of –0.4% (95% CI, −0.6, −0.2; p=0.004) during the follow-up suggesting additional benefits to hypoglycaemia reduction associated with Flash wear. IMPACT study was perform in well or suboptimum T1DM patients with HbA1c levels less than 7.5% (screening mean HbA1c 6.7±0.5%) whereas our initial mean HbA1c was higher (7.4±0.7%). Initial HbA1c is a well-known limiting factor in T1DM patients study results, well-controlled T1DM patients (as IMPACT patients) may not improve further by adding new technologies.25 Thus, our start HbA1c levels could have allowed finding these differences. Differences found herein can be considered both relevant and trustworthy.
The use of Flash therapy had previously demonstrated a reducing hypoglycemia effect without increasing HbA1c values in patients with T1DM.26 In Bolinder et al. report, the average time spent in hypoglycaemia was reduced by 38%, dropping by 1.30h/24h from 3.4h/24h at baseline to 2h/24h compared with no changes in control group.19 These results came from interstitial glucose monitoring records. Nevertheless, we could not find any hypoglycemia benefits associated with Flash use. Our hypoglycemia results came from isolated capillary data and not from 24-hour interstitial glucose reports since a comparable basal interstitial glucose monitoring was not possible due to retrospective study characteristic. It seems conceivable that a higher information amount could be necessary to attain possible hypoglycemia better results. Added to this, loss of information justified by less SMBG frequency in Flash group (−1.8 test/day; 95% CI, −3.0, −0.7; p=0.01) could be behind this lack of effect. Moreover, RT-CGM more effectively reduces time spent in hypoglycaemia in T1DM patients and impaired awareness of hypoglycaemia.27 Our data are supported by this study conducted by Reddy et al. where Flash patients did not showed hypoglycemic reduction associated to intermittent glucose monitoring use.
SMBG frequency was maintained by control cohort patients along the study, whereas it was decreased in the Flash group and replaced with sensor scanning. Flash-exposed cohort showed a three-times increase in daily self-monitoring of glucose control (17.8±9.9 scans/day). Our scanning frequency was similar to previously reported in the IMPACT study.19 This corroborated high confidence in using current, historic, and trend sensor Flash glucose data for self-management.
Flash patients showed an increase of 0.9 boluses/day (95% IC, 0.3, 1.6; p=0.01). However, at the end of IMPACT study there were no differences in total daily doses of insulin or bolus/basal insulin ratios between the study groups.19 Basal differences in bolus frequency between our both cohorts probably limit better understanding of insulin pump use during the study.
Our last set of analysis was aimed at assessing Flash safety. As reported in the IMPACT study, we did not find any serious adverse event related to the device or DKA.19 Thus, Flash technology can be considered a secure diabetes treatment.
There are, nevertheless, some evident limitations inherent to this study. First, these data correspond to a small sized cohort study. The study size was not estimated to detect any of the studied endpoints (including security assessment) because of the low expected frequency of patients receiving combined therapy with Flash plus CSII. In addition, we only observed the effect of Flash on the study subjects, so we play no role in assigning exposure to the study subjects.28 Previously published data reported 60–70% RT-CGM adherence as the lower limit to obtain HbA1c reductions, thus we settled a minimum of 70% of time Flash wear as exclusion criteria. This criterion limited our results and conclusions to higher Flash users. Moreover, early technology adopters are more likely to maintain high Flash wear. In fact, just one patient (4.4%) from the initial screened sample used Flash less than 70% of the time, whereas previously reported interstitial glucose monitoring device adherence was lower (20–30% patients using less than 60–70% of the possible time).9–16 The interventions were known to participants and investigators given the nature of medical devices. Finally, assessment of severe hypoglycemia relied on patient medical records. Patients recall of episodes or official clinical register, such as emergency assist records, were not investigated to check severe hypoglycemia frequency.
Nevertheless, our humble cohort study may give an idea about Flash clinical efficacy in CSII-treated T1DM patients. This information is necessary for proper planning of the randomized controlled trials. Often, RCT will confirm what has been found in the preceding observation studies. Sometimes, RCT are delayed due to different market strategies among technological developers involved.
ConclusionsIn conclusion, we suggest Flash technology as a novel approach that may help improve diabetes control in CSII-treated T1DM patients.
Funding sourcesNo funding or sponsorship was received for this study or publication of this article.
Conflict of interestThe authors declare that they have no conflicts of interest concerning this article.
The authors are very grateful to all patients for their altruistic collaboration.